ABSTRACT
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma is rare among lung neoplasia cases, representing only 0.5%–1% of newly diagnosed primary lung lymphoma. MALT lymphoma with relapsed refractory and malignant transformation is highly heterogeneous and consensus therapy remains undetermined. We report a 55 year-old woman with a 3 year history of primary pulmonary MALT lymphoma confined to the lung presenting with massive pleural effusion. After two cycles of R-CHOP and six cycles of R2-CHOP, pleural effusion disappeared but the pulmonary mass remained persistent. Second-line therapies R2-GemOx failed to make any substantial improvement. Core-needle puncture biopsy of the pulmonary mass was obtained and pathological testing revealed transformed diffuse large B-cell lymphoma of germinal center B-cell subtype. Next-generation sequencing confirmed BN2 subtype. The mass showed no reduction after three cycles of R-MINE, following which the BTK inhibitor ibrutinib was administered to this patient. Unfortunately, after two months of ibrutinib treatment, the patient rapidly developed an enlarged mass and hyperprogressive disease, to which she subsequently succumbed.
Introduction
Mucosa-associated lymphoid tissue (MALT) lymphoma has been described in virtually all tissues and accounts for 7%–8% of all malignant B-cell lymphomas. MALT lymphoma is a low-grade neoplasm derived from post-germinal center marginal zone B-cells, which frequently involves the gastrointestinal tract but is less commonly described in the lung.Citation1,Citation2 Furthermore, of all the reports regarding MALT lymphoma, only a few cases were found to have transformed into diffuse large B-cell lymphoma (DLBCL), an occurrence so infrequent that the precise frequency of transformation to DLBCL remains unknown and the involved mechanisms of histologic transformation are unclear.
MALT lymphoma is a low-grade malignancy associated with good prognosis. However, the presence of pleural effusion has been found to be a risk factor adversely affecting survival and conversion to DLBCL is significantly associated with poor survival outcome.Citation2 Lee et al.Citation3 reported that the clinical stage of most patients with primary pulmonary MALT lymphoma is IE (76.5%; 39/51 patients), but with disease progression, these patients are more likely to suffer from dyspnea and B symptoms as well as a higher international prognostic index (IPI) score. A growing number of clinical trials have demonstrated that primary pulmonary MALT lymphoma with pleural effusion may be treated with watchful waiting for low-grade patients and advanced patients with low tumor burden. However, systemic chemotherapy is considered to be the first choice of treatment in patients with refractory disease accompanied by significant disease progression and diffuse dissemination.Citation4 The overall response rate of transformed DLBCL with lung/pleural involvement is significantly lower than that observed in patients without pleural infiltration. Available treatments that have been reported so far include surgical resection, watchful waiting, immunotherapy, radiation therapy and chemotherapy; however, owing to the rarity of this disease, there are still no encouraging measures to prevent the conversion of MALT lymphoma to DLBCL, and the optimal protocol for treatment of malignant transformation to DLBCL remains to be established.Citation5
In the present report, we describe a rare case of BN2-DLBCL originating from primary lung/pleural MALT lymphoma. The patient achieved a period of relief after R-CHOP in combination with lenalidomide as first-line chemotherapy, but the disease eventually progressed into invasive DLBCL. R2-GemOx and later enhanced R-MINE as the last salvage therapy failed to substantially improve the patient’s prognosis. Next-generation sequencing results showed that the patient suffered from BN2-type DLBCL that was susceptible to ibrutinib. However, the patient developed resistance and disease progression two months after using ibrutinib.
