ABSTRACT
Alterations of the Retinoblastoma (Rb) pathway are frequent in ovarian cancer, typically resulting from CDKN2A down-regulation, CCNE1 amplification, CCND1/2 amplification, and RB1 loss. However, bi-allelic CDKN2A mutation or homozygous deletion is a very rare event, concerning less than 5% of patients.
Initial trials with palbociclib in serous ovarian cancer have shown very modest benefit in unselected patient populations, thus underlining the need for a biomarker predicting response. We report the case of a heavily pre-treated patient with a serous ovarian tumor harboring a homozygous deletion of the CDKN2A gene that derived significant, prolonged clinical benefit from palbociclib, a CDK4/6 oral inhibitor, with letrozole. Treatment with palbociclib and letrozole started on February 2018, with an ongoing response after 12 months.
In conclusion, homozygous CDKN2A deletion is rare and could be used to predict response to CDK4/6 inhibitors in association with other genomic features. We encourage further trials in this direction.
Introduction
Ovarian cancer is the fourth most frequent cause of cancer death for women in developed countries.Citation1 Serous carcinomas, 90% of which are high grade, are the most common histological type. Despite extensive use of chemotherapy, the prognosis of recurrent disease remains unfavorable.Citation1
CDK4/6 inhibitors are currently being investigated in ovarian cancer and other tumor types,Citation2,Citation3 but their clinical efficacy is modest in an unselected population and predicting which patient will benefit is difficult. The combination of CDK4/6 inhibitors with fulvestrant or aromatase inhibitors was associated with longer progression-free survivalCitation4 and overall survivalCitation5 in hormone receptor-positive breast cancer but the identification of predictive biomarkers is still lacking.
Here we report the case of a patient with platinum-refractory, high grade serous ovarian cancer with bi-allelic CDKN2A loss, intact RB1 and without CCNE1 amplification who had a durable response to the association of palbociclib and letrozole.
Case presentation
The patient was diagnosed in 2011 with high-grade serous ovarian cancer (HGSC) stage IIIC and has been managed in our institution since then. She was 49 years old at diagnosis, and her family history was not informative. Germline BRCA1/BRCA2 testing did not reveal a pathogenic variant.
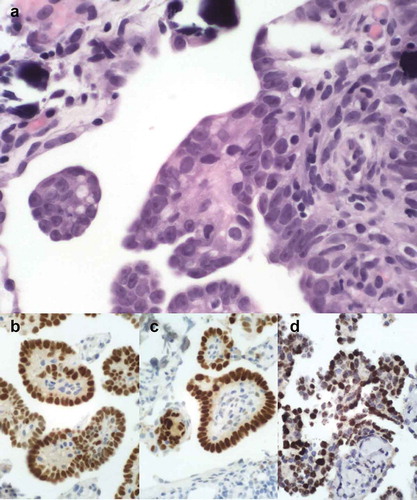
The patient initially presented with ascites and a radiological image of “omental cake”. She received chemotherapy with carboplatin AUC 5 and paclitaxel 175 mg/m2, without clinical or radiological response. She then received a second line of chemotherapy with gemcitabine 1000 mg/m2 for ten cycles, resulting in radiologically stable disease, a decrease of ascites and of CA-125 concentration. Radiological progression was observed 6 weeks after the last treatment cycle, justifying the introduction of third-line chemotherapy with liposomal doxorubicin 20 mg/m2 for 4 cycles, without clinical benefit. The fourth-line chemotherapy with weekly intravenous topotecan 4 mg/m2 resulted in a good clinical and radiological response after 4 cycles. We observed the complete regression of ascites, reduction of an ovarian mass, and the decline of CA-125 from 314 to 36 kU/L. This allowed the patient to undergo debulking surgery in May 2013, which was unfortunately incomplete and left a 2 cm residual tumor. The histological review of the surgical specimen showed a morphological and immunohistochemical pattern of high-grade serous ovarian cancer (), consistent with the initial diagnosis. After three additional cycles of weekly topotecan, the patient attained a complete clinical and biological remission, which lasted until June 2014. At this moment, the appearance of localized symptomatic ascites led the medical team to perform paracentesis, which cytologically confirmed the recurrence. Given the excellent response to weekly topotecan, the patient was again treated with the same regimen from June 2014 to February 2015, and once more in October 2015 (4 cycles), with good clinical response and a decrease of ascites.
