ABSTRACT
Background: Liver metastasis of colon cancer is strongly affected by the tumor microenvironment (TME), with interactions between tumor cells and cancer-associated fibroblasts (CAFs) in particular. TGF-β is well known for its ability to mediate the CAF phenotype, and CXCR4 expression is closely correlated to poor prognosis in CRC. The relationship between these two signaling pathways remains to be delineated in liver metastasis of colon cancer.
Methods: Immunohistochemistry was employed to investigate CXCR4 expression in 45 human specimens of primary colorectal cancer (CRC) and liver metastasis. The functions of SDF-1 released by hepatic stellate cells (HSCs) on CXCR4 and TGF-β1 in CRC cells were investigated in vitro. The effects of CRC on HSCs differentiation into CAFs were confirmed using co-culture technology and expression analysis of CAFs markers by qPCR, western blot and immunofluorescence. The involvement of CXCR4 and TGF-β1 was verified with addition of CXCR4 inhibitor AMD3100 and TGF-β1 inhibitor cyclophosphamide (Cy) both in vitro and in vivo.
Results: There were more CXCR4-positive cells at the liver metastatic tissues compared to the primary sites. CRC cells activated and transformed HSCs to CAFs after co-cultivating with HSCs. Activated HSCs stimulated TGF-β1 secretion from CRC cells after co-culture with CRC cells in vitro. Moreover, the expression of CAFs markers was increasing in the activated HSCs. In a mouse hepatic metastasis model, treated with AMD3100 or Cy blocked the metastatic potential of HCT116 cells and the hepatic CAFs differentiation.
Conclusions: These results indicated that CXCR4/TGF-β1 axis plays an important role in CRC liver metastasis through mediating HSCs differentiation into CAFs, providing preclinical evidences that blockade of the axis might be beneficial for anti-metastasis therapy in CRC.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second in females worldwide.Citation1 Approximately 50% of the patients with CRC develop liver metastases synchronously or metachronously, and in the advanced stage of disease, the development of hepatic metastases is primarily responsible for the mortality of CRC.Citation2 It is therefore imperative to understand the molecular mechanisms contributing to liver metastases to achieve an effective and comprehensive therapy, especially against advanced and metastatic CRC.
Tumor metastasis is a multistep process that involves the escape of tumor cells from the primary tumor, the survival of these cells in the circulation, and their seeding and growth at distant sites.Citation3,Citation4 Each of these processes involves rate-determining steps that are affected by nonmalignant cells of the tumor microenvironment (TME).Citation5 In the advanced and metastatic CRC, it is the tumor–stroma interaction within the TME that often facilitates tumor invasion and metastasis via chemokine signaling.Citation6 Previous studies have suggested the SDF-1/CXCR4 axis as one of the key chemokine signaling that promotes metastasis in lung, pancreatic, and breast cancers, as well as CRC.Citation7–Citation12 And in clinical studies, the expression of CXCR4 was demonstrated to increase disease recurrence and liver metastasis of CRC.Citation11–Citation15
Carcinoma-associated fibroblasts (CAFs) that consist of stromal fibroblasts and α-smooth muscle actin (α-SMA)-positive myofibroblasts are the main constituents in TME associated with primary and metastatic CRC.Citation16,Citation17 Previous studies have correlated the number of stromal myofibroblasts, Citation18 vimentin expression,Citation19 and fibroblast activation protein (FAP) expressionCitation20 with CRC prognosis. Furthermore, a previous study has revealed that SDF-1 was secreted from α-SMA-positive myofibroblasts in CRC metastasis.Citation21 In that study, α-SMA-positive myofibroblasts generated from hepatic stellate cells (HSCs), the liver-specific pericytes released SDF-1 and promoted liver metastasis through the SDF-1/CXCR4 axis.Citation21 When or how HSCs are activated in the process of metastasis, however, remains unsolved.
Autocrine transforming growth factor beta (TGF-β) can directly contribute to stromal-epithelial crosstalk and fibroblast-myofibroblast differentiation.Citation22 TGF-β has been associated with the upregulation of CXCR4 and the sensitization of hepatoma cells to respond to SDF-1 in vivo .Citation23 Similar crosstalk exists in human liver tumorigenesis, in which enhancement of CXCR4/SDF-1 expression correlates with activation of the TGF-β pathway.Citation24 Of note, the necessity of CXCR4 signaling for the TGF-β-induced CAF myofibroblasts has been reported in breast cancer.Citation25 And active colon CAFs display an expression profile similar to TGF-β-activated fibroblasts.Citation26 This might provide a possibly homing mechanism whereby the SDF-1-CXCR4-TGF-β pathway mediates the HSCs differentiation into CAFs in liver metastasis of CRC.
In the present study, we first quantitatively evaluated CXCR4 and CAFs markers expression in human CRC specimens from primary and metastatic colorectal cancer. We then revealed that HSCs secreted SDF-1 in liver, and studied the direct interaction between HSCs and CRC cells with the respect to TGF-β signaling and consequent regulation of CAFs formation in vitro and in vivo. Finally, we examined the effect of specific CXCR4/TGF-β1 antagonists AMD3100/Cy on CAFs formation in metastatic mouse experiments. We demonstrate that the establishment of two autocrine signaling loops, mediated by CXCR4 and TGF-β1, endows resident HSCs with the tumor-promoting myofibroblastic phenotype, thereby prompting their differentiation into CAFs.