Case report
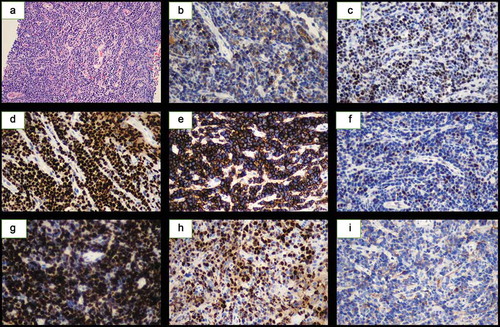
A 55-year-old woman with a 3-year history of untreated primary pulmonary MALT lymphoma was admitted to the Nantong University Affiliated Hospital in December 2017 with coughing and asthma. Pulmonary biopsy obtained at a previously attended hospital revealed the following results: LCA (+), CD20 (+), CD79a (+), Ki-67 (20%), CD21 (+), CD5 (-), cyclinD1 (-), CD43 (-), CKpan (-) and CK18 (-). In combination with computed tomography (CT) and B-ultrasound results, the patient was diagnosed with primary pulmonary MALT lymphoma (stage IV, group B). She did not experience symptoms such as night sweats or fever during this period. Multiple laboratory tests showed abnormal results, including white blood cell count exceeding 16 × 109/L and platelet count of 450 × 109/L. Serum lactate dehydrogenase reached an extremely high level of 1,392 U/L, while liver biochemistry showed a slight increase in aspartate transaminase and alanine transaminase. Chest CT examination revealed a lesion in the right middle lobe soft tissue accompanied by a large amount of pleural effusion on the right side with insufficiency. Multiple mediastinal lymph node enlargement indicated the presence of tumor with a high degree of malignancy. Pleural effusion flow cytometry analysis demonstrated clonal B-cells expressing CD19 (+), CD10 (-), CD20 (+), CD22 (+), Kappa (+) and Lambda (-) on the cell surface. The patient was started on a traditional R-CHOP chemotherapy regimen for two courses, but pleural effusion persisted even though the mass shrank slightly. Lenalidomide, 25 mg daily on days 1 through 10, was added to the R-CHOP regimen. The pleural effusion disappeared completely, but the lung mass was 6.5 × 3.9 cm after six cycles of R2-CHOP (–). PET/CT evaluation showed the right lung soft tissue mass was 6.2 × 4.4 × 5.2 cm with a high FDG uptake value of 22.3 ().
Figure 1. Computed tomography scan at time of admission (a). Over two cycles of R-CHOP and six cycles of R2-CHOP treatment, the patient demonstrated disappearance of pleural effusion and gradual shrinkage of the tumor mass, followed by subsequent recurrence and refractory disease (b–d). Subsequent chemotherapy regimens did not benefit the patient and there were no substantial changes in the lung masses (e). Within two months of using ibrutinib, the patient developed rapid DR and disease progression, and the lung mass increased progressively (f).
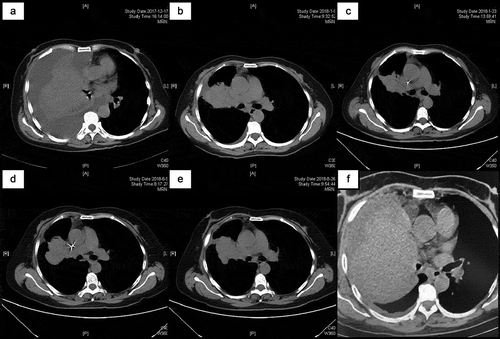
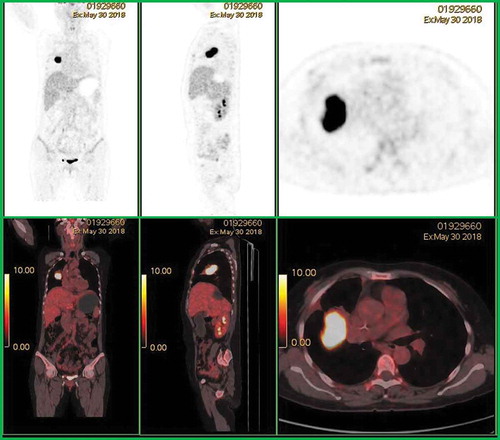
Figure 2. After administration of R2-CHOP chemotherapy regimens, positron emission tomography-computed tomography showed a soft tissue mass adjacent to the right upper pulmonary lobe with high fluorodeoxyglucose uptake, suggesting progression of tumor activity.
