ABSTRACT
Malignant peritoneal mesothelioma is a very rare tumor originating from the peritoneal serous mesothelium. Meningeal metastasis of malignant peritoneal is even more rare. Here, we reported a case of a 60-year-old female patient with a history of exposure to asbestos for 10 years who presented with massive peritoneal effusion followed by disorder of consciousness and symptoms of cranial nerve injury. The patient was diagnosed as peritoneal mesothelioma with meningeal metastasis through neurological symptoms, cytological finding of cerebrospinal fluid combined with cranial magnetic resonance imaging (MRI). The patient received systemic chemotherapy and total craniospinal irradiation. The follow up visits showed that the survival time of patient after diagnosis of meningeal metastasis from peritoneal mesothelioma was 5 months. To our knowledge, this is the first case of menigeal metastasis of peritoneal mesothelioma. We hope this particular case may be helpful in providing some experience to the treatment of peritoneal mesothelioma with meningeal metastasis.
Introduction
Mesothelioma is a rare tumor originating from serous cavities, including the pleura, peritoneum, pericardium, and capsule. In fact, mesothelioma can occur in anybody cavity covered with mesothelial cells.Citation1,Citation2 Peritoneal mesothelioma was first reported by Miller and Wynn in 1908 and is the second most common mesothelioma following pleural mesothelioma, accounting for 12.5–25% of all mesotheliomas.Citation3,Citation4 Peritoneal mesothelioma is more common in males with a history of asbestos exposure.Citation2 In addition, the insidious onset and a lack of symptom specificity often lead to misdiagnosis.
Meningeal metastasis, also known as carcinomatous meningitis, is a serious complication of malignant tumors. The incidence of meningeal metastases varies among different types of tumors and is about 5% of all malignant tumors. However, autopsy studies suggest that 19% of cancer patients with neurological symptoms and signs have meningeal metastasis.Citation5 The clinical manifestations of meningeal metastasis differ greatly among patients, the most common being symptoms and signs of cranial nerve injury and cranial nerve paralysis.Citation6,Citation7 The most used methods for meningeal metastases diagnosis are magnetic resonance imaging (MRI) and cytological study of tumor cells in the cerebral spinal fluid (CSF), the latter being the gold standard of diagnosis.Citation8
With the improvement in cancer therapeutics and diagnostic methods, the incidence of meningioma has been increasing in recent years.Citation9 The incidence of peritoneal mesothelioma with meningeal metastasis is particularly rare. Here, we presented such a case and described the diagnosis, treatment and the outcome. To our knowledge, this is the first case report of its kind.
Case report
The patient was a 60-year-old woman who was admitted to the Department of Radiation and Medical Oncology (Gynecological Oncology Ward) at Zhongnan Hospital of Wuhan University (Wuhan, China) on February 25,th 2017. Previously, she was treated in local hospital on January 10,th, 2017 because of progressive abdominal distension for half a month. Computed tomography (CT) scan and ultrasonography of the abdomen showed massive ascites. Having excluded possible ascites caused by cardiac failure, liver of kidney dysfunctions and other disorders, an exploratory laparotomy was performed. Extensive miliary nodules were found in the pelvic cavity. Then, a total hysterectomy, bilateral adnexectomy, pelvic lymph node dissection, greater omentum resection, appendectomy, and peritoneal lesion resection were performed. Histopathological examination demonstrated a disseminated peritoneal mesothelioma.
For family history, we noted that her mother diagnosed as gastric cancer at the age of 63, and her aunt diagnosed as breast cancer at the age of 60. Otherwise, the patient had no special past medical or surgical history except for a career exposure to asbestos for about 10 years.
The patient was well recovered after operation and came to our hospital for further treatment. The routine tests of blood cell counts, coagulation functions, hepatic and renal functions were normal. The tests of hepatitis B, hepatitis C, human immunodeficiency virus and syphilis were all negative. The tumor marker detection showed high serum level of CA125 of 96.4 U/mL (reference range: 0–35 U/ml), CA153 of 54.5 U/mL (reference range: 0–31.3 U/mL), CA724 of 12.51 U/mL (reference range: 0–6.9 U/mL), HE4 148.2 pmol/L (reference range: 0–74.3 pmol/L), while other markers including CEA, AFP, CA199, SCC, β-hCG, were at normal levels. Chest CT examination showed no obvious abnormality. CT examination of the whole abdomen showed postoperative changes of peritoneal mesothelioma and a little effusion in the abdominal and pelvic cavity. No obvious signs of recurrence or metastasis were observed. In addition, we re-examined the pathological specimens in our hospital and confirmed the diagnosis of a malignant mesothelioma (). Then, the patient was treated with a systemic chemotherapy regimen with pemetrexed 700 mg on day 1; cis-platinum 45 mg on days 1–3. From February 2017 to October 2017, the patient received nine cycles of chemotherapies in our hospital. The treatment was well tolerated. CT and MRI were performed regularly. No obvious recurrence or metastasis was found.
