ABSTRACT
Background
Transmembrane-4-L-six-family-1 (TM4SF1) functions to regulate cell growth and mobility and TM4SF1 expression was upregulated in pancreatic cancer. This study further investigated the role of TM4SF1 in regulating pancreatic cancer epithelial-mesenchymal transition (EMT) and angiogenesis and the underlying molecular events.
Methods
Tissue specimens were collected from 90 pancreatic cancer patients for immunohistochemical and qRT-PCR analysis of miR-141 and TM4SF1 levels, respectively. Pancreatic cancer cell lines were used for in vitro assays, while nude mice were used for the in vivo assay.
Results
TM4SF1 expression was upregulated, whereas miR-141 expression was lost in pancreatic cancer tissues, both of which was associated with advanced clinicopathological features and poor survival of pancreatic cancer patients. Furthermore, miR-141 was able to target and reduce TM4SF1 expression in pancreatic cancer cells and miR-141 expression inhibited pancreatic cancer cell EMT in vitro and Matrigel plug angiogenesis and lung metastasis in nude mice. At the gene level, miR-141 directly targeted and reduced TM4SF1 expression and in turn induced E-cadherin expression and reduced VEGF-A expression by suppressing activation of the AKT signaling pathway.
Conclusions
This study demonstrated that upregulated TM4SF1 and lost miR-141 expression were associated with advanced clinicopathological features and poor survival of pancreatic cancer patients. Lost miR-141 expression but induced TM4SF1 expression altered expression of VEGF-A and E-cadherin and promoted pancreatic cancer cell EMT and angiogenesis via the AKT signaling pathway, suggesting that targeting of miR-141 and TM4SF1 may be a potential therapeutic strategy to control pancreatic cancer.
Background
Pancreatic cancer is the most lethal malignancy in the world with extremely poor survival rate and increased cancer incidence. The most recent worldwide data showed that there were 330,000 pancreatic cancer-related deaths in 2012 globally vs. 200,000 pancreatic cancer-related deaths in 1990.Citation1,Citation2 Pancreatic cancer risk factors include tobacco smoking, obesity, chromic pancreatitis, alcohol consumption, and family history.Citation3-Citation5 Histologically, 95% of pancreatic cancer originates from the exocrine portion of the pancreas as pancreatic adenocarcinoma.Citation3 To date, most pancreatic cancer patients are diagnosed at the advanced stages of the disease, for which curable surgical resection is not possible, and chemoradiotherapy of pancreatic cancer shows limited success.Citation3-Citation5 The prognosis of pancreatic cancer is stage-dependent; for example, more than 12% of clinical stage I patients could survive for five years, whereas only 1% of stage IV patients survived for 5 years [https://www.cancer.org/cancer/pancreatic-cancer/detection-diagnosis-staging/survival-rates.html]. Thus, research to better understand the biology and molecular mechanisms of pancreatic carcinogenesis and angiogenesis and metastasis could help us develop novel strategies in the prevention, early detection, prognosis and treatment responses, as well as treatment options for pancreatic cancer patients.
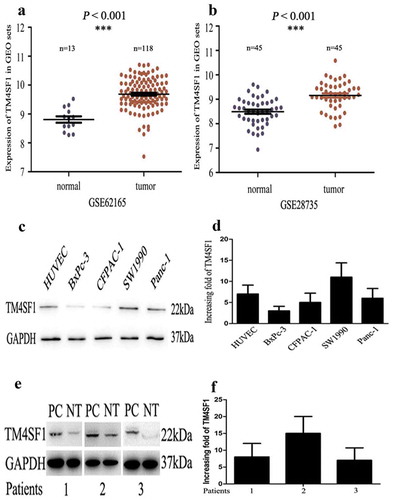
Figure 1. Upregulation of TM4SF1 expression in pancreatic cancer tissues and cell lines. (a) and (b). The GEO profile datasets. TM4SF1 expression was upregulated in 118 and 45 pancreatic cancer samples (Tumor) compared with that of 13 and 45 normal tissue samples (Normal). (c) and (d). Western blot and qRT-PCR. The level of TM4SF1 mRNA and protein was assessed using qRT-PCR and Western blot, respectively, in pancreatic cancer cell lines (SW1990, PANC-1, BxPC-3 and CFPAC-1) and HUVECs. E and F. Western blot and qRT-PCR. Level of TM4SF1 mRNA and protein was determined in the three pairs of pancreatic cancer and normal tissue samples using qRT-PCR and Western blot, respectively.