Thereafter, re-implement CT-guided puncture biopsy revealed CD10 (+), CD20 (+), CD21 (+), CD43 (+), CD5 (+), Bcl-2 (+), CD38 (+), IgM (+), Pax-5 (+), EBER (-), TdT (-), SOX11 (-), CD23 (-), Bcl-6 (-), CyclinD1 (-), CD3 (-) and Ki-67 (40%+). Given the patient showed some benefit from R2-CHOP but did not achieve a satisfactory result, we then treated this patient with R2-GemOx. Unfortunately, after two courses of treatment, the lung mass did not shrink and there were signs of continued growth, with the tumor size increasing to 6.4 × 6.0 cm (). On August 22, CT-guided percutaneous core needle biopsy of pulmonary tissue demonstrated the following: CD3 (-), CD5 (-), CD43 (-), CD20 (+), CD79a (+), Pax-5 (+), Bcl-2 (50%+), Bcl-6 (+), CD10 (+), MUM1 (+), C-myc (40%+), CD21 (+), CD23 (+), CD30 (-), Cyclin D1 (-), Ki67 (90%+), CD38 (-) and CD138 (-). In situ hybridization remained negative for EBER. Combined with hematoxylin and eosin staining, it became apparent that the patient had progressed to malignant B-cell lymphoma, and the immunophenotype favored DLBCL (). Next generation sequencing data revealed that the patient showed a variety of gene abnormalities, including IGHJ1 rearrangement and BCL6 fusion, NOTCH2 mutations, deletion of TNFAIP3 and amplification of NOTCH2 and MYD88. Combining the gene expression characteristics and pathological results, this patient was then diagnosed with germinal center B-cell type DLBCL derived from MALT lymphoma. Three courses of intensive R-MINE chemotherapy were used as the last salvage therapy but she did not respond. The patient’s genetic and phenotypic characteristics strongly suggested BN2-DLBCL, which is sensitive to ibrutinib. Unfortunately, after using ibrutinib for two months for this patient, the disease progressed rapidly and the lung mass increased significantly (). The patient died one month later.
Discussion
MALT lymphoma originating in the lungs accounts for only 0.5–1% of primary lung neoplasms.Citation5 At present, MALT lymphoma with relapsed refractory and malignant transformation is quite rare and highly heterogeneous, thus there is no universally accepted treatment guideline.
This transformed DLBCL patient demonstrated an unsatisfactory response to various chemotherapy regimens. Next-generation sequencing of the tumor tissue after disease conversion showed that the gene subtype was BN2-DLBCL. Schmitz et al.Citation6 reported that the BN2 subtype is rich in NOTCH2 mutations and BCL6 fusion, which is dependent on “chronic active” B-cell receptor signaling. These factors render this subtype responsive to Bruton’s tyrosine kinase (BTK) inhibitors such as ibrutinib. Firstly, the interaction of the Notch receptor with its ligand upregulates the expression of NF-κB2, and the NF-κB signaling pathway regulates the expression levels of Notch2 or Notch2 pathway components.Citation7 Consistent with these data, Saito et al found that BCL6 gene expression is regulated by the CD40-NF-κB signaling pathway and confirmed that the activation of NF-κB might represent a common pathway by which multiple stimuli can induce the transcription factor IRF4 and directly suppress BCL6 expression through IRF4 in normal germinal center B-cells. However, this negative regulatory mechanism might be blocked in DLBCL harboring BCL6 gene alterations.Citation8,Citation9 These findings suggest that the interaction of NOTCH2 mutations and BCL6 fusions with the BCR signaling pathway plays a key role in disease progression. Thus, we speculate that the BTK inhibitor ibrutinib, which targets the B-cell antigen receptor (BCR) pathway, may play an important role in disease remission.