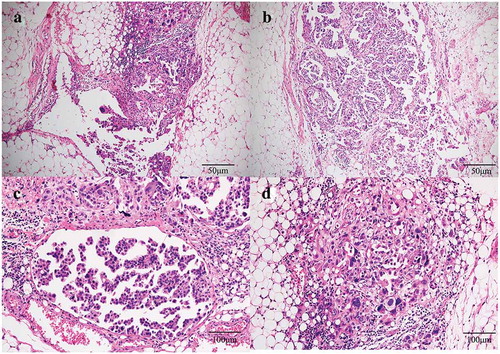
Figure 1. Histopathology staining of malignant peritoneal mesothelioma from patient. (a) and (b): HE staining showing a migration between malignant tumor cells and normal mesenteric mesothelial cells. Invasive growth and obvious nuclear atypia are shown in tumor cells (100×). (c) and (d): HE staining showing the tumor cells are in the epithelioid cell morphology, with large nuclear staining, coarse chromatin and obvious nucleoli (200×).
However, the patient returned to our hospital with back pain for 6 days on December 15,th 2018. Serum tumor marker was detected and abnormally high levels of some tumor markers were noted: CA 125 452.8 U/mL, CA199 64.09 U/mL, HE4, 252.9 pmol/L. Positron Emission Tomography-CT(PET-CT) revealed recurrence of disease. The image showed there were multiple peritoneum thickening in the greater omentum, the mesentery and the pelvic floor with increased FDG uptake (). At the same time, the head MRI revealed multiple abnormal signals in the sulci of bilateral cerebral hemisphere and vermis with abnormal enhancement foci (). Four days later, the patient experienced two times of temporary loss of consciousness and blindness after waking up. Neurologically, bilateral pupil dilation occurred that recovered later, but a left facial paralysis persisted. Tumor cells were found via pasteurization staining in the cerebral spinal fluid (CSF). CSF cytology conducted in the laboratory at Zhongnan Hospital showed that a group of cells were suspected tumor cells. They had larger bodies and irregular nuclei, visible nucleolus, abundant cytoplasm, and deep staining (). The tumor cell finding in CSF is the only reliable basis for diagnosis of meningeal metastasis. Therefore, a diagnosis of meningeal metastasis from peritoneal mesothelioma was identified. Considering the patient’s family history of cancer, we performed genetic testing on patient’s DNA from specimen of tumor tissue after surgery using next-generation sequencing (NGS) targeting over 600 cancer-relevant genes. The assay was done using a commercial test (No. 18s4146315-1, BGI Genomics, BGI-Shenzhen, China.). Genomic DNA from the whole blood sample was used as germline control. We detected several genomic alterations known to be associated with cancer, and six gene mutations were identified, including TP53 (c.725G>T), ARAF (c.176G>Cc), ITGB2 (c.2081-2A>G), PDGFRB (c.976C>T), POLQ (c.7571G>T), PALB2 (c.1269_1283del). The detail information is shown in . After a thorough discussion of multi-disciplinary team (MDT), the patient was treated with craniospinal irradiation by Tomotherapy. The target was given 32.5Gy in total in 18 fractions. The dose distribution is shown in . The patient completed the planned radiotherapy without significant side effect. By the end of radiotherapy, symptoms like back pain, disorder of consciousness, and blindness improved significantly. Then, the patient received three cycles of temozolomide treatment and was under scheduled follow-up later. Disease progression appeared in April 2019 and the patient died on May 15,th 2019.
Figure 2. PET-CT showing recurrence and metastasis of malignant mesothelioma. Increased FDG uptake was shown in omentum (a) mesentery (b), pelvic floor (c and d).