Materials and methods
Clinical samples
Forty-five patients who underwent surgical resection for primary colorectal and metastatic hepatic tumor were selected in Hunan Provincial People’s Hospital institutional databases from 2017.3 to 2018.2. Tissue specimens of tumors were obtained from untreated patients of CRC during colectomy and hepatectomy. The protocol was approved by the ethical committee of Hunan Provincial People’s Hospital, with written informed consent obtained from all patients.
Cell culture and drug treatment
The human CRC cell lines HCT-116 and HT-29 were purchased from American Type Culture Collection (ATCC, Manassas VA, USA) and a human HSCs line LX2 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin and streptomycin (Beyotime, China) at 37 °C supplied with 5% CO2 humidified atmosphere.
To ascertain the effects of soluble factors secreted by CRC cells on HSCs differentiation, HSCs were co-cultured with CRC cells in two-chamber dishes allowing the exchange of soluble diffusible factors while preventing their direct contact. HCT116 and HT29 (1 × 104 cells per well) were seeded onto a polycarbonate Transwell membrane with 0.45 μm pores coated with collagen (Corning Costar, Cambridge, MA) and LX2 cells lawns were grown in the lower chambers (24-well plates), and incubated for 24, 48 and 72 h in RPMI 1640 medium with 2% FBS. LX2 cells were collected afterward for performing downstream assays. AMD3100 (1, 10, 50, 100, and 200 μM) (Sigma, St. Louis, MO) and cyclophosphamide (Cy) (0.5, 1, 5, 10 and 50 μM) dissolved in 1% dimethyl sulfoxide (DMSO) were added to HCT116 and HT29 cells with different concentrations as indicated and these cells were incubated for 48 h. The control group was treated with 1% DMSO alone.
siRNA design and transfection
siRNAs for human CXCR4 and TGFB1 genes were designed and synthesized by GenePharma (Shanghai, China). The target sequences of CXCR4 by three siRNAs are listed as following: siCXCR4-1, 5ʹ-CCGACTTCATCTTTGCCAA-3ʹ; siCXCR4-2, 5ʹ-CCTCAAGATCCTCTCCAAA-3ʹ; siCXCR4-3; 5ʹ-CCTTCATCCTCCTGGAAAT-3ʹ. And the target sequences of TGFB1 by another three siRNAs are listed as following: siTGFB1-1, 5ʹ-GCAGAGTACACACAGCATA-3ʹ; siTGFB1-2, 5ʹ-GCAACAATTCCTGGCGATA-3ʹ; siTGFB1-3, 5ʹ-CCTGTGACAGCAGGGATAA-3ʹ. RNA oligonucleotides were transfected into HCT116 and HT29 cells by Lipofectamine 3000 (Invitrogen, SanDiego, CA, USA) according to the manufacturer’s instructions.
Western blot analysis
Cultured cells were harvested and lysed in ice-cold radioimmunoprecipitation assay buffer (RIPA, Beyotime, Beijing, China) supplied with 0.001% protease inhibitor cocktail (Roche, Pleasanton, CA, USA) and incubated on ice for 30 min. BCA Protein assay kit (Beyotime) was then used to detect the concentrations of protein samples. Equal amounts of protein extracts were run on a 10% SDS-PAGE gels and transferred electrophoretically to polyvinylidene fluoride membranes (Millipore, Shanghai, China). The membranes were blocked with 5% nonfat milk, followed by incubation with primary antibodies at 37°C overnight. The primary antibodies used were anti-CXCR4 (ab181020), anti-TGF-β1 (ab92486), anti-α-SMA (ab5694), anti-FSP1 (ab197896), anti-FAP (ab53066) were purchased from Abcam (Cambridge, England), and anti-Vimentin (#5741), anti-β-actin (#3700) were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). Membranes were then incubated with horseradish peroxidase (HRP)-conjugated secondaryantibodies for 1 h at room temperature. Finally, the protein bands were developed using an ECL reagent (EMD Millipore) and visualized by a chemiluminescence system (Bio-Rad, USA). The protein relative expression levels were quantified by integrated gray values of the bands normalized with the internal reference β-actin.
Immunohistochemistry
Immunostaining was performed on the paraffin-embedded tumor tissues fixed with 10% formaldehyde (ZSGB-BIO, Beijing, China). The primary antibodies used were antibodies specific against CXCR4 (D4Z7W) (Cell Signaling; dilution 1:800), α-SMA (Cell Signaling; dilution 1:640) and vimentin (Cell Signaling; dilution 1:100)., and the slides were incubated in 37°C overnight. The secondary antibody was anti-mouse IgG-peroxidase antibody at a dilution of 1:200 (Sigma-Aldrich). Sections were formalin-fixed and paraffin-embedded and analyzed using a Streptavidin labeled peroxidase (S-P) kit (MaiXin Fuzhou, China). Positive immunostaining was assessed when granular brown color was observed in the cytoplasm.
RNA extraction and qPCR assays
Total RNA was prepared using TRIzol Reagent (Invitrogen™, China) Complementary DNA was synthesized with random primers using a reverse transcription kit PrimeScript RT reagent Kit (Takara Biomedical Technology, Dalian, China). Quantitative real-time PCR (qPCR) analysis was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) by primers listed in . The ΔΔCt method for relative quantification of gene expression was used by normalized to GAPDH as an internal reference.
