ABSTRACT
Cytosolic phospholipase A2alpha (cPLA2α) is a key mediator of tumorigenesis. In this study, by using a combination of pharmacological and genetic approaches in cell models and patient samples, we identify cPLA2α as a selective target to increase chemosensitivity in cervical cancer. We found that transcript and protein levels of cPLA2α but not other forms of cPLA2 (e.g., cPLA2β and cPLA2αδ) were consistently increased in all tested malignant cervical cancer cells and tissues compared to normal counterparts, suggesting that cPLA2α upregulation is a common feature in cervical cancer. We further found that promoting growth and survival rather than invasion were the predominant roles of cPLA2α on cervical cancer. In addition, chemotherapeutic agents achieved ~100% inhibition efficacy in cPLA2α-depleted cervical cancer cells, demonstrating the important role of cPLA2α in chemoresistance. Importantly, we identify that β-catenin is critically involved in the molecular mechanism of cPLA2α’s action in cervical cancer. In summary, our work demonstrates the multiple essential roles of cPLA2α in cervical cancer, particularly in chemoresistance, via a β-catenin-dependent manner. Our work also suggests that targeting cPLA2α has a therapeutic value in overcoming chemoresistance in cervical cancer or other cPLA2α-regulated cancers.
1. Introduction
Cervical cancer is the most common cause of cancer-related death in women worldwide despite advances in prevention, screening, diagnosis, and treatment.Citation1 The chemotherapeutic agent cisplatin is the standard treatment for advanced cervical cancer patients but failed to achieve complete response.Citation2 Patients with advanced cervical cancer have poor prognosis and rapidly developed resistance to chemotherapy.Citation3 The molecular mechanisms underlying chemoresistance are complicated and include overexpression of anti-apoptotic proteins, aberrant activation of eukaryotic translation initiation factor 4E (eIF4E)/β-catenin, and epithelial–mesenchymal transition.Citation3–Citation5 Novel agents targeting these molecular pathways are currently being studied. Alternative approaches are needed to overcome chemoresistance.
Phospholipases A2s (PLA2s) are a group of enzymes which cleave phospholipids to release arachidonic acid and lysophospholipids. Cytosolic phospholipase A2 alpha (cPLA2) is one of the major isoforms of PLA2s. In addition, cPLA2 family consists of six members including cPLA2α, -β, -γ, -δ, -ε, and -ζ.Citation6 cPLA2α is a predominant source of arachidonic acid and plays a key role in pathophysiology of cancer and inflammation.Citation7,Citation8 cPLA2α has been identified to be upregulated in various human cancers including lung, breast, colon, and prostate cancers.Citation9–Citation12 Diverse stimuli such as calcium mobilization and activation of mitogen-activated protein kinases can activate cPLA2α.Citation13 The activated cPLA2α mediates cellular activities through modulating FOXO1 and ERK1/2.Citation14–Citation16 Particularly in tumor cells, cPLA2α has been shown to activate PI3K/AKT and inhibit Smad2/3 during epithelial–mesenchymal transition of hepatocellular carcinoma cells.Citation17 Targeting cPLA2α also inhibits colorectal cancer cell growth via suppressing constitutively activated Akt.Citation18
In this study, we analyzed the expression of cPLA2α and its other isoforms in normal and malignant cervical tissues and cells. Combining both genetic and pharmacological approaches, we further investigated the role of cPLA2α in cervical cancer growth, survival, metastasis, and chemo-response and analyzed the possible underlying mechanism of cPLA2α inhibition. We are the first to demonstrate that cPLA2α can be served as a therapeutic target to increase chemosensitivity in cervical cancer.
2. Materials and methods
2.1 Cells and materials
Human cervical cancer cell lines were cultured as previously reported in our study.Citation19 Immortalized normal cervical epithelial cell line Ect1/E6E7 (ATCC® CRL-2614™) and primary normal human cervical epithelial cell HCvEpC (Cell Applications Inc., US) were cultured as per manufacturer's protocol. Paclitaxel and cisplatin were purchased from Sigma, US. Pyrrophenone and RSC-3388 were purchased from Santa Cruz Biotechnology, US. Lithium chloride (LiCl) was purchased from Sigma, US. Antibodies for cPLAα, p-Akt, Akt, p-eIF4E, eIF4E, β-catenin, and β-actin were from Santa Cruz, US; cPLAβ antibody was from Biorbyt, US; and cPLAδ antibody was from St John’s Laboratory, US.