Figure 1. Histological and immunohistochemical images of the tumor, consistent with high grade papillary serous carcinoma. The tumor showed a typical morphology with numerous papillary formations and psammoma bodies. The tumor cells are atypical with irregular nuclei and macro-nucleoli (A). They stain positive for the estrogen (B) and progesterone receptors (C) and for PAX8 (D) .
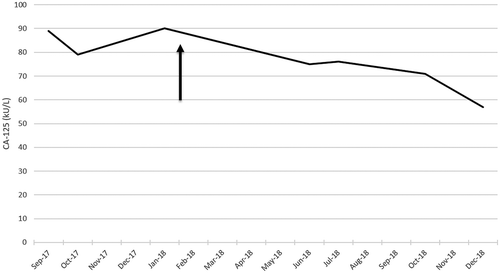
In June 2017, the patient received topotecan for the fourth time but the disease progressed during treatment with the appearance of ileus, requiring the placement of a nasogastric tube. Surgery could not be performed because of extensive peritoneal carcinomatosis. The patient was hospitalized for 2 months and received parenteral nutrition, with minimal oral intake. She received seven cycles of weekly paclitaxel 80 mg/m2. Bevacizumab was omitted because of therapeutic anticoagulation for deep vein thrombosis and the risk of intestinal perforation in the context of sub-ileus. A computed tomography (CT)-scan in January 2018 showed stable disease (), and the CA-125 concentration remained stable around 90 kU/L ().
Figure 2. CT-scans in January 2018 (A) in October 2018 (B) and in February 2019 (C), showing a tumor reduction (reaching criteria for partial response according to RECIST) and the resolution of the pathological intestinal dilation (white arrows) .
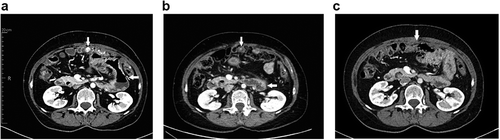
Figure 3. Evolution of CA-125 concentration (kU/L) after introduction of palbociclib and letrozole (arrow) .
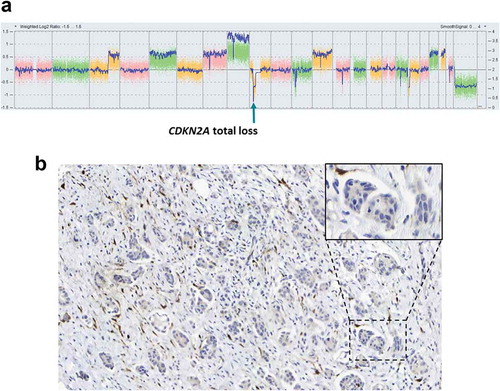
Molecular tumor testing by next-generation sequencing of 50 genes and copy number variation analysis performed previously had shown a bi-allelic focal deletion of CDKN2A (), which was also confirmed by the absence of p16 expression in immunohistochemistry (). We did not find any other pathogenic mutation nor other targetable focal copy number alterations. Specifically, there was no amplification in CCND1, CCNE1 and no loss in RB1. We did find a modest diffuse copy number gain in 7p14 (three copies of CDK6), 12q13 (three copies of CCND2) and 12q13 (three copies of CDK4). The tumor also expressed estrogen receptors (ER, 80%) and progesterone receptors (PgR, 30%) by immunohistochemistry.
Figure 4. (A) Genomic profile generated by SNP array showing the total loss of CDKN2A. (B) Representative P16INK4a immunohistochemistry image showing the loss of its expression in cancer cells. Inset corresponds to a zoomed portion of the image.
The patient maintained a good performance status (ECOG 1) despite the need for parenteral nutrition support. Therefore, we proposed a combination of palbociclib (125mg daily for 3 weeks, then a week off) and letrozole (2.5mg daily). This treatment produced significant clinical benefit, including the recovery of a normal intestinal transit, allowing the progressive introduction of exclusive oral nutrition. A first CT-scan in April 2018 showed the resolution of pathological intestinal dilation and a slight tumor reduction which did not meet the RECIST criteria for a partial response. A follow-up CT in October 2018 showed an ongoing response () and the latest CT scan in February 2019 showed a further decrease of 46% from baseline in two measurable lesions, corresponding to a partial response (). The treatment was well-tolerated, without nausea or mucositis. Transient G3 neutropenia occurred twice, after cycles 7 and 10, and resolved with a one-week treatment pause.