Table 1. Paired primer sequences used in qPCR.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
HCT116 and HT29 cells (1 × 104) were seeded into 96-well plates and grown overnight. The media were then replaced with DMEM supplemented with 1% FBS. AMD3100 (1, 10, 50, 100, and 200 μM) (Sigma, St. Louis, MO) and cyclophosphamide (Cy) (0.5, 1, 5, 10 and 50 μM) dissolved in 1% DMSO were added to the low serum medium with different concentrations as indicated and these cells were incubated for 48 h. The control group was treated with 1% DMSO alone. After replaced with a fresh medium, each well of cells was added with 20 μl MTT solution (5 mg/ml) and incubated for another 4 h at 37°C. Then, 100 μl DMSO was added and the samples were shaken in the dark for 30 min to dissolve the formazan crystals. The absorbance was measured using a microtiter plate reader (Bio-Tek Instruments) at 490 nm (a reference wavelength was subtracted at 600 nm).
Immunofluorescence
After treatment as indicated, cells were fixed with 4% paraformaldehyde (PFA), permeabilized with 0.01%Triton X-100 and blocked in 2% BSA, followed by overnight incubation with α-SMA and vimentin primary antibodies. Secondary fluorescent antibodies were added for extra 1 h incubation. DAPI was used for nuclear counterstaining. Images were captured by a SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA).
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of TGF-β1 secreted from colon cancer cells and SDF-1 secreted from LX2 were measured by commercialized enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, England) by the manufacturer’s printed instructions.
Colon cancer liver metastases in nude mice
Five- to six-week-old, male nude mice were purchased from Hunan Provincial Laboratory Animal Public Service Center (Changsha, China). They were bred and acclimated under specific pathogen-free conditions. All of the animal studies were conducted in accordance with the Institutional Animal Care and Use Committee of Hunan Provincial People’s Hospital.
Firstly, based on the group, HCT116 were pretreated with 10μM AMD3100 or 5 μM Cy (Sigma, St. Louis, MO) or 1% DMSO for 24 h. Afterward, these pre-treated-HCT116 cells (5 × 106) were suspended in 100 μl PBS and then injected into the tail vein of the mice under anesthesia. The survival rate of these mice after injection was confirmed by statistical analysis. The metastatic liver tissues were separated at the 30th day, and the pathological and molecular expression analyses of the metastatic tumor were further performed.
Statistical analysis
Data analysis was performed using SPSS 18.0 (Chicago, USA) and GraphPad Prism (California, USA). Data were expressed as mean ± standard deviation (SD) based on at least three repeats. Group difference was assessed by Student’s t test and one-way ANOVA analysis. P value less than 0.05 was considered as statistically significant.
Results
CXCR4 and CAFs markers were highly expressed in metastatic liver specimens of colorectal cancer patients
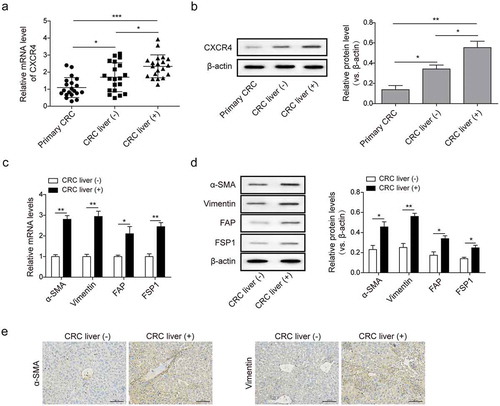
We first quantitatively evaluated the expression of CXCR4 in primary tumor and non-metastatic or metastatic liver specimens from 45 patients with CRC. As expected, CXCR4 mRNA and protein expression in liver metastatic specimens were markedly higher than in those without liver metastasis ( and ). We then examined the expressions of a series of CAFs makers with known roles in liver metastasis. In the 20 paired metastatic and non-metastatic liver specimens from patients with CRC, the mRNA and protein expression of α-SMA, vimentin, FSP1, and FAP were two-fold higher in liver metastatic specimens than that in paired non-metastatic ones ( and ). IHC revealed the abundant α-SMA and vimentin expression in the cytoplasm and/or the cell membrane in liver metastatic CRC (). These data confirm the involvement of CXCR4 and CAFs in the process of liver metastasis of CRC.
Figure 1. CXCR4 and CAFs markers were highly expressed in metastatic liver specimens of colorectal cancer patients (a) CXCR4 mRNA and (b) protein expression in primary tumor and metastatic or non-metastatic liver specimens from CRC patients measured by qPCR and western blot analysis. (c) The mRNA transcription and (d) protein levels of CAFs markers α-SMA, vimentin, FSP1, and FAP in metastatic and non-metastatic liver specimens from CRC patients measured by qPCR and western blot analysis, respectively. (e) The levels of α-SMA and vimentin in paired-liver specimens as described in C and D detected by immunohistochemistry (IHC); scale bar = 100μm. Primary CRC: primary CRC tumor tissues from CRC patients; CRC liver (+): metastatic liver specimens from CRC patients; CRC liver (-): non-metastatic liver specimens from CRC patients. The asterisks show difference significant as * p < .05, ** p < .01, ***p < .001 compared with the two groups showed by a horizontal line.