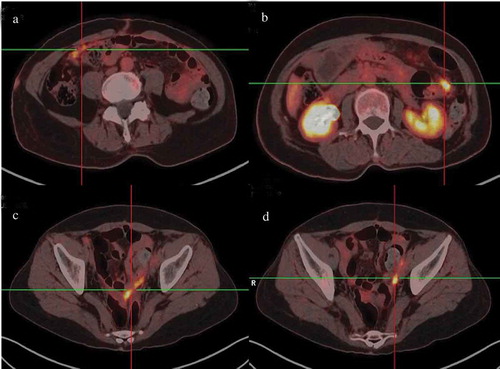
Figure 3. Head magnetic resonance imaging (MRI) scans from patient. (a) and (b) an enhanced T1-weighted imaging (T1WI) images demonstrated abnormal hyperintense signal representing leptomeningeal metastatic disease in the cerebellar folia. (c) Axial T2 fluid-attenuated inversion recovery (FLAIR) images of leptomeningeal showing the same enhancement. (d) Sagittal postcontrast T1-weighted images demonstrated the evidence of metastases in leptomeningeal.
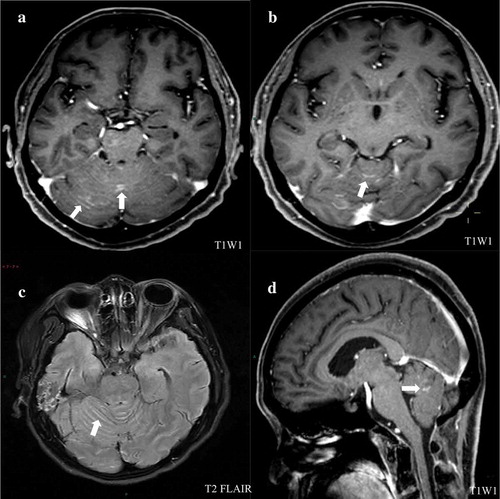
Figure 4. Cytological examination of cerebrospinal fluid staining confirmed meningeal metastasis from peritoneal mesothelioma. Cerebrospinal fluid staining showing that cells had larger bodies and irregular nuclei, visible nucleolus, abundant cytoplasm, and deep staining (200×).
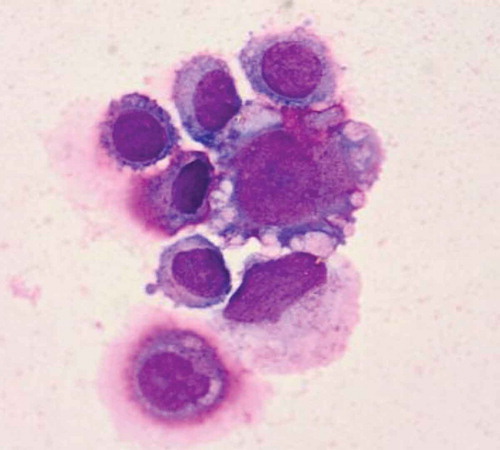
Figure 5. Tomotherapy plan for patient. (a) Axial, coronal, and sagittal distributions for patient, the areas in red received 32.5 Gy. (b) Dose–volume histograms showed OARs were well protected.
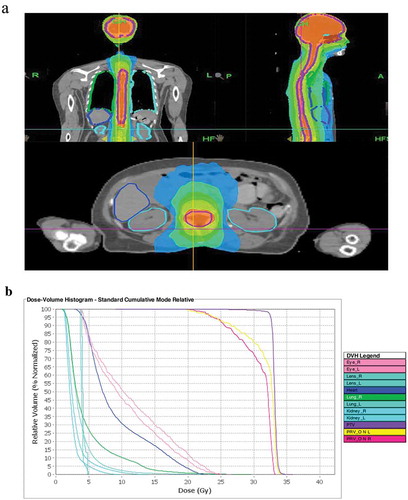
Table 1. Somatic mutation.