Discussion
Activated CDK4 and CDK6 phosphorylate the Rb protein (RB1 gene), releasing the E2F transcription factors which mediate cell cycle progression. The Cyclin D-CDK4/6 complex is inhibited by p16 (CDKN2A gene) in response to DNA damage or other stressors. CDKN2A is often mutated or lost in head and neck cancer, pancreatic cancer and melanoma, resulting in inappropriate CDK4/6 activation and excessive proliferation.Citation6 The loss of function by epigenetic mechanisms is far more frequent in several other cancer types.Citation7 This lends theoretical support to the use of CDK4/6 inhibitors although their efficacy depends on an intact downstream Rb protein.Citation7,Citation8
The use of cyclin-dependent kinases 4 and 6 inhibitors, such as palbociclib, is being investigated in ovarian cancer after in vitro studies demonstrated growth inhibition in cells with p16 loss.Citation9 Little evidence exists regarding the clinical benefit of CDK4/6 inhibitors in ovarian cancer. One phase I study in a Japanese population reported a clinical benefit of palbociclib 100 mg once daily in an ovarian cancer patient, with stable disease and a progression-free survival (PFS) of 4 months.Citation10 One prospective phase II study of 40 molecularly unselected patients with high-grade ovarian cancer demonstrated good tolerance of the CDK4/6 inhibitor as a single agent, with a modest median PFS of 3.7 months, corresponding to 30% at 6 months.Citation2 Only one patient attained an objective radiological response (1 of 26 evaluable). An additional three patients attained a response using the GCIG CA125 criteria (4/30 evaluable). Despite an undeniable signal of efficacy for very few patients, the use of palbociclib cannot be recommended for unselected ovarian cancer patients.
Reliable predictive biomarkers are needed, but predicting response to palbociclib is difficult. Several genomic alterations are thought to result in excessive CDK4/6 activity and should render tumors sensitive to CDK4/6 inhibitors,Citation11 but this was not reflected in the biomarker assessment of clinical trials.Citation12–Citation14 For example, in the phase II PALOMA-1 trial, the combination of palbociclib and letrozole was equally efficacious in tumors with CCND1 amplification or CDKN2A loss, and in tumors without these features.Citation12 Additional complications arise from the use of different techniques for biomarker assessment, for example, gene expression, immunohistochemistry, and DNA sequencing. Predicting resistance to CDK4/6 inhibitors appears to be somewhat clearer, since downstream events, such as loss of RB1 or amplification of CCNE1 bypass the drug’s mechanism of function and have emerged as resistance mechanisms in multiple studies.Citation15–Citation17
Interestingly, even though the alterations affecting the Rb pathway can be found in the majority of ovarian cancers (up to 67%), the bi-allelic deletion of CDKN2A that we saw in this patient is a rare event (2%).Citation18 Furthermore, the bi-allelic deletion of CDKN2A was focal, limited to a narrow region of 1728 kb, and therefore a plausible driver event. In addition, our patient did not have CCNE1 amplification, which is found in more than 20% of patients, or RB1 loss (2% of patients). Both of these alterations would be likely to induce resistance to palbociclib. The copy number gains of CDK4, CDK6, and CCND2 were diffuse and resulted only in a single additional gene copy, a presentation that would be consistent with a passenger alteration and should not reduce the tumor’s reliance on CDK4/6 activation. Overall, the constellation of molecular findings reported in our patient is rare and consistent with an expectation of benefit from palbociclib.
Noteworthy, we did not find any hotspot mutation of TP53, which is known to be mutated in over 90% of HGSOC.Citation18 A study based on The Cancer Genome Atlas (TCGA) data identified 15 patients of 316 with TP53 wild-type HGSC.Citation19 This finding was associated with poor prognosis and chemoresistance, possibly explaining the fact that our patient’s disease was refractory to first-line platinum-based therapy. A post hoc pathological review of the TCGA data suggests that p53 wild-type tumors may be misclassified as HGSOC and could correspond to other disease entities.Citation20 In our case, a histological review of the surgical specimen from May 2013 confirmed the initial diagnosis of HGSOC () and has been reviewed by two expert pathologists. Finally, it is important to note that our NGS panel includes only hotspot mutations and that is possible that a rare TP53 mutation in a not included region exists.