2.2 Patient tissue specimens and immunohistochemistry
This study was approved by the ethics committee of Huangjiahu Hospital of the Hubei University of Chinese Medicine. Samples were obtained from the Department of Tissue Repository. The sectioned tissues were deparaffinized and hydrated by incubating in xylene and then in decreasing concentrations of ethanol solution. Antigen retrieval on sections was performed using citrate acid followed by staining using designated primary (1: 200 X dilution) and secondary antibodies (1: 1000X dilution). Sections were counterstained with hematoxylin (Sigma, US). Quantification of staining was conducted by using ImageJ software. Results were analyzed in a blinded fashion.
2.3 CPLAα enzyme activity assay
Cells were treated with drugs for 24 h. Cells were homogenized, and the enzyme activity of cPLA2 was determined using the cPLA2 Assay Kit (Cayman Chemical Inc., US) according to manufacturer’s instructions. Arachidonoyl thio-PC was used as the substrate to measure cPLA2 activity. The absorbance value was measured at 414 nm and normalized to protein concentration.
2.4 Proliferation and apoptosis assays
Cells were treated with drugs for 72 h. Cell proliferation activity was determined using BrdU Cell Proliferation Assay Kit (Abcam, US). The absorbance was measured using a microplate reader. Apoptosis was determined using Annexin V-FITC and PI Apoptosis Detection Kit (BD Pharmingen, US), followed by flow cytometry (Beckman Coulter, US).
2.5 Boyden chamber migration assay
Cell migration assay was performed using Boyden chamber (Cell Biolabs Inc., US) as per manufacturer's protocol. Briefly, drugs at various concentrations and cells at 10,000/well with media (5% Fetal Bovine Serum (FBS)) were added into a cylindrical cell culture insert nested inside the well of a cell culture plate. Twenty percent FBS was placed into the lower chamber. After 6 h incubation in cell culture incubator, cells on the upper surface of the insert were then removed with a cotton swab. Migratory cells on the lower surface of the insert were fixed with 4% formaldehyde (Sigma, US), stained with 0.4% Giemsa, and counted under a microscope (Zeiss, Germany).
2.6 Transfection
Transfection was done using DharmaFECT1 reagent as per manufacturer’s protocol. Specific cPLA2α knockdown was achieved by transfecting cells with two siRNAs against different regions of human cPLA2α. siRNA sequence was customized and purchased from Ambion (Ambion Inc., CA, USA) (Supplementary Table 1). Cell assays were performed after 48 h post-transfection. Overexpression of β-catenin was achieved by transfecting cells with 1.5 μg pcDNA or pcDNA-β-cat (human β-catenin pcDNA3 plasmid, a kind gift from Dr. Hui Li).Citation20 Cell assays were performed after 24 h post-transfection. For TOPflash report assay, cells transfected with an M50 Super 8x TOPFlash plasmid. After 24 h post-transfection, cells were treated with drugs for another 24 h prior to assessing β-catenin activity using the Luciferase Reporter Assay System (Promega, US) as per manufacturer’s protocol. β-catenin activity was quantified by normalizing SuperTopFlash to β-gal.
2.7 RNA extraction and real-time PCR
RNA purification, cDNA preparation, and quantitative real-time PCR were carried out using the same protocol as reported in our past study.Citation19 The sequences of the primers are designed and obtained from the BioRad Inc., US (Supplementary Table 2). The relative mRNA expression levels of designated genes were first normalized with GAPDH levels.
2.8 Statistical analyses
All data are expressed as mean and standard deviation to indicate data variability. Statistical analyses were performed by paired Student’s t test. A p-value <0.05 was considered statistically significant.
3. Results
3.1 cPLA2α is specifically upregulated in cervical carcinoma in vitro and in vivo
The expression level of cPLA2α was analyzed in normal and malignant cervical tissue specimens by immunohistochemistry. We observed a consistent low-to-moderate expression level of cPLA2α in normal cervical tissues from 16 healthy donors ()). In contrast, moderate-to-high expression level of cPLA2α was detected in malignant cervical tissues from 20 patients with cervical cancer, suggesting that cPLA2α is upregulated in cervical cancer. In addition, we did not observe any significant correlation between the degree of cPLA2α upregulation and disease stages (data not shown). We next investigated the expression level of other cPLA2 isoforms such as cPLA2β and cPLA2δ. We found that cPLA2β and cPLA2δ expression levels were similar in normal and malignant cervical tissues ()). Quantification of section staining also demonstrated that cPLA2α but not cPLA2β or cPLA2δ was significantly increased in cervical carcinoma than normal cervical tissues ()). In line with the in vivo findings, both mRNA and protein levels of cPLA2α but not cPLA2β or cPLA2δ were significantly increased in a panel of human cervical cancer cell lines including human papillomavirus (HPV)-positive (CaLo, SiHa, and CaSki) and HPV-negative (ViBo) than immortalized human normal cervical epithelial cell line (Ect1/E6E7) or primary human normal cervical epithelial cell (HCvEpC)( and Supplementary Figure 1). These results demonstrate the specific upregulation of cPLA2α but not other cPLA2 isoforms in cervical cancer. In addition, the cell lines used in our study are representative of cervical cancer tissues in patients.