Discussion
Malignant peritoneal mesothelioma (MPM) is a rare kind of cancer which originating from peritoneal mesothelial cells. Hodgson et al. reported that the annual incidence of malignant peritoneal mesothelioma in Europe is about 1 to 2 in 1 million, and the number of new cases in the United States is 250 every year.Citation10 The age of onset is mostly between 50 and 60 years old, and patients younger than 20 years old account for only 2%~5%.Citation11 As the second most common mesothelioma following pleural mesothelioma, malignant peritoneal mesothelioma is considered to be associated with long-term exposure to asbestos. In Turkey, the incidence of malignant peritoneal mesothelioma is relative higher than that in other places which may be attributed to the use of asbestos-containing buildings.Citation12 On the other side, Attanoos et al. reported that only 50% of patients with peritoneal mesothelioma had a history of asbestos exposure, suggesting other risk factors also may be involved in the oncogenesis in MPM.Citation13 For instances, Simian virus 40 (SV40), erionite, genetic disorders, radiation exposure, and other factors may also be related to the development of mesothelioma. Indeed, SV40 can act as a synergistic factor with asbestos fiber or as an independent risk factor of malignant peritoneal mesothelioma development.Citation14–Citation17
Meningeal metastasis is a serious complication of malignant tumors, which confers poor prognosis to the majority of these patients. Among solid tumors, melanoma had a strongest tendency of meningeal metastasis, but breast cancer and lung cancer are becoming the most common tumors tending to develop meningeal metastasis due to their higher incidence. It is reported that about 5% of breast cancers, 9%~25% of lung cancers, 20% of melanoma, and 1.7%~5.5% of gastric cancers would eventually develop meningeal metastases.Citation18–Citation20 To our knowledge, there has been no previous literature describing meningeal metastasis from malignant peritoneal mesothelioma. We reported first case of its kind that was confirmed by cytological findings.
Patients with peritoneal malignancies usually have symptoms such as abdominal pain, abdominal distension, abdominal enlargement, loss of appetite and weight, and ascites. A few patients have low fever, night sweats, and high blood clots.Citation21,Citation22 Diagnosis of peritoneal malignancies mainly depends on pathological biopsy, including cytology examination of ascites and postoperative biopsy. In addition, it is reported that hyaluronic acid and CA125 are helpful for the diagnosis of peritoneal malignancies, and CA125 is positively correlated with the degree of tumor damage and can be used as an indicator for diagnosis, efficacy evaluation, and prognosis.Citation23 The clinical signs and symptoms of meningeal metastasis are highly variable because they affect the entire neuraxis. Most patients have symptoms and signs of cranial nerves, brain, and spinal cord when diagnosed. But dementia or confusion state is difficult to be attributed to meningeal metastasis. Therefore, supplementary examinations are required for an accurate diagnosis.Citation24–Citation27 Contrast-enhanced MRI scanning is the main imaging methods for the diagnosis of meningeal metastasis. The finding of malignant tumor cells in cerebrospinal fluid (CSF) is the gold standard for the diagnosis of meningeal metastasis.Citation28
For patients with peritoneal mesothelioma, tumor reduction surgery combined with intraperitoneal hyperthermic perfusion is the mainstream treatment at present, which could inhibit ascites generation, reduce tumor load but produces less adverse reactions than systemic chemotherapy. Additionally, it is also effective for patients with recurrent disease and may improve the overall survival.Citation29,Citation30 In addition, preoperative induction chemotherapy, intraoperative and postoperative adjuvant chemotherapy are also shown to significantly reduce tumor recurrence and bring survival benefit. Currently, pemetrexed combined with platinum (cisplatin or carboplatin) is a recommended regimen worldwide. Nevertheless, patients with meningeal metastasis have a significantly poor prognosis. The median survival of those patients without any treatment is only 4 to 6 weeks and is 4 to 6 months for patients with treatment.Citation5,Citation31 The treatment of meningeal metastases is palliative at present. Radiotherapy of symptomatic lesions is often effective in improving symptoms. Craniospinal irradiation is associated with high mortality and severe myelosuppression. Intrathecal chemotherapy has become the main treatment method for meningeal metastasis. However, few drugs are effective. These include MTX, ara-c and tiotipide, as well as some biological agents such as lL-2, IFN and LAK.Citation32 Many researches concerning gene therapy. The genetic test from this patient indicated that there were six gene mutations. According to the researches, the mutation of TP53 (c.725g >T) can lead to the loss of the protein’s trans-activation ability, and affect the binding of DNA and the stability of p53 protein.Citation33–Citation35 This mutation may be related to the occurrence and development of the disease. In addition, Melaiu reported that PDGFRB silencing will reduce the proliferation rate of malignant pleural mesothelioma cell lines, reduce the ability of colony formation, and promote apoptosis.Citation36 Therefore, the patient’s gene mutation such as PDGFRB c.976C>T may have important influence on gene function. The other four mutated genes could also be deleterious mutations. However, according to the results of gene testing, no available targeted therapeutic drugs were found.