The benefit of hormonotherapy in ovarian cancer is generally anecdotal and remains somewhat controversial.Citation20,Citation21 This tumor expressed both estrogen and progesterone receptors, which could favor response to hormonotherapy.Citation22,Citation23 Pre-clinical data demonstrated that ER-positive breast cancer cell lines were also more sensitive to palbociclib than other breast cancer cell lines.Citation11 In addition, the action of tamoxifen and palbociclib appears to be synergistic in cell lines sensitive to anti-estrogen therapy and overcomes resistance in those who have acquired a resistant phenotype. The combination of an anti-estrogen with palbociclib in ER-positive breast cancer reinforces the effect of inhibition of Rb phosphorylation, diminishes the expression of key transcription factors as E2F and FOXM1, and increases the expression of senescence markers.Citation24
Our case demonstrates durable clinical benefit with the association of palbociclib and letrozole in heavily pretreated, platinum-refractory ovarian cancer positive for ER/PgR and harboring bi-allelic CDKN2A loss. Given the rarity of CDKN2A loss in ovarian cancer, our report supports further research in this direction for the discovery of reliable predictive biomarkers.
Materials and methods
The patient provided written informed consent for publication of this case report, in accordance with institutional policy.
Next-generation sequencing
The sequencing of tumor tissue was done with the IonAmpliseq Cancer Hotspot Panel v2 (ThermoFisher, cat# 4475346) on an IonTorrent Proton sequencer. Variant calling was performed with the TorrentVariantCaller then manually curated. Variants with allele frequency below 5% or present in normal DNA were not reported.
Copy number variation analysis
Copy-number variation (CNV) analysis was done on 80ng genomic DNA isolated from FFPE tumor tissues with the Affymetrix OncoScan® FFPE Assay Kit according to the manufacturer’s instructions. CEL files from the scanned array images were imported into the Chromosome Analysis Suite (ChAS 3.3) software to generate probe set analysis results. Genomic copy number variations and LOH of regions as small as 25kb were automatically highlighted by the software and were further evaluated manually.
P16INK4a immunohistochemical staining
Immunohistochemical staining for p16 was carried out on 4 μm FFPE sections using the CINtec Histology Kit (Ventana) according to the manufacturer’s instructions.
High grade papillary serous carcinoma immunohistochemical staining
Stainings were performed on a Ventana benchmark ultra automat using the following antibodies (estrogen receptor – SP1 pre-diluted Roche Ventana Tucson, progesterone receptor – 1E2 pre-diluted Roche Ventana Tucson, PAX8 – BCL12, ABCAM, Cambridge UK) and the following protocols (estrogen receptor – buffer CC1 at 96C for 36 minutes, progesterone receptor – buffer CC1 64 minutes at 96C, PAX8 – diluted 1:20 buffer CC1 48 minutes at 96C).
Disclosure of interest
The authors report no conflict of interest.
Additional information
Funding
References
- Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C. ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv259–iv259. doi:10.1093/annonc/mdy157.
- Konecny GE, Wahner Hendrickson AE, Jatoi A, Burton JK, Paroly J, Glaspy JA, Dowdy SC, Slamon DJ. A multicenter open-label phase II study of the efficacy and safety of palbociclib a cyclin-dependent kinases 4 and 6 inhibitor in patients with recurrent ovarian cancer. J Clin Oncol. 2016;34:5557. doi:10.1200/JCO.2016.34.15_suppl.5557.
- ClinicalTrials.gov. Ribociclib and Letrozole in Treating Patients With Relapsed ER Positive Ovarian, Fallopian Tube, Primary Peritoneal, or Endometrial Cancer - Full Text View - ClinicalTrials.gov [Internet]. [accessed 2018 Dec 3]. https://clinicaltrials.gov/ct2/show/NCT02657928.
- Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi:10.1056/NEJMoa1607303.
- Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi:10.1056/NEJMoa1810527.