Experimental studies have shown that ibrutinib is effective with few toxic side effects for the treatment of patients with B-cell malignancies. Noy et al.Citation10 showed that the overall rate of efficacy of ibrutinib in 60 patients with relapsed/refractory marginal zone lymphoma (MZL) was 48%, and the median progression-free survival was 14.2 months. In a DLBCL Phase II trial, the overall response rate to ibrutinib in 80 patients with relapsed refractory ABC-type DLBCL was 37%.Citation11 Recent phase III trials have shown that ibrutinib combined with R-CHOP significantly improves survival in patients younger than 60 years of age with non-GCB subtypes compared with conventional R-CHOP chemotherapy regimens.Citation12 The NCCN guidelines have indicated ibrutinib as a second-line treatment for MZL and DLBCL. The novelty of our current report is that BN2-DLBCL was originally a pathological type that was sensitive to ibrutinib treatment, but who developed rapid disease progression and significant drug resistance (DR) after two months of treatment with ibrutinib. Previous reports have indicated that if a patient suffers poor prognosis or refractory relapse after treatment with ibrutinib, the response rate to subsequent rescue treatment is extremely low, with a 1-year survival rate of only 22%.Citation13,Citation14 At present, the prognosis of patients with MALT lymphoma undergoing treatment with ibrutinib has not been systematically evaluated. Therefore, exploring the possible mechanism of ibrutinib resistance (IR) is essential for further treatment of patients with DLBCL arising from MALT lymphoma.
We have described the mechanism of primary or early acquired resistance to ibrutinib (treatment time <5 months) in B-cell lymphoma from three aspects and herein provide a theoretical basis for the study of new targeted drugs and combination therapies in such patient populations (). Importantly, although tumors respond to kinase inhibitors, they can rapidly bypass the signaling network targeting oncoproteins and continue to transmit tumor proliferation signals through alternative pathways such as PI3K-AKT-mTOR to avoid targeted therapies. Chiron et al.Citation15 found that resistance to ibrutinib in six patients with mantle cell lymphoma (MCL) maintained tumor progression by activating the BCR-PI3K-AKT signaling pathway. Thereafter, Ma et al.Citation16 confirmed their results in MCL cell lines, ibrutinib effectively inhibited BTK phosphorylation, but only the degree of P-ERK or P-AKT inhibition was linearly related to cell death. In the case of BTK inhibition, cells can still survive and grow through conduction of the PI3K-AKT-mTOR signaling pathway. Activation of this pathway suggests that the combination of the PI3K inhibitor idelalisib and ibrutinib may overcome DR and show promising responses in transforming MALT lymphoma.Citation17
Table 1. The mechanism of ibrutinib resistance in B-NHL and inhibitors of key pathway molecules that may overcome drug resistance.
In addition to the signal bypass described above, recent reports suggest that patients with primary resistance to ibrutinib are more dependent on the alternative NF-κB pathway than the BTK-mediated classical pathway. This patient revealed the presence of a TNFAIP3 (A20) mutation. TNFAIP3 is a negative regulator of the NF-κB pathway and mutations in A20 can abolish its function and stabilize MAP3K14 (also known as NIK), thereby facilitating the processing of p100 into NF-κB2 and ultimately activating the NF-κB pathway.Citation18 Another study showed that silencing NIK blocks activation of the canonical and alternative NF-κB pathways and that depletion of this molecule results in significant cell apoptosis in NIK-overexpressing cell lines, accompanied by reduction in the expression of multiple pro-survival and anti-apoptotic factors.Citation19,Citation20 Therefore, we hypothesize that the combined use of NIK inhibitors may enable MALT lymphoma patients to overcome IR.
In addition, the changes in these signaling networks are partially regulated by external signals from the tumor microenvironment (TME), and the interaction between tumor cells and the TME potentially causes hyperprogressive disease (HPD) after treatment with ibrutinib. It is well known that in addition to BTK, ibrutinib also inhibits IL-2-inducible T-cell kinase (ITK), which negatively regulates amplification of IL-2-induced Foxp3 regulatory T-cells (Treg) in the TME.Citation21,Citation22 Down-regulation of ITK by ibrutinib also up-regulates the expression of PD-1 in human Foxp3+ CD4+ T-cells, and greatly enhances the proliferation and immunosuppressive ability of Foxp3+ Tregs in the TME which have lost ITK.Citation23 This effect therefore inhibits the antitumor activity of cytotoxic T-cells. Furthermore, Chiron et al.Citation24 suggest that the TME interacts with a variety of specific growth factors and is the main force that promotes cell proliferation and enhances DR. The authors showed that the CD40-NFkB-Bcl-xL signaling pathway induces down-regulation of mitochondrial primers, which is a major driver of extracellular resistance. Zhao and colleagues explored a cross-linking network centered on PI3K-AKT-mTOR/integrin b1-ILK, which is a bridge between components of the TME and plays an indispensable role in mediating DR.Citation25 These findings show that TME-mediated mechanisms of primary and acquired DR, as well as the compositional relationships in their vast signaling networks cause tumors to expand, which ultimately leads to rapid deterioration of the disease and HPD. Therefore, we hypothesize that depletion of Tregs in combination with ibrutinib may provide a means by which to prevent and treat HPD in this patient population.