Figure 1. cPLA2α is specifically upregulated in cervical cancer tissues.
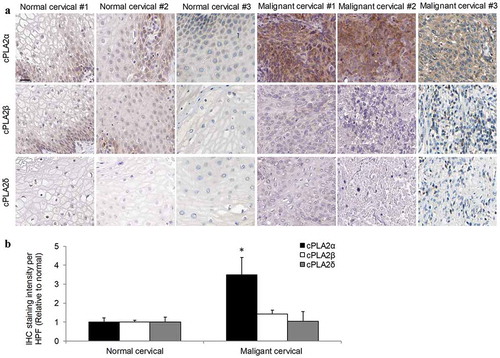
Figure 2. cPLA2α is upregulated in cervical cancer cells.
3.2 cPLA2α inhibition suppresses growth and survival without affecting migration in cervical cancer cells
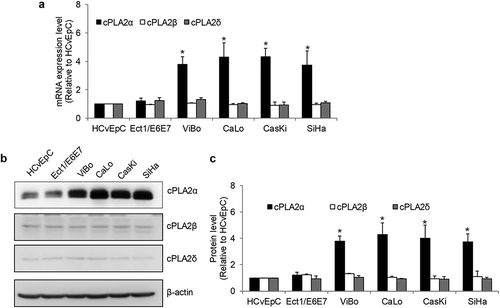
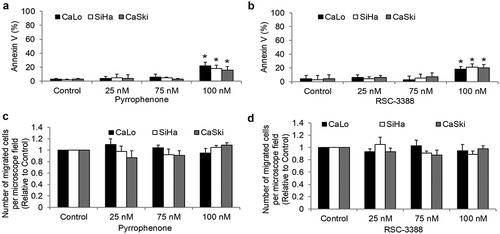
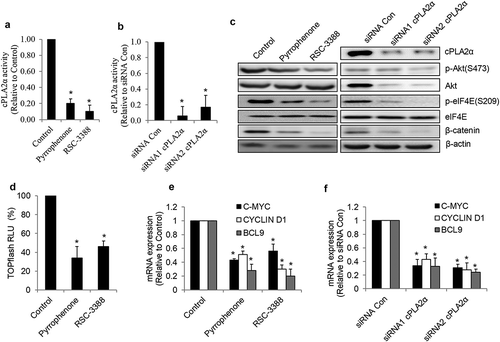
To understand the possible functions of cPLA2α in cervical cancer, we inhibited cPLA2α using pharmacological inhibitors pyrrophenone and RSC-3388 and analyzed the multiple biological aspects of cervical cancer cells including growth, survival, and migration. Pyrrophenone and RSC-3388 are potent and specific cPLA2α inhibitors with an IC50 of ~35 nM in enzyme assays.Citation21,Citation22 We showed that pyrrophenone and RSC-3388 at 25, 75, and 100 nM dose-dependently decreased cPLA2α enzyme activity in cervical cancer cells, with IC50 at ~30 nM (). The maximal concentration of pyrrophenone and RSC-3388 used in this study is 100 nM which has been shown to specifically inhibit cPLA2α activity without off-target effects.Citation23 We found that pyrrophenone and RSC-3388 at 75 and 100 nM inhibited proliferation and at 100 nM induced apoptosis in CaLo, SiHa, and CaSki cells () and ). In addition, pyrrophenone and RSC-3388 up to 100 nM do not affect cervical cancer migration (). These results suggest that promoting growth and survival rather than invasion are the predominant roles of cPLA2α on cervical cancer.
Figure 3. Inhibition of cPLA2α inhibits the growth of cervical cancer cells. cPLA2α inhibitors pyrrophenone (a) and RSC-3388 (b) significantly inhibit cPLA2α enzyme activity. cPLA2α inhibitors pyrrophenone (c) and RSC-3388 (d) significantly inhibit proliferation of cervical cancer cells.
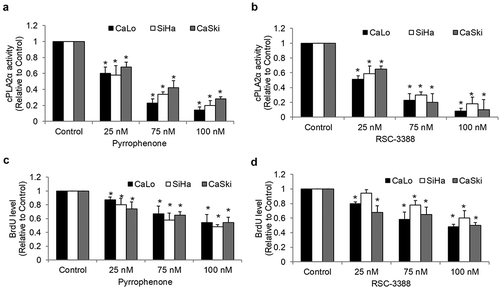
Figure 4. Inhibition of cPLA2α inhibits survival without affecting migration of cervical cancer cells. Pyrrophenone (a) and RSC-3388 (b) at 100 nM significantly induce apoptosis in cervical cancer cells. Pyrrophenone (c) and RSC-3388 (d) up to 100 nM do not affect cervical cancer migration.