- US National library of Medicine – Genetic Home. Cyclin dependent kinase inhibitor 2A [Internet]. [accessed 2018 Dec 3]. https://ghr.nlm.nih.gov/gene/CDKN2A.
- Zhao R, Choi BY, Lee M-H, Bode AM, Dong Z. Implications of Genetic and Epigenetic Alterations of CDKN2A (p16INK4a) in Cancer. EBioMedicine. 2016;8:30–39. doi:10.1016/j.ebiom.2016.04.017.
- Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–4032. doi:10.1038/onc.2010.154.
- Konecny GE, Winterhoff B, Kolarova T, Qi J, Manivong K, Dering J, Yang G, Chalukya M, Wang H-J, Anderson L, et al. Expression of p16 and retinoblastoma determines response to CDK4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17:1591–1602. doi:10.1158/1078-0432.CCR-10-2307.
- Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, et al. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016;107:755–763. doi:10.1111/cas.2016.107.issue-6.
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, et al. Open Access PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77.
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2014;16:25–35. doi:10.1016/S1470-2045(14)71159-3.
- DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C, et al. CDK 4/6 Inhibitor Palbociclib (PD0332991) in Rb+ Advanced Breast Cancer: phase II Activity, Safety, and Predictive Biomarker Assessment. Clin Cancer Res. 2015;21:995–1001. doi:10.1158/1078-0432.CCR-14-2258.
- Finn R, Liu Y, Martin M, Rugo H, Dieras V, Im S-A, Gelmon K, Harbeck N, Zhu Z, Lu D, et al. Abstract P2-09-10: comprehensive gene expression biomarker analysis of CDK 4/6 and endocrine pathways from the PALOMA-2 study. Cancer Res. 2018;78:09–10. P2-09-10-P2-.
- Condorelli R, Spring L, O’Shaughnessy J, Lacroix L, Bailleux C, Scott V, Dubois J, Nagy RJ, Lanman RB, Iafrate AJ, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2018;29:640–645. doi:10.1093/annonc/mdx784.
- Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, DeMichele A, Harbeck N, André F, Zhang Z, et al. Abstract CT039: cyclin E1 (CCNE1) expression associates with benefit from palbociclib in metastatic breast cancer (MBC) in the PALOMA3 trial. Cancer Res. 2018;78:CT039–CT039.
- Turner NC, Liu Y, Zhu Z, Loi S, Colleoni M, Loibl S, DeMichele A, Harbeck N, André F, Bayar MA, et al. Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J Clin Oncol. 2019. JCO1800925. doi:10.1200/JCO.18.00925.
- Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615.
- Wong -K-K, Izaguirre DI, Kwan S-Y, King ER, Deavers MT, Sood AK, Mok SC, Gershenson DM. Poor survival with wild-type TP53 ovarian cancer? Gynecol Oncol. 2013;130:565–569. doi:10.1016/j.ygyno.2013.06.016.
- Vang R, Levine DA, Soslow RA, Zaloudek C, Shih I-M, Kurman RJ. Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma. Int J Gynecol Pathol. 2016;35:48–55. doi:10.1097/PGP.0000000000000207.
- Williams C, Simera I, Bryant A, Platt J. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev. 2010.17(3):CD001034.
- Voutsadakis IA. Hormone Receptors in Serous Ovarian Carcinoma: prognosis, Pathogenesis, and Treatment Considerations. Clin Med Insights Oncol. 2016;10:17–25. doi:10.4137/CMO.S32813.
- Bonaventura A, OʼConnell RL, Mapagu C, Beale PJ, McNally OM, Mileshkin LR, Grant PT, Hadley AM, Goh JCH, Sjoquist KM, et al. Paragon (ANZGOG-0903): phase 2 Study of Anastrozole in Women With Estrogen or Progesterone Receptor-Positive Platinum-Resistant or -Refractory Recurrent Ovarian Cancer. Int J Gynecol Cancer. 2017;27:900–906. doi:10.1097/IGC.0000000000000978.
- Lee NV, Yuan J, Eisele K, Cao JQ, Painter CL, Chionis J, Liu C, Shields DJ, Kan JLC, Arndt K, et al. Abstract LB-136: mechanistic exploration of combined CDK4/6 and ER inhibition in ER-positive breast cancer. Cancer Res. 2014;74:LB-136-LB–136.