CRC cells activated HSCs and promoted secretion of SDF-1, which in turn binds to CXCR4 and induced tgf-β1 expression and secretion in CRC cells
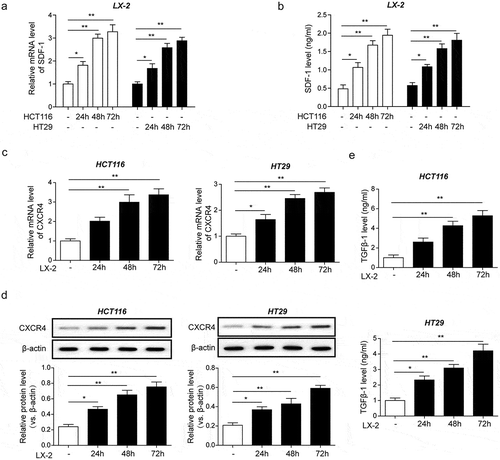
We next evaluated how CRC cells and HSCs interact with each other within the TME by co-culturing HCT116 or HT29 cells with LX2 cells at increasing time intervals (0 to 72 h). Q-PCR results revealed time-dependent enhancement of SDF-1 expression from LX2 cells after co-cultured with HCT116 or HT29 (). An ELISA also showed progressively elevated levels of SDF-1 secreted by LX2 cells after co-cultured with HCT116 or HT29 (). Moreover, qPCR and western blot analysis revealed progressive elevation of CXCR4 in HCT116 and HT29 cells after co-cultured with LX2 ( and ). Of note, we also observed time-dependent enhancement of TGF-β1 secretion in HCT116 and HT29 cells after co-cultured with LX2 ( and ). At all time intervals, the enhancement was significant from baseline (P < .005). Collectively, these data suggest a direct interaction between CRC cells and HSCs in vitro, wherein CRC cells activate HSCs to secret SDF-1 that in turn binds to CXCR4 and facilitates TGF- β1 secretions.
Figure 2. CRC cells activated HSCs and promoted secretion of SDF-1, which in turn binds to CXCR4 and induced TGF-β1 expression and secretion in CRC cells. (a) Time-dependent mRNA expression of SDF-1 in LX2 cells co-cultured with CRC cell lines HCT116 and HT29 for 72 h detected by qPCR. (b) Time-dependent secretion of SDF-1 from LX2 cells co-cultured with HCT116 and HT29 for 72 h measured by ELISA. (c) Time-dependent mRNA transcription and (d) protein expression of CXCR4 in HCT116 and HT29 cells co-cultured with LX2 cells for 72 h detected by qPCR and western blot analysis, respectively. (e) Time-dependent secretion of TGF-β1 from HCT116 and HT29 cells co-cultured with LX2 cells for 72 h measured by ELISA. The asterisks show difference significant as * p < .05, ** p < .01 compared with the two groups showed by a horizontal line.
CRC cells mediated hscs differentiation into CAFs in vitro by increasing the expression of CAFs markers
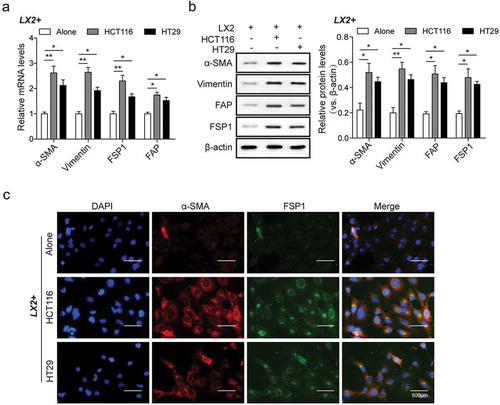
In the above-mentioned studies, HSCs interacts with CRC cells to facilitate TGF-β1 secretions (). Myofibroblasts can be derived from fibroblasts via differentiation following exposure to TGF-β in vitroCitation22 and we speculated that HSCs could potentially convert into CAFs in vitro following exposure to tumor-derived TGF-β. To test this hypothesis, we measured the expression of CAFs markers in LX2 co-cultured with CRC cells. Levels of mRNA and protein expression of α-SMA, vimentin, FSP1, FAP significantly increased ( and ). The expression of α-SMA and FSP1 in LX2 cells was also improved markedly (). These observations suggest that the interaction between HSCs and CRC cells results in activation of TGF-β1 in CRC cells, resulting in increased α-SMA expression in HSCs and therefore HSCs differentiation into CAFs.
Figure 3. CRC cells mediated HSCs differentiation into CAFs in vitro by increasing the expression of CAFs markers. (a) The mRNA levels and (b) protein levels of α-SMA, vimentin, FSP1, FAP in LX2 cells co-cultured with CRC cells as compared with single cultivation, measured by qPCR and western bot analysis, respectively. (c) LX2 cells positively expressed α-SMA and FSP1 determined by immunofluorescent staining. The asterisks show difference significant as * p < .05, ** p < .01 compared with the two groups showed by a horizontal line.