When a patient with history of long-term exposure to asbestos shows symptoms of progressive abdominal distension, the possibility of peritoneal mesothelioma should be taken into consideration. When symptoms of cranial nerve injury occurred in patients with peritoneal mesothelioma, cytological examination of the CSF should be carried out to evaluate the existence of meningeal metastasis. This is the first paper that presents a rare case with metastasis of peritoneal mesothelioma to meningeal. We hope this particular case may be helpful in improving treatment of peritoneal mesothelioma with meningeal metastasis. More studies are needed to improve the treatment of malignant peritoneal mesothelioma with or without meningeal metastasis.
Conclusion
This is a rare case of peritoneal mesothelioma with meningeal metastasis. More attention should be attached to peritoneal mesothelioma and meningeal metastasis when symptom of progressive abdominal distension and cranial nerve injury occur, respectively. More studies are needed to normalize the treatment of malignant peritoneal mesothelioma and/or with meningeal metastasis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Dessy E, Falleni M, Braidotti P, Del Curto B, Panigalli T, Pietra GG. Unusual clear cell variant of epithelioid mesothelioma. Arch Pathol Lab Med. 2001;125:1588–1590. doi:10.1043/0003-9985(2001)125<1588:UCCVOE>2.0.CO;2.
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi:10.1056/NEJMra050152.
- Munkholm-Larsen S, Cao CQ, Yan TD. Malignant peritoneal mesothelioma. World J Gastrointest Surg. 2009;1:38–48. doi:10.4240/wjgs.v1.i1.38.
- Remon J, Reguart N, Corral J, Lianes P. Malignant pleural mesothelioma: new hope in the horizon with novel therapeutic strategies. Cancer Treat Rev. 2015;1:27–34. doi:10.1016/j.ctrv.2014.10.007.
- Ray-Coquard I, Biron P, Bachelot T, Guastalla JP, Catimel G, Merrouche Y, Droz JP, Chauvin F, Blay JY. Vinorelbine and cisplatin (CIVIC regimen) for the treatment of metastatic breast carcinoma after failure of anthracycline- and/or paclitaxel-containing regimens. Cancer. 1998;82:134–140.
- Morikawa A, Jordan L, Rozner R, Patil S, Boire A, Pentsova E, Seidman AD. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17:23–28. doi:10.1016/j.clbc.2016.07.002.
- Lee S, Ahn HK, Park YH, Nam DH, Lee JI, Park W, Choi DH, Huh SJ, Park KT, Ahn JS, et al. Leptomeningeal metastases from breast cancer: intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat. 2011;129:809–817. doi:10.1007/s10549-011-1682-0.
- Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013;Suppl 4:S265–288.
- Kokkoris CP. Leptomeningeal carcinomatosis how does cancer reach the pia-arachnoid? Cancer. 1983;51:154–160. doi:10.1002/1097-0142(19830101)51:1<154::aid-cncr2820510130>3.0.co;2-k.
- Hodgson JT, McElvenny DM, Darnton AJ, Price MJ, Peto J. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92:587–593. doi:10.1038/sj.bjc.6602307.
- Coffin CM, Dehner LP. Mesothelial and related neoplasms in children and adolescents: a clinicopathologic and immunohistochemical analysis of eight cases. Pediatr Pathol. 1992;12:333–347.
- Marchevsky AM, Wick MR. Current controversies regarding the role of asbestos exposure in the causation of malignant mesothelioma: the need for an evidence-based approach to develop medicolegal guidelines. Ann Diagn Pathol. 2003;7:321–332.
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology. 1997;30:403–418.
- Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, Baris YI. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7:147–154. doi:10.1038/nrc2068.
- Carbone M, Pass HI, Miele L, Bocchetta M. New developments about the association of SV40 with human mesothelioma. Oncogene. 2003;22:5173–5180. doi:10.1038/sj.onc.1206552.
- Amin AM, Mason C, Rowe P. Diffuse malignant mesothelioma of the peritoneum following abdominal radiotherapy. Eur J Surg Oncol. 2001;27:214–215. doi:10.1053/ejso.2000.1024.
- Kane AB. Animal models of malignant mesothelioma. Inhal Toxicol. 2006;18:1001–1004. doi:10.1080/08958370600835393.