In the 1970’s, Folkman et al. reported a novel strategy to target the “tumor angiogenic factor”, and such factors could be responsible for initiating tumor angiogenesis, the targeting of which was able to block the formation of new blood vessels in tumor lesions and therefore inhibit tumor cell growth and survival.Citation6 Since then, tumor angiogenesis has been a hot field for cancer research. A previous study showed that vascular endothelial growth factor (VEGF) was identified as the primary tumor angiogenic factor,Citation7 although clinically targeting VEGF and its receptor showed limited success in controlling solid human cancers;Citation8,Citation9 thus, additional targets could be needed. For example, bevacizumab in combination with fluorouracil-based chemotherapy resulted in clinical improvement in the survival of metastatic colorectal cancer patients.Citation10 Furthermore, tumor cell epithelial-mesenchymal transition (EMT) and angiogenesis could be equally important in promoting tumor cell growth, invasion, and metastasis.Citation11 During tumor cell EMT, it loses the epithelial phenotypes, such as cell polarity and cell-cell adhesion; however, it gains mesenchymal phenotypes, such as migration and invasion capacity. Molecularly, EMT tumor cells lose E-cadherin expression but show increased expression of N-cadherin, fibronectin and vimentin.Citation11,Citation12 Nevertheless, recent studies demonstrated that Transmembrane-4-L-six-family (TM4SF1), a member of the transmembrane 4 superfamily, was able to regulate pancreatic cancer cell EMT and angiogenesis.Citation13–Citation15 TM4SF1is a 22 Kd four-transmembrane-domain protein with 202 amino acids (also known as L6-Ag), which was originally identified in 1986 as a tumor cell antigen recognized by the mouse monoclonal antibody L6Citation16and cloned in 1992.Citation17 TM4SF1 was highly expressed in many cancer cells (lung, breast, colon, pancreatic, and ovarian cancers) and endothelial cellsCitation16,Citation17 and functions to regulate the motility and intercellular adhesion of tumor and endothelial cells.Citation13,Citation18,Citation19 More recent studies have shown that the expression of TM4SF1 could be targeted by miR-141, a class of small non-coding and single-stranded RNAs with 19–24 nucleotides in length.Citation20 mRNA functions to regulate gene expression by binding to the3ʹ-untranslated region of the target mRNA to block the translation of mRNA to protein and/or degrade mRNA.Citation21 In our previous study, we demonstrated that miR-141 expression was able to suppress pancreatic cancer cell migration and invasion by targeting TM4SF1.Citation20
In this study, we further investigated the role of TM4SF1 in regulating the pancreatic cancer EMT and angiogenesis as well as the underlying molecular events. We expected to establish the miR-141/TM4SF1/AKT axis in pancreatic cancer progression and to determine whether this gene signaling could be a potential therapeutic strategy for pancreatic cancer.
Methods
Patients and tissue specimens
In this study, we retrospectively collected normal and cancerous tissue specimens from 90 pancreatic cancer patients who underwent pancreatic resection in Gaochun People’s Hospital (Nanjing, China) between January 2015 and January 2016. All patients were histologically diagnosed as pancreatic adenocarcinoma according to the criteria of the updated World Health Organization classification,Citation22and tumor stages were classified according to the UICC classification, 7th edition.Citation23 Patients did not receive any pre-surgery chemoradiotherapy, and the clinicopathological characteristics of all patients were collected from their medical records (). This study was approved by the Ethics Committee of Gaochun People’s Hospital. All participants provided written informed consent form before enrollment into this study.
Table 1. Association of TM4SF1 and miR-141 expression with clinicopathological features of pancreatic cancer patients.