Knockdown of CXCR4 and TGF-β1 in CRC cells inhibits HSCs differentiation into CAFs in vitro
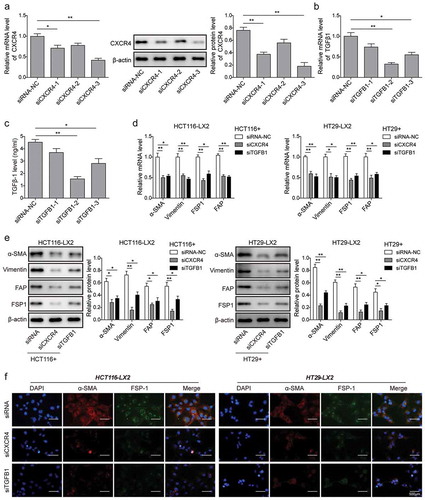
We investigated the functional roles of CXCR4/TGF-β1 axis through knocking down their expressions in CRC cells. Our screening studies showed that siCXCR4-3 reduced the mRNA and protein levels of CXCR4 in HCT116 cells to the maximum extent (). As to reduction in the mRNA and secretion levels of TGF-β1, siTGFB1-2 displayed the strongest effect ( and ). We then transfected the effective siCXCR4-3 (short as siCXCR4) and siTGFB1-2 (short as siTGFB1) into HCT116 and HT29 cell lines for 6 h, and then co-cultured with LX-2 cells for 48 h. Finally, we collected LX-2 cells and analyzed the expression of CAFs markers with qPCR, western blot and immunofluorescence. The results showed that knockdown of CXCR4 and TGF-β1 by siRNAs significantly reduced the expression levels of α-SMA, vimentin, FAP and FSP1 ( and ). Immunofluorescence staining confirmed the attenuated expression levels of α-SMA and FSP-1 in LX2 cells co-cultured with HCT116 or HT29 cells treated with siCXCR4 or siTGFB1 (). These data suggest that CXCR4/TGF-β1 axis in CRC cells promotes HSCs differentiation into CAFs in vitro.
Figure 4. Knockdown of CXCR4 and TGF-β1 in CRC cells inhibits HSCs differentiation into CAFs in vitro. (a) The mRNA and protein expression levels of CXCR4 in HCT116 cells treated with siRNA-NC, siCXCR4-1, siCXCR4-2 or siCXCR4-3 for 48 h by qPCR and western blot analysis, respectively. (b) The mRNA expression levels of TGF-β1 in HCT116 cells treated with siRNA-NC, siTGFB1-1, siTGFB1-2 or siTGFB1-3 for 48 h by qPCR. (c) The secretion amount of TGF-β1 in HCT116 cells treated with siRNA-NC, siTGFB1-1, siTGFB1-2 or siTGFB1-3 for 48 h by ELISA. (d) Changes in mRNA and (e) protein levels of α-SMA, vimentin, FSP1, and FAP in LX2 cells after 48 h co-cultured with CXCR4/TGF-β1 siRNA-treated HCT116 and HT29 by qPCR and western blot analysis, respectively. (f) Changes of LX2 cells positively expressed α-SMA and FSP-1 determined by immunofluorescent staining. The asterisks show difference significant as * p < .05, ** p < .01 compared with the two groups showed by a horizontal line.
Blockade of CXCR4/TGF-β1 axis in CRC cells inhibits HSCs differentiation into CAFs in vitro
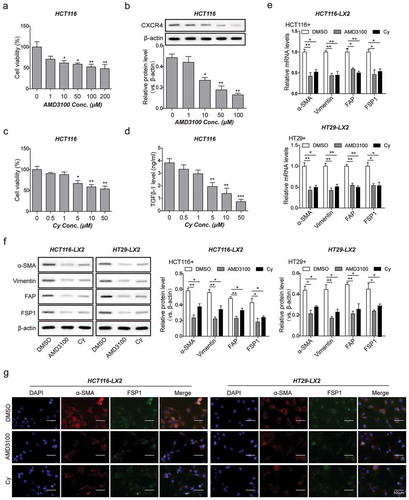
To further validate the promoting effects of CXCR4/TGF-β1 axis in HSCs differentiation, we treated HCT116 and HT29 cells with AMD3100, a CXCR4-specific inhibitor and Cy, a TGF-β1-specific inhibitor for 2 h and then co-cultured the cells with LX2 for 48 h to dissert the expression of CAFs markers. Both ADM3100 and Cy displayed time- and dose-dependent toxicity on CRC cells (-). A dose-dependent reduction in CXCR4 expression was observed in ADM3100-treated CRC cells, with the optimal ADM3100 concentration being 10 μM. At this concentration, ADM3100 exerted relatively low toxicity with a 20% inhibition of cell viability and a 50% inhibition of CXCR4 expression ( and ). Meanwhile, a dose-dependent reduction in TGF-β1 secretions was observed in Cy-treated CRC cells, with the optimal Cy concentration being 5 μM. At this concentration, Cy exerted relatively low toxicity with a 15% inhibition of cell viability and a 75% inhibition of TGF-β1 secretions ( and ). As expected, CXCR4/TGF-β1-mediated induction of CAFs markers expression (α-SMA, vimentin, FSP1, and FAP) was inhibited by AMD3100 or Cy ( and ). Immunofluorescence staining confirmed the attenuated expression levels of α-SMA and FSP1 in LX2 cells co-cultured with HCT116 or HT29 cells treated with AMD3100 or Cy (). Taken together, these various observations indicated that CXCR4/TGF-β1 axis is a necessity for promoting the CAFs phenotype in HSC cells.