3.3 cPLA2α inhibition remarkably increases chemosensitivity in cervical cancer cells
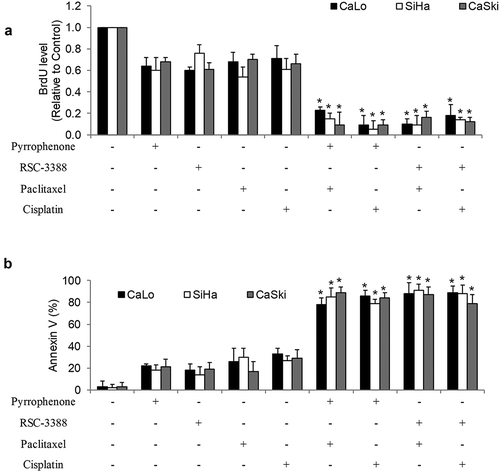
To understand whether cPLA2α plays a role in chemoresistance, we investigated the combinatory effects of cPLA2α inhibition with chemotherapeutic agents: paclitaxel and cisplatin, which are mostly used for cervical cancer treatment. In order to observe the combinatory effects, the concentration of each drug we used for combination studies is the concentration that induces subtoxic inhibitory effects. We found that the combination of paclitaxel with pyrrophenone or RSC-3388 is significantly more effective than a single drug alone (). It is worth noting that the complete inhibition of proliferation and induction of apoptosis was observed in the combination group. In addition, the remarkable enhancement is not limited to the combination with paclitaxel but other chemotherapeutic agents such as cisplatin ().
Figure 5. cPLA2α inhibition using a pharmacological approach enhances chemosensitivity in cervical cancer cells. cPLA2α inhibitors pyrrophenone and RSC-3388 significantly enhance chemotherapeutic agents’ (paclitaxel and cisplatin) effects in inhibiting proliferation (a) and inducing apoptosis (b) in CaLo, SiHa, and CaSki cells. RSC-3388 and pyrrophenone at 100 nM and paclitaxel at 10 nM and cisplatin at 100 nM were used in the combination studies.
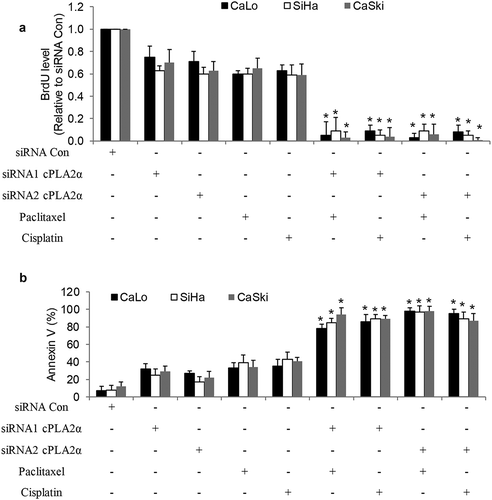
To further confirm the significant role of cPLA2α in chemoresistance, we conducted cPLA2α knockdown using two siRNAs that specifically target different regions of cPLA2α. Similar to cPLA2α inhibitors, cPLA2α siRNA knockdown alone mildly inhibited ~40% proliferation and induced ~30% apoptosis in cervical cancer cells (). However, paclitaxel or cisplatin almost achieved ~100% inhibition in cPLA2α-depleted cervical cancer cells, demonstrating that cPLA2α inhibition remarkably increases chemosensitivity. These results also suggest that cPLA2α might play an important role in cervical cancer chemoresistance.
Figure 6. cPLA2α inhibition using a genetic approach enhances chemosensitivity in cervical cancer cells.
3.4 cPLA2α inhibition acts on cervical cancer cells in a β-catenin-dependent manner
We and others have demonstrated that eukaryotic translation initiation factor 4E (eIF4E)/β-catenin axis is critically involved in the chemoresistance in various cancers.Citation19,Citation24,Citation25 Given the importance of cPLA2α in cervical cancer chemoresistance, we examined the eIF4E/β-catenin signaling in CaLo cells after cPLA2α inhibition. We found that inhibition of cPLA2α enzyme activity by both pharmacological and genetic approaches decreased phosphorylation of eIF4E and β-catenin in CaLo () and Supplementary Figures 2–5), suggesting that cPLA2α inhibition suppresses eIF4E/β-catenin signaling in cervical cancer cells. Consistently, we further observed that cPLA2α inhibition decreased p-Akt which regulates eIF4E phosphorylation and active β-catenin levels.Citation26 In addition, we found that β-catenin activity and the transcriptional levels of Wnt/β-catenin-mediated genes (e.g., BCL9, C-MYC, and CYCLIN D) were decreased in CaLo cells treated with cPLA2α inhibitors or transfected with cPLA2α siRNA ().