Immunohistochemistry
The Expose HRP/DAB detection system kit from Abcam(Shanghai, China) was used to immunohistochemically to detect TMSF1 expression in normal and cancerous tissue blocks from pancreatic cancer patients according to the manufacturer’s protocol. anti-TM4SF1/VEGF-A/p-AKT antibodies were purchased from Sigma-Aldrich (Cat# SAB3500723; Shanghai, China) and diluted at 1:100 and then visualized using the 3,3ʹ-diaminobenzidine solution. The immunostained tissue sections were reviewed under a light microscope (BX41; Olympus, Japan) with the LangjiaPatho Graphic system (Wuxi, Jiangsu, China) and scored according to a previous study.Citation24
RNA isolation and qRT-PCR
Levels of TM4SF1 mRNA and hsa-miR-141 were assessed using qRT-PCR. Briefly, total cellular RNA was isolated from pancreatic tissues and cell lines using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA using the PrimeScript RT Reagent (Takara, Dalian, China) according to the manufacturers’ protocols. qPCR was performed using the 2△△ct method with the SYBR Green kit (Takara) and primers in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) following a previous study.Citation20 The primer sequences were TM4SF1, 5ʹ-ACCACTATGTCTTGATTCCCTC-3ʹ and 5ʹ-ATTGTGGCTCTGTCCTGGGT-3ʹ; miR-141, 5ʹ-CGCTAACACTGTCTGGTAAAG-3ʹ and 5ʹ-GTGCAGGGTCCGAGGT-3ʹ; GAPDH, 5ʹ-TCACCCACACTGTGCCCATCTACGA-3ʹ and 5ʹ-CAGCGGAACCGCTCATTGCCAATGG-3ʹ.
Western blot
The expression of TM4SF1, AKT, VEGF-A, and E-cadherin in pancreatic cancer tissues and cells was determined using Western blot with antibodies against TM4SF1, AKT, VEGF-A, and E-cadherin (Abcam, Cambridge, MA, USA), while the anti-GAPDH antibody (Beyotime, Jiangsu, China) was used as a loading control. The protein extraction and Western blot procedures followed those of our previous study.Citation20
Cell lines and culture
Human umbilical vein endothelial cells (HUVECs), and human pancreatic cancer SW1990, PANC-1, BxPC-3, and CFPAC-1 cell lines were obtained from Shanghai Cell Bank (Shanghai, China) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Wisent, St-Bruno, QC, Canada) supplemented with 2% fetal bovine serum (FBS; Wisent) for HUVECs or 10% FBS for all other cell lines, 2 mM glutamine, 100 unit/ml penicillin, and 100 µg/ml streptomycin in a humidified chamber with 5% CO2 at 37°C.
miRNA, siRNA, and cell infection
We obtained lentiviruses carrying miR-141 mimic (141M), miR-141 mimic negative control (141M-NC), miR-141 inhibitor (141I), miR-141 inhibitor negative control (141I-NC) as well as TM4SF1 siRNA (siTM4SF1) or a plasmid carrying TM4SF1 cDNA (oeTM4SF1) from GenePharma (Shanghai, China). For cell infection, SW1990 and BxPc-3 cells were seeded in 6-well plates and grown overnight to reach 50% confluency and then infected with the lentiviruses carrying the above named constructs for 48 h following the manufacturer’s instructions. After that, cells with 90% of the fluorescent index were harvested to assess TM4SF1 mRNA and protein levels or subjected to other assays. In the indicated experiments, the AKT activator Recilisib (cat #HY-101625; Cell Signaling Technology, Beverly, MA, USA) and an AKT inhibitor Perifosine (cat #KRX-0401; Cell Signaling Technology) were dissolved in dimethyl sulfoxide (DMSO) and used to treat cell lines at a 30 µM dose and various periods of time.
Tube formation assay
To assess the tube formation capacity of HUVECs in conditioned media from SW1990-141M-NC, SW1990-141M, SW1990-141M-oeTM4SF1, BxPc-3-141I-NC, BxPc-3-141I, and BxPc-3-141I-siTM4SF1 cell cultures, we first prepared these conditioned media. In particular, we grew these cells to reach 80% confluency and then cultured them in the DMEM without FBS for additional 48 hand collected the supernatant from these cell cultures as conditioned media (CM) and stored them at −20°C. Next, the Matrigel (Cat# 356230; BD, Bedford, MA, USA) was thawed at 4°C overnight and on the next day was used to coat 96-well plates. The plates were then incubated at room temperature for at least 30 min. Meanwhile, HUVECs were suspended at a density of 2 × 105 cells/ml in these CMs and seeded into the Matrigel-precoated plates with 100 µL of HUVEC suspensions and grown for 18 h. The formed endothelial cell networks were assessed, photographed, and quantified using Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA) according to a previous study.Citation