Figure 5. Blockade of CXCR4/TGF-β1 axis in CRC cells inhibits HSCs differentiation into CAFs in vitro. (a) Cell viability of HCT116 cells treated with ADM3100 at increased concentrations (0 to 100 μM) for 24 h by MTT assay. (b) The protein levels of CXCR4 in HCT116 cells treated with ADM3100 as described in A detected by western blot analysis. (c) Cell viability of HCT116 cells treated with cyclophosphamide (Cy) at increased concentrations (0 to 50 μM) for 24 h by MTT assay. (d) The secretion amount of TGF-β1 from HCT116 cells treated with Cy as described in C measured by ELISA. (e) Changes in mRNA and (f) protein levels of α-SMA, vimentin, FSP1, and FAP in LX2 cells co-cultured with DMSO-, ADM3100- or Cy-treated HCT116 and HT29 detected by qPCR and western blot analysis, respectively. (g) Changes of LX2 cells positively expressed α-SMA and FSP1 determined by immunofluorescent staining. The asterisks show difference significant as * p < .05, ** p < .01, *** p < .001 compared with the Blank group (0 μM) or between two groups showed by a horizontal line.
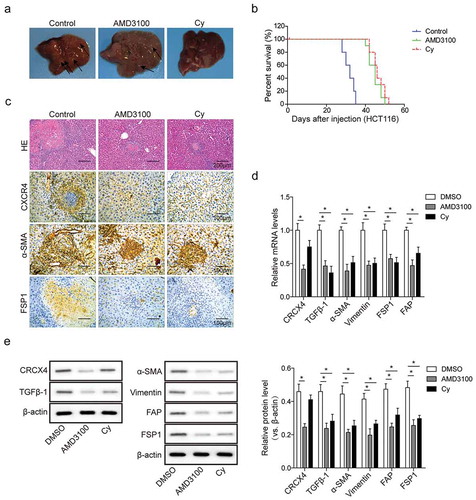
Blockade of CXCR4/TGF-Β1 axis inhibits colorectal liver metastases and hepatic CAFs differentiation in vivo
To determine the in vivo importance of CXCR4/TGF-β1 axis to HSCs differentiation into CAFs, we constructed nude mouse models of liver metastases from colon cancer by tail vein injection with HCT116 cells which were pre-treated with DMSO, AMD3100 and Cy for 24 h before inoculation. In the control group, HCT116 cells exhibited high liver metastatic potential, and large metastatic nodules were observed, but AMD3100 or Cy treatment significantly reduced the metastatic potential of HCT116 cells, leading to a decrease in metastatic nodules (). Kaplan-Meier survival analysis demonstrated that nude mice with ADM3100 or Cy treatment had markedly better overall survival, compared with mice without these treatments (). Obvious histopathological changes in the liver surrounding metastatic lesions were observed by H&E staining (). In the control group, significantly large and numerous tumor nodules were visible in the liver, with the liver cells loosely structured and irregular in shape. After AMD3100/Cy treatment, however, the tumor nodules were significantly reduced, with the liver cells tightly arranged and regular in shape (). Of note, blockage of CXCR4 expression by AMD3100 also led to a significant decrease in TGF-β1 expression in HCT116 cells ( and ). In contrast, inhibition of TGF-β1 expression by Cy had no apparent effect on CXCR4 expression ( and ), indicating that CXCR4 may be an upstream molecule of TGF-β1 and mediate TGF-β1 expression. Both AMD3100 and Cy decreased the expression of CAFs markers (α-SMA and FSP1) (-). Collectively, these observations suggest the requirement of CXCR4 for the TGF-β1-induced HSCs differentiation into CAFs within the liver metastasis of CRC.
Figure 6. Blockade of CXCR4/TGF-β1 axis inhibits colorectal liver metastases and hepatic CAFs differentiation in vivo. (a) Macroscopic images of livers from nude mice administered with DMSO-, AMD3100- and Cy-pretreated HCT116 cells by tail vein injection. Arrows indicated metastatic tumor nodules. (b) Kaplan–Meier curves for overall survival of these CRC nude mice as described in A. (c) The histopathological analysis of hepatic metastatic tumor nodules (circled by lines) from these CRC nude mice as described in A by hematoxylin-eosin (H&E, scale bar = 500μm) and immunohistochemistry (IHC, scale bar = 100μm) staining. (d) The mRNA levels and (e) protein levels of CXCR4, TGF-β1, and CAFs in liver metastasis tissues from these CRC nude mice as described in A determined by qPCR and western blot analysis, respectively. The asterisks show difference significant as * p < .05 compared with the two groups showed by a horizontal line.
Discussion
Although colorectal liver metastasis is generally considered to be regulated by interactions between tumor cells and tumor-activated stromal factors, especially with CAFs within the TME, Citation27 the origin, location, and function of CAFs remain to be determined.Citation28 Using in vitro and in vivo models of liver metastasis of CRC, we demonstrated that: (1) CAFs increase during CRC progression, particularly during the liver metastasis; (2) CXCR4/SDF-1 binding induces TGF-β1 expression in CRCs to promote live metastasis; (3) HSCs acquire a CAF-like phenotype as a consequence of autocrine TGF-β1 signaling, and (4) Blockage of CXCR4/TGF-β1 axis by AMD3100/Cy abolishes HSCs-to-CAF differentiation and liver metastasis. The CXCR4/TGF-β1 axis has not been previously reported in liver metastasis of CRC.