Figure 7. cPLA2α inhibition suppresses Akt/eIF4E/β-catenin signaling in cervical cells. cPLA2α inhibition by inhibitors (a) or siRNA (b) significantly decreases cPLA2α enzyme activity in cervical cancer cells.
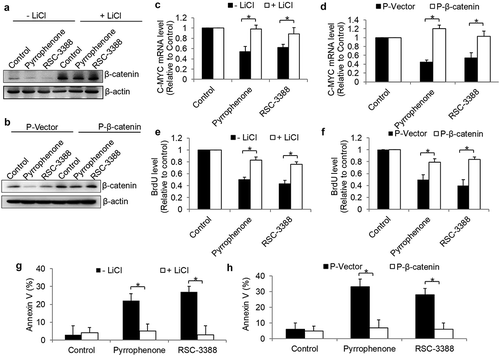
We next rescued β-catenin levels in CaLo cells in the presence of pyrrophenone and RSC-3388 using both pharmacological (e.g., LiCl) and genetic (e.g., β-catenin overexpression) approaches to investigate whether β-catenin stabilization can reverse the inhibitory effects of cPLA2α inhibitors. LiCl is a known inhibitor of glycogen synthase kinase-3β (GSK-3β) that activates Wnt signaling through inhibiting β-catenin degradation.Citation27 We treated cervical cancer cells with cPLA2α inhibitor in the absence or presence of LiCl. We observed a significant decrease in β-catenin levels in cells treated with cPLA2α inhibitor, whereas no significant decrease in β-catenin levels was detected in the presence of both LiCl and cPLA2α inhibitors (). Additionally, we achieved a good efficacy of β-catenin overexpression as no decrease in β-catenin was detected in the presence of pyrrophenone or RSC-3388 in β-catenin-overexpressing cervical cancer cells and Supplementary Figures 6 and 7). Importantly, we found that in the cells treated with LiCl or overexpressing β-catenin, pyrrophenone or RSC-3388 was ineffective in decreasing transcription of β-catenin-targeted gene (e.g., C-MYC), inhibiting proliferation, and inducing apoptosis (–h)). These results confirm that cPLA2α inhibition acts on cervical cancer cells in a β-catenin-dependent manner.
Figure 8. cPLA2α inhibition acts on cervical cancer cells in a β-catenin-dependent manner.
4. Discussion
Although combination therapy of cisplatin and paclitaxel is the most effective chemotherapeutic regimen to treat patients with advanced or recurrent cervical cancer, patients still develop resistance in a fast manner and continue to relapse.Citation28 Better understanding of the molecular mechanism underlying chemoresistance is essential to the development of targeted therapy to overcome resistance in cervical cancer. Our previous study has shown that eIF4E-β-catenin axis is aberrantly activated and plays a critical role in the development of cervical cancer chemoresistance.Citation19 In this work, we further demonstrate that cPLA2α may serve as an upstream regulator of eIF4E-β-catenin axis in cervical cancer. In addition, cPLA2α inhibition remarkably increases chemosensitivity in cervical cancer.
We found that cPLA2α overexpression is a consistent feature in 20 out of 20 tested samples with cervical cancer tissues obtained from patients regardless of disease stages compared to normal counterparts ( and and data not shown). cPLA2α has been reported to be overexpressed in non-small lung cancer cells, invasive breast cancer tissues, and highly metastatic hepatocellular carcinoma cells compared to their normal counterparts.Citation17,Citation29,Citation30 We and others, on the differential expression pattern of cPLA2α in malignant versus normal cancer, suggest that cPLA2α is a selective therapeutic target in cancer. The aberrant overexpression and activation of cPLA2α in invasive and highly metastatic carcinoma cells suggest that cPLA2α may play important role in cancer metastasis. Interestingly, this feature is limited to cPLA2α but not cPLA2β and cPLA2δ ( and ). This is consistent with the findings that only cPLA2α but not other isoforms of cPLA2 plays role in pathophysiology of cancers.Citation31
Using both pharmacological and genetic approaches, we identified that cPLA2α is involved in cervical cancer growth and survival but not migration ( and ). This finding is consistent with the previous work on the biological roles of cPLA2α on prostate, colon, and lung cancers.Citation10–Citation12 Interestingly, knockdown of cPLA2α inhibited migration of hepatocellular carcinoma cellsCitation17 without affecting migration of cervical cancer cells (), suggesting that the role of cPLA2α might be cancer type specific. Notably, although cPLA2α inhibition alone shows moderate anti-proliferative and pro-apoptotic effects, its combination with chemotherapeutic agents achieved almost complete inhibition on cervical cancer growth and survival ( and ). This demonstrates the critical role of cPLA2α in cervical cancer chemoresistance. While most studies of cPLA2α have focused on its roles in cancer transformation and development,Citation17,Citation32–Citation34 the possible role of cPLA2α in cancer chemoresistance has recently garnered attention. Li et al.’s work demonstrated that cPLA2α is activated in breast cancer patients after chemotherapy and cPLA2α blockage overcomes breast cancer chemoresistance.Citation23 Expression of cPLA2α has been recently demonstrated to be associated with resistance to chemotherapy in glioblastoma.Citation35 In agreement with Li et al.’s and Yang et al.’s works, we show that cPLA2α plays a critical role in cancer cell response to chemotherapy. We and others suggest that cPLA2α is an attractive target in combination with chemotherapy in cancer.