Conclusion
Patients with transformed DLBCL demonstrate an aggressive clinical course and poor prognosis. We provides a new perspective on the clinical features of BN2-DLBCL and the prognosis after treatment with ibrutinib. The development of DR is a stepwise process. A patient may already have a mutation in a DR gene such as A20 before the drug is administered. Additionally, the emergence of DR is strongly driven by the complex interactions of multiple factors such as the TME, BCR signal bypass and gene mutation, which ultimately lead to unlimited proliferation and invasion of tumor cells. we have detailed the possible causes of IR in this patient and highlights the need for urgent exploration of new targeted drugs for this patient population to treat or even prevent the occurrence of HPD after development of resistance to ibrutinib.
Disclosure of Potential Conflicts of Interest
The authors declare no conflicts of interest.
Ethics approval and consent to participate
The enrolled patient provided written informed consent.
Acknowledgments
We thank Professors Jianyong Li and Wei Xu at Jiangsu Province Hospital in Nanjing for their generous help. We thank Gillian Campbell, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Additional information
Funding
References
- Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66:153–171. doi:10.3322/caac.21330.
- Mian M, Wasle I, Gritsch S, Willenbacher W, Fiegl M. B cell lymphoma with lung involvement: what is it about? Acta Haematol. 2015;133:221–225. doi:10.1159/000365778.
- Lee H, Yang B, Nam B, Jeong B-H, Shin S, Zo JI, Shim YM, Kwon OJ, Lee KS, Kim H, et al. Treatment outcomes in patients with extranodal marginal zone B-cell lymphoma of the lung. J Thorac Cardiovasc Surg. 2017;154:342–349. doi:10.1016/j.jtcvs.2017.03.043.
- Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol. 2002;3:97–104. doi:10.1016/S1470-2045(02)00651-4.
- Nahorecki A, Chabowski M, Straszak E, Teplicki A, Szuba A, Langfort R, Janczak D. Primary pulmonary MALT lymphoma - case report and literature overview. Eur Rev Med Pharmacol Sci. 2016;20:2065–2069.
- Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, et al. Genetics and pathogenesis of diffuse large B-Cell lymphoma. N Engl J Med. 2018;378:1396–1407. doi:10.1056/NEJMoa1801445.
- Troen G, Wlodarska I, Warsame A, Hernandez Llodra S, De Wolf-Peeters C, Delabie J. NOTCH2 mutations in marginal zone lymphoma. Haematologica. 2008;93:1107–1109. doi:10.3324/haematol.11635.
- Green MR, Vicente-Duenas C, Romero-Camarero I, Long Liu C, Dai B, Gonzalez-Herrero I, García-Ramírez I, Alonso-Escudero E, Iqbal J, Chan WC, et al. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat Commun. 2014;5:3904. doi:10.1038/ncomms4904.
- Valls E, Lobry C, Geng H, Wang L, Cardenas M, Rivas M, Cerchietti L, Oh P, Yang SN, Oswald E, et al. BCL6 antagonizes NOTCH2 to maintain survival of human follicular lymphoma cells. Cancer Discov. 2017;7:506–521. doi:10.1158/2159-8290.CD-16-1189.
- Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, Collins GP, Ma S, Coleman M, Peles S, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224–2232. doi:10.1182/blood-2016-10-747345.
- Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, Lih C-J, Williams PM, Shaffer AL, Gerecitano J, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–926. doi:10.1038/nm.3884.
- Younes A, Sehn LH, Johnson P, Zinzani PL, Hong X, Zhu J. Randomized Phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37:1285–1295. doi:10.1200/JCO.18.02403.
- Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126:739–745. doi:10.1182/blood-2015-03-635326.
- Epperla N, Shana’ah AY, Jones D, Christian BA, Ayyappan S, Maddocks K, Woyach JA. Resistance mechanism for ibrutinib in marginal zone lymphoma. Blood Adv. 2019;3:500–502. doi:10.1182/bloodadvances.2018029058.
- Chiron D, Di Liberto M, Martin P, Huang X, Sharman J, Blecua P, Mathew S, Vijay P, Eng K, Ali S, et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014;4:1022–1035. doi:10.1158/2159-8290.CD-14-0098.
- Ma J, Lu P, Guo A, Cheng S, Zong H, Martin P, Coleman M, Wang YL. Characterization of ibrutinib-sensitive and -resistant mantle lymphoma cells. Br J Haematol. 2014;166:849–861. doi:10.1111/bjh.2014.166.issue-6.
- Hershkovitz-Rokah O, Pulver D, Lenz G, Shpilberg O. Ibrutinib resistance in mantle cell lymphoma: clinical, molecular and treatment aspects. Br J Haematol. 2018;181:306–319. doi:10.1111/bjh.2018.181.issue-3.
- Rahal R, Frick M, Romero R, Korn JM, Kridel R, Chan FC, Meissner B, Bhang H-E, Ruddy D, Kauffmann A, et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat Med. 2014;20:87–92. doi:10.1038/nm.3435.
- Odqvist L, Sanchez-Beato M, Montes-Moreno S, Martin-Sanchez E, Pajares R, Sanchez-Verde L, Ortiz-Romero PL, Rodriguez J, Rodriguez-Pinilla SM, Iniesta-Martinez F, et al. NIK controls classical and alternative NF-kappaB activation and is necessary for the survival of human T-cell lymphoma cells. Clin Cancer Res. 2013;19:2319–2330. doi:10.1158/1078-0432.CCR-12-3151.
- Ahmed M, Lorence E, Wang J, Jung D, Zhang L, Nomie K, Wang M.Interrogating B cell signaling pathways: A quest for novel therapies for mantle cell lymphoma. Sci Signal. 2019;12:eaat4105. doi:10.1126/scisignal.aat4105.
- Huang W, Jeong AR, Kannan AK, Huang L, August A. IL-2-inducible T cell kinase tunes T regulatory cell development and is required for suppressive function. J Immunol (Baltimore, Md: 1950). 2014;193:2267–2272. doi:10.4049/jimmunol.1400968.
- Mamontov P, Eberwine RA, Perrigoue J, Das A, Friedman JR, Mora JR. A negative role for the interleukin-2-inducible T-cell kinase (ITK) in human Foxp3+ TREG differentiation. PLoS One. 2019;14:e0215963. doi:10.1371/journal.pone.0215963.
- Eken A, Cansever M, Somekh I, Mizoguchi Y, Zietara N, Okus FZ, Erdem S, Canatan H, Akyol S, Ozcan A, et al. Genetic deficiency and biochemical inhibition of ITK affect human Th17, treg, and innate lymphoid cells. J Clin Immunol. 2019;39:391–400. doi:10.1007/s10875-019-00632-5.
- Chiron D, Bellanger C, Papin A, Tessoulin B, Dousset C, Maiga S, Moreau A, Esbelin J, Trichet V, Chen-Kiang S, et al. Rational targeted therapies to overcome microenvironment-dependent expansion of mantle cell lymphoma. Blood. 2016;128:2808–2818. doi:10.1182/blood-2016-06-720490.
- Zhao X, Lwin T, Silva A, Shah B, Tao J, Fang B, Zhang L, Fu K, Bi C, Li J, et al. Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma. Nat Commun. 2017;8:14920. doi:10.1038/ncomms14920.