- Zachariah B, Zachariah SB, Varghese R, Balducci L. Carcinomatous meningitis: clinical manifestations and management. Int J Clin Pharmacol Ther. 1995;33:7–12.
- Taillibert S, Laigle-Donadey F, Chodkiewicz C, Sanson M, Hoang-Xuan K, Delattre JY. Leptomeningeal metastases from solid malignancy: a review. J Neurooncol. 2005;75:85–99. doi:10.1007/s11060-004-8101-x.
- Forman A. Leptomeningeal carcinomatosis. Am J Clin Oncol. 1990;13:536–540.
- Baker PM, Clement PB, Young RH. Malignant peritoneal mesothelioma in women: a study of 75 cases with emphasis on their morphologic spectrum and differential diagnosis. Am J Clin Pathol. 2005;123:724–737. doi:10.1309/2h0n-vrer-pp2l-jdua.
- Acherman YI, Welch LS, Bromley CM, Sugarbaker PH. Clinical presentation of peritoneal mesothelioma. Tumori. 2003;89:269–273.
- Baratti D, Kusamura S, Martinetti A, Seregni E, Oliva DG, Laterza B, Deraco M. Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2007;14:500–508. doi:10.1245/s10434-006-9192-8.
- Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, Fuks J, Portenoy R. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol. 1990;9:225–229.
- Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49:759–772. doi:10.1002/1097-0142(19820215)49:4<759::aid-cncr2820490427>3.0.co;2-7.
- Little JR, Dale AJ, Okazaki H. Meningeal carcinomatosis Clinical manifestations. Arch Neurol. 1974;30:138–143. doi:10.1001/archneur.1974.00490320026003.
- Wolfgang G, Marcus D, Ulrike S. LC: clinical syndrome in different primaries. J Neurooncol. 1998;38:103–110.
- Glantz MJ, Cole BF, Glantz LK, Cobb J, Mills P, Lekos A, Walters BC, Recht LD. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82:733–739. doi:10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z.
- Chase LG, Yang S, Zachar V, Yang Z, Lakshmipathy U, Bradford J, Boucher SE, Vemuri MC. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl Med. 2012;1:750–758. doi:10.5966/sctm.2012-0072.
- Baratti D, Kusamura S, Deraco M. Diffuse malignant peritoneal mesothelioma: systematic review of clinical management and biological research. J Surg Oncol. 2011;103:822–831. doi:10.1002/jso.21787.
- Lorusso V, Crucitta E, Silvestris N, Guida M, Misino A, Latorre A, De Lena M. A phase I study of capecitabine in combination with vinorelbine in advanced breast cancer. Clin Breast Cancer. 2003;4:138–141.
- Gieschke R, Reigner B, Blesch KS, Steimer JL. Population pharmacokinetic analysis of the major metabolites of capecitabine. J Pharmacokinet Pharmacodyn. 2002;29:25–47.
- Jordan JJ, Inga A, Conway K, Edmiston S, Carey LA, Wu L, Resnick MA. Altered-function p53 missense mutations identified in breast cancers can have subtle effects on transactivation. Mol Cancer Res. 2010;8:701–716. doi:10.1158/1541-7786.MCR-09-0442.
- Kunkele A, De Preter K, Heukamp L, Thor T, Pajtler KW, Hartmann W, Mittelbronn M, Grotzer MA, Deubzer HE, Speleman F, et al. Pharmacological activation of the p53 pathway by nutlin-3 exerts anti-tumoral effects in medulloblastomas. Neuro Oncol. 2012;14:859–869. doi:10.1093/neuonc/nos115.
- Yu X, Blanden AR, Narayanan S, Jayakumar L, Lubin D, Augeri D, Kimball SD, Loh SN, Carpizo DR. Small molecule restoration of wildtype structure and function of mutant p53 using a novel zinc-metallochaperone based mechanism. Oncotarget. 2014;5:8879–8892. doi:10.18632/oncotarget.2432.
- Melaiu O, Catalano C, De Santi C, Cipollini M, Figlioli G, Pelle L, Barone E, Evangelista M, Guazzelli A, Boldrini L, et al. Inhibition of the platelet-derived growth factor receptor beta (PDGFRB) using gene silencing, crenolanib besylate, or imatinib mesylate hampers the malignant phenotype of mesothelioma cell lines. Genes Cancer. 2017;8:438–452. doi:10.18632/genesandcancer.129.