A significant finding of our work is the identification of β-catenin suppression as a consequence of cPLA2α inhibition in cervical cancer cells, possibly through Akt/eIF4E inhibition. We observed the decreased phosphorylation of Akt, eIF4E, and β-catenin levels as well as reduced Wnt/β-catenin activities in cPLA2α-depleted cervical cancer cells or cells exposed to cPLA2α inhibitors (). The decreased Akt phosphorylation by cPLA2α inhibition observed in our study is consistent with the previous work on the molecular signaling analysis of cPLA2α activation or inhibition in cancer cells.Citation17,Citation23 Additionally, inhibition of cPLA2α has been shown to result in a reduction in phosphorylated Akt and expression of cyclin D1.Citation12 Our study further extends the previous work by showing that targeting cPLA2α results in β-catenin inhibition in cervical cancer cells might be via suppressing Akt/eIF4E. The rescue of cPLA2α inhibitors’ effects by β-catenin stabilization using both LiCl and β-catenin overexpression further confirms that cPLA2α inhibition acts on cervical cancer cell via β-catenin-dependent manner (). Notably, LiCl is a known inhibitor of GSK-3β through Akt activation, connecting Akt and β-catenin.Citation27 This finding is consistent with our previous work that eIF4E-β-catenin axis confers cervical cancer chemoresistance and inhibition of eIF4E-β-catenin axis overcomes chemoresistance.Citation19 This also well explains the action of cPLA2α inhibition in sensitizing cervical cancer cell response to chemotherapeutic agents. It is known that Wnt/β-catenin signaling is essential for cancer stem cell functions.Citation36–Citation39 The suppression of Wnt/β-catenin by cPLA2α inhibition suggests a possible role of cPLA2α in the cancer stem cell.
In conclusion, our findings demonstrate that cPLA2α upregulation confers cervical cancer chemoresistance and that inhibition of cPLA2α effectively increases chemosensitivity. In addition, we are the first to demonstrate that β-catenin is a downstream effector of cPLA2α in cervical cancer, likely via Akt/eIF4E. Because normal stem cells also depend on β-catenin signaling for proper functions, strategies to specifically target cancer stem cells will require identification of target capable of distinguishing between normal and tumor stem cells. Our results on the differential expression pattern of cPLA2α suggest that cPLA2α is a selective target and cPLA2α inhibitors are likely to inhibit β-catenin in tumor stem cells without affecting normal stem cell function. These results suggest that cPLA2α inhibitors have utility in treating patients with cervical cancer and possibly with other β-catenin-driven cancers.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (3.1 MB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:5–29.
- Kumar L, Harish P, Malik PS, Khurana S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr Probl Cancer. 2018. doi:10.1016/j.currproblcancer.2018.01.016.
- Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther. 2016;10:1885–1895. doi:10.2147/DDDT.S106412.
- Chen J, Xiong J, Liu H, Chernenko G, Tang SC. Distinct BAG-1 isoforms have different anti-apoptotic functions in BAG-1-transfected C33A human cervical carcinoma cell line. Oncogene. 2002;21:7050–7059. doi:10.1038/sj.onc.1205845.
- Chao CC. Enhanced excision repair of DNA damage due to cis-diamminedichloroplatinum(II) in resistant cervix carcinoma HeLa cells. Eur J Pharmacol. 1994;268:347–355.
- Leslie CC. Cytosolic phospholipase A(2): physiological function and role in disease. J Lipid Res. 2015;56:1386–1402. doi:10.1194/jlr.R057588.