In recent years, increasing emphasis has been placed on the role of chemokines and their receptors in the pathological process, such as cancer metastasis.Citation29 Of all the chemokine receptors, CXCR4/SDF-1 axis has been implicated as a pivotal signaling pathway engaged in the spreading and progression of multiple cancers, including CRC.Citation30 CXCR4 expression predicted disease recurrence and CRC-specific death in clinical CRCs.Citation11 Moreover, immunohistochemistry studies showed that overexpressed CXCR4 was closely related to the early relapse of CRC.Citation21 In addition, enhancement of SDF-1 expression has been found in CAF myofibroblasts both in vitro and in vivo.Citation31–Citation33 Our findings indicate that SDF-1 is likely derived from the CRCs-containing HSCs and appears to act as an autocrine signaling factor activating more niche cells, and to function as a paracrine factor to attract and maintain CAFs in HSCs. Consistently, blockage of CXCR4 expression – in our in vitro and in vivo studies – was able to suppress stromal cell recruitment to tumors and abolish the induction of CAFs in the livers of mice with CRC.
TGF-β1 has been connected to the activation of fibroblasts, resulting to the production of CAFs.Citation22 In CRC, the shift in TGF-β signaling from epithelial cells to CAFs has been confirmed by immunohistochemistry.Citation34 Moreover, TGF-β1silencing has been reported to reverse the growth advantages conferred by CAFs.Citation35 Previous studies have documented the existence of a crosstalk between the TGF-β and CXCR4 in liver tumors, in which elevated CXCR4 expression is strongly associated with TGF-β activation.Citation24 In addition, the establishment of TGF-β/SDF-1 signaling pathway engenders CAF myofibroblasts during tumor progression.Citation25 However, the potential initiating steps for TGF-β1 formation remain inconclusive. In breast cancer, osteopontin initiates TGF-β1 secretion and subsequently the production of CAF in mesenchymal stem cells.Citation36 In CRC, the interaction between tumor cells and CAF-derived factors induces TGF-β signaling and growth of CRCs, possibly via the activation of latent TGF-β1.Citation37 Our findings demonstrated that CXCR4/SDF-1 binding induces tumor-derived TGF-β1 and consequently the formation of CAF phenotype in HSCs in liver metastasis of CRC. Accordingly, blockage of TGF-β significantly abrogated CAFs markers expression in HSCs both in vitro and in vivo.
Although CAFs could arise from diverse origins including local fibroblasts, preadipocytes, smooth muscle cells, or bone marrow-derived progenitor cells, Citation36 HSCs are liver-specific pericytes that can be activated and differentiate into myofibroblasts when micrometastases in the sinusoidal region of liver lobules occur.Citation38 Like the activation process following liver damage, static and non-dividing HSCs adapt marked phenotypic changes due to activation by tumor cells, and differentiate into myofibroblasts. Nevertheless, HSCs activation within the TME is a complicated process that involves the participation of intracellular factors in HSCs and paracrine stimuli of tumor cells, Citation39 with TGF-β being one of the most important regulatory factors of HSC activation in vivo .Citation27 In cholangiocarcinoma cells, incubation with tumor-conditioned medium-endowed HSCs with a CAF-like phenotype, including persistent expression of α-SMA-positive stress fibers.Citation40 In CRC, conditioned medium from activated HSCs has been reported to enhance cell proliferation, migration, and invasion.Citation39 In addition, activated HSCs released SDF-1 in colorectal liver metastases, and this was further accompanied by enhanced CXCR4 expression in CRC cells.Citation21 In the current study, we demonstrated that HSCs were activated by CRCs expressing CXCR4. The activated HSCs highly expressed SDF-1 that binds to CXCR4 and stimulates TGF-β1secretion in CRCs. This results in the formation of CAFs-like phenotype in HSCs and thereby promoting colorectal liver metastases. The importance of the CXCR4/TGF-β1 axis for CRC metastasis was further confirmed with knockdown experiments using AMD3100 and Cy. These inhibitors suppressed the metastatic ability of HCT116 cells both in vitro and in vivo. Of note, inhibition of CXCR4 was sufficient to abolish the TGF-β1-induced CAF-phenotype, suggesting the requirement of CXCR4 signaling to engender HSCs-to-CAF differentiation in the liver microenvironment.
In conclusion, we have identified a signal pathway by which tumor-derived CXCR4 activates HSCs to release SDF-1, resulting in TGF-β1 secretion in CRC cells to induce HSCs differentiation into CAFs and promote liver metastasis of CRC both in vitro and in vivo. Blockade of CXCR4/TGF-β1 axis by AMD3100/Cy effectively abrogates this pathway. Therefore, CXCR4/TGF-β1 blockage may be an effective clinical strategy for live metastasis of colon cancer. Additional studies are warranted to evaluate the effects of coordinating chemotherapy, radiotherapy, or surgery with TGF-β antagonists targeting HSCs differentiation into CAFs on suppressing liver metastases and enhancing the survival benefit of patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi:10.3322/caac.21262.
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi:10.3322/caac.21208.
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi:10.1038/nrc2622.
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi:10.1016/j.cell.2011.09.024.
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi:10.1038/nrc2618.
- Itatani Y, Kawada K, Inamoto S, Yamamoto T, Ogawa R, Taketo M, Sakai Y. The role of chemokines in promoting colorectal cancer invasion/metastasis. Int J Mol Sci. 2016;17:E643. doi:10.3390/ijms17050643.
- Kucia M, Reca R, Miekus K. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi:10.1634/stemcells.2004-0342.
- Muller A, Homey B, Soto H. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi:10.1038/35065016.
- Saur D, Seidler B, Schneider G. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237–1250. doi:10.1053/j.gastro.2005.06.056.
- Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–3839.
- Kim J, Takeuchi H, Lam ST. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi:10.1200/JCO.2005.07.078.