- Hanel AM, Schuttel S, Gelb MH. Processive interfacial catalysis by mammalian 85-kilodalton phospholipase A2 enzymes on product-containing vesicles: application to the determination of substrate preferences. Biochemistry. 1993;32:5949–5958.
- Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI. Brain lipid metabolism in the cPLA2 knockout mouse. J Lipid Res. 2003;44:109–117.
- Caiazza F, Harvey BJ, Thomas W. Cytosolic phospholipase A2 activation correlates with HER2 overexpression and mediates estrogen-dependent breast cancer cell growth. Molecular Endocrinology. 2010;24:953–968. doi:10.1210/me.2009-0293.
- Blaine SA, Wick M, Dessev C, Nemenoff RA. Induction of cPLA2 in lung epithelial cells and non-small cell lung cancer is mediated by Sp1 and c-Jun. J Biol Chem. 2001;276:42737–42743. doi:10.1074/jbc.M107773200.
- Parhamifar L, Jeppsson B, Sjolander A. Activation of cPLA2 is required for leukotriene D4-induced proliferation in colon cancer cells. Carcinogenesis. 2005;26:1988–1998. doi:10.1093/carcin/bgi159.
- Patel MI, Singh J, Niknami M, Kurek C, Yao M, Lu S, Maclean F, King NJ, Gelb MH, Scott KF, et al. Cytosolic phospholipase A2-alpha: a potential therapeutic target for prostate cancer. Clin Cancer Res. 2008;14:8070–8079. doi:10.1158/1078-0432.CCR-08-0566.
- Shimizu T, Ohto T, Kita Y. Cytosolic phospholipase A2: biochemical properties and physiological roles. IUBMB Life. 2006;58:328–333. doi:10.1080/15216540600702289.
- Naini SM, Choukroun GJ, Ryan JR, Hentschel DM, Shah JV, Bonventre JV. Cytosolic phospholipase A2alpha regulates G1 progression through modulating FOXO1 activity. FASEB J. 2016;30:1155–1170. doi:10.1096/fj.15-278416.
- Hua S, Yao M, Vignarajan S, Witting P, Hejazi L, Gong Z, Teng Y, Niknami M, Assinder S, Richardson D, et al. Cytosolic phospholipase A2alpha sustains pAKT, pERK and AR levels in PTEN-null/mutated prostate cancer cells. Biochim Biophys Acta. 2013;1831:1146–1157. doi:10.1016/j.bbalip.2013.02.011.
- Thomas W, Coen N, Faherty S, Flatharta CO, Harvey BJ. Estrogen induces phospholipase A2 activation through ERK1/2 to mobilize intracellular calcium in MCF-7 cells. Steroids. 2006;71:256–265. doi:10.1016/j.steroids.2005.10.010.
- Fu H, He Y, Qi L, Chen L, Luo Y, Chen L, Li Y, Zhang N, Guo H. cPLA2alpha activates PI3K/AKT and inhibits Smad2/3 during epithelial-mesenchymal transition of hepatocellular carcinoma cells. Cancer Lett. 2017;403:260–270. doi:10.1016/j.canlet.2017.06.022.
- Zheng Z, He X, Xie C, Hua S, Li J, Wang T, Yao M, Vignarajan S, Teng Y, Hejazi L, et al. Targeting cytosolic phospholipase A2 alpha in colorectal cancer cells inhibits constitutively activated protein kinase B (AKT) and cell proliferation. Oncotarget. 2014;5:12304–12316. doi:10.18632/oncotarget.2639.
- Xu H, Wang Z, Xu L, Mo G, Duan G, Wang Y, Sun Z, Chen H. Targeting the eIF4E/beta-catenin axis sensitizes cervical carcinoma squamous cells to chemotherapy. Am J Transl Res. 2017;9:1203–1212.
- Li H, Jiao S, Li X, Banu H, Hamal S, Wang X. Therapeutic effects of antibiotic drug tigecycline against cervical squamous cell carcinoma by inhibiting Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2015;467:14–20. doi:10.1016/j.bbrc.2015.09.140.
- Yamamoto M, Haruna T, Imura K, Hikita I, Furue Y, Higashino K, Gahara Y, Deguchi M, Yasui K, Arimura A. Inhibitory effect of a potent and selective cytosolic phospholipase A2alpha inhibitor RSC-3388 on skin inflammation in mice. Pharmacology. 2008;81:301–311. doi:10.1159/000117816.
- Yun B, Lee H, Ewing H, Gelb MH, Leslie CC. Off-target effect of the cPLA2alpha inhibitor pyrrophenone: inhibition of calcium release from the endoplasmic reticulum. Biochem Biophys Res Commun. 2016;479:61–66. doi:10.1016/j.bbrc.2016.09.033.
- Li Z, Qu M, Sun Y, Wan H, Chai F, Liu L, Zhang P. Blockage of cytosolic phospholipase A2 alpha sensitizes aggressive breast cancer to doxorubicin through suppressing ERK and mTOR kinases. Biochem Biophys Res Commun. 2018;496:153–158. doi:10.1016/j.bbrc.2018.01.016.
- Li Z, Sun Y, Qu M, Wan H, Cai F, Zhang P. Inhibiting the MNK-eIF4E-beta-catenin axis increases the responsiveness of aggressive breast cancer cells to chemotherapy. Oncotarget. 2017;8:2906–2915. doi:10.18632/oncotarget.13772.
- Lim S, Saw TY, Zhang M, Janes MR, Nacro K, Hill J, Lim AQ, Chang CT, Fruman DA, Rizzieri DA, et al. Targeting of the MNK-eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc Natl Acad Sci USA. 2013;110:E2298–2307. doi:10.1073/pnas.1301838110.
- Wang X, Yue P, Chan C-B, Ye K, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Fu H, Khuri FR, Sun S-Y. Inhibition of mammalian target of rapamycin induces phosphatidylinositol 3-kinase-dependent and Mnk-mediated eukaryotic translation initiation factor 4E phosphorylation. Mol Cell Biol. 2007;27:7405–7413. doi:10.1128/MCB.00760-07.
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668.
- Lorusso D, Petrelli F, Coinu A, Raspagliesi F, Barni S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol Oncol. 2014;133:117–123. doi:10.1016/j.ygyno.2014.01.042.
- Sundarraj S, Kannan S. Immunohistochemical expression of cytosolic phospholipase A2alpha in non-small cell lung carcinoma. Asian Pac J Cancer Prev. 2010;11:1367–1372.
- Chen L, Jin T, Zhu K, Piao Y, Quan T, Quan C, Lin Z. PI3K/mTOR dual inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A synergistically exert anti-tumor activity in breast cancer. Oncotarget. 2017;8:11937–11949. doi:10.18632/oncotarget.14442.
- Yarla NS, Bishayee A, Vadlakonda L, Chintala R, Duddukuri GR, Reddanna P, Dowluru KS. Phospholipase A2 isoforms as novel targets for prevention and treatment of inflammatory and oncologic diseases. Curr Drug Targets. 2016;17:1940–1962.
- Chen L, Fu H, Luo Y, Chen L, Cheng R, Zhang N, Guo H. cPLA2alpha mediates TGF-beta-induced epithelial-mesenchymal transition in breast cancer through PI3k/Akt signaling. Cell Death Dis. 2017;8:e2728. doi:10.1038/cddis.2017.518.
- Nakanishi M, Rosenberg DW. Roles of cPLA2alpha and arachidonic acid in cancer. Biochim Biophys Acta. 2006;1761:1335–1343. doi:10.1016/j.bbalip.2006.09.005.
- Gresham A, Masferrer J, Chen X, Leal-Khouri S, Pentland AP. Increased synthesis of high-molecular-weight cPLA2 mediates early UV-induced PGE2 in human skin. Am J Physiol. 1996;270:C1037–1050. doi:10.1152/ajpcell.1996.270.4.C1037.
- Yang L, Zhang H. Expression of cytosolic phospholipase a2 alpha in glioblastoma is associated with resistance to chemotherapy. Am J Med Sci. 2018;356:391–398. doi:10.1016/j.amjms.2018.06.019.
- Xu L, Zhang L, Hu C, Liang S, Fei X, Yan N, Zhang Y, Zhang F. WNT pathway inhibitor pyrvinium pamoate inhibits the self-renewal and metastasis of breast cancer stem cells. Int J Oncol. 2016;48:1175–1186. doi:10.3892/ijo.2016.3337.
- Boone JD, Arend RC, Johnston BE, Cooper SJ, Gilchrist SA, Oelschlager DK, Grizzle WE, McGwin G Jr., Gangrade A, Straughn JM Jr., et al. Targeting the Wnt/beta-catenin pathway in primary ovarian cancer with the porcupine inhibitor WNT974. Lab Invest. 2016;96:249–259. doi:10.1038/labinvest.2015.150.
- Bello JO, Nieva LO, Paredes AC, Gonzalez AM, Zavaleta LR, Lizano M. Regulation of the wnt/beta-catenin signaling pathway by human papillomavirus E6 and E7 oncoproteins. Viruses. 2015;7:4734–4755. doi:10.3390/v7082842.
- Ring A, Kim YM, Kahn M. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev. 2014;10:512–525. doi:10.1007/s12015-014-9515-2.