- Schimanski CC, Schwald S, Simiantonaki N. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750. doi:10.1158/1078-0432.CCR-04-1195.
- Ottaiano A, Franco R, Talamanca AA, Liguori G, Tatangelo F, Delrio P, Nasti G, Barletta E, Facchini G, Daniele B, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–2803. doi:10.1158/1078-0432.CCR-05-2142.
- Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer. 2013;132:276–287. doi:10.1002/ijc.27670.
- Yopp AC, Shia J, Butte JM, Allen PJ, Fong Y, Jarnagin WR, DeMatteo RP, D’Angelica MI. CXCR4 expression predicts patient outcome and recurrence patterns after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2012;19:S339–S346. doi:10.1245/s10434-011-1774-4.
- Mueller L, Goumas FA, Affeldt M, Sandtner S, Gehling UM, Brilloff S, Walter J, Karnatz N, Lamszus K, Rogiers X, et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol. 2007;171:1608–1618. doi:10.2353/ajpath.2007.060661.
- Herrera M, Islam AB, Herrera A, Martin P, Garcia V, Silva J, Garcia JM, Salas C, Casal I, de Herreros AG, et al. Functional heterogeneity of cancer-associated fibroblasts from human colon tumors shows specific prognostic gene expression signature. Clin Cancer Res. 2013;19:5914–5926. doi:10.1158/1078-0432.CCR-13-0694.
- Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. doi:10.1158/1078-0432.CCR-06-2191.
- Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda J-I, Konishi K, Takemasa I, Ikeda M, Sekimoto M, et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi:10.1038/sj.bjc.6603651.
- Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, Chen W-T, Cheng JD. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. doi:10.1158/1078-0432.CCR-06-1746.
- Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Nakano K, Itami A, Kawada K, Nagayama S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi:10.1245/s10434-009-0599-x.
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi:10.1038/nrc1926.
- Bertran E, Caja L, Navarro E, Sancho P, Mainez J, Murillo MM, Vinyals A, Fabra À, Fabregat I. Role of CXCR4/SDF-1alpha in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-beta. Cell Signal. 2009;21:1595–1606. doi:10.1016/j.cellsig.2009.06.006.
- Bertran E, Caja L, Navarro E, Caja L, Lopez-Luque J, Navarro E, Egea G, Lastra R, Serrano T, Ramos E, et al. Overactivation of the TGF-β eta pathway confers a mesenchymal-like phenotype and CXCR4-dependent migratory properties to liver tumor cells. Hepatology. 2013;58:2032–2044. doi:10.1002/hep.26597.
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi:10.1073/pnas.1013805107.
- Torres S, Garcia-Palmero I, Herrera M, Bartolome RA, Pena C, Fernandez-Acenero MJ, Padilla G, Pelaez-Garcia A, Lopez-Lucendo M, Rodriguez-Merlo R, et al. LOXL2 is highly expressed in cancer-associated fibroblasts and associates to poor colon cancer survival. Clin Cancer Res. 2015;21:4892–4902. doi:10.1158/1078-0432.CCR-14-3096.
- Kang N, Gores GJ, Shah VH. Hepatic stellate cells: partners in crime for liver metastases? Hepatology. 2011;54:707–713. doi:10.1002/hep.24384.
- Mishra PJ, Humeniuk R, Medina DJ, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–4339. doi:10.1158/0008-5472.CAN-08-0943.
- Sheu BC. Cytokine regulation networks in the cancer microenvironment. Fron Biosci. 2008;13:6255–6268. doi:10.2741/3152.
- Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–213. doi:10.1002/(ISSN)1096-9896.
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi:10.1016/j.ccr.2004.06.010.
- Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi:10.1016/j.cell.2005.02.034.
- Ao M, Franco OE, Park D, Raman D, Williams K, Hayward SW. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi:10.1158/0008-5472.CAN-06-3946.
- Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman JHN, Mesker W, Ten Dijke P, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2012;33:97–107. doi:10.1038/onc.2012.536.
- Su G, Sung KE, Beebe DJ, Friedl A. Functional screen of paracrine signals in breast carcinoma fibroblasts. PLoS One. 2012;7:e46685. doi:10.1371/journal.pone.0046685.
- Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MAC, Franzen CA, Gupta GN, Osipo C, Zlobin A, et al. Osteopontin mediates an MZF1-TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015;34:4821–4833. doi:10.1038/onc.2014.410.
- Hawinkels L, Paauwe M, Verspaget H, Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman JHN, Mesker W, Ten Dijke P, et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi:10.1038/onc.2012.536.
- Vidal-Vanaclocha F. The prometastatic microenvironment of the liver. Cancer Microenviron. 2008;1:113–129. doi:10.1007/s12307-008-0011-6.
- Shimizu S, Yamada N, Sawada T, Ikeda K, Kawada N, Seki S, Kaneda K, Hirakawa K. In vitro interactions between human colon carcinoma cells and hepatic stellate cells. Jpn J Cancer Res. 2000;91:1285–1295. doi:10.1111/cas.2000.91.issue-12.
- Okabe H, Beppu T, Hayashi H, Ishiko T, Masuda T, Otao R, Horlad H, Jono H, Ueda M, Ando Y, et al. Hepatic stellate cells accelerate the malignant behavior of cholangiocarcinoma cells. Ann Surg Oncol. 2011;18:1175–1184. doi:10.1245/s10434-010-1391-7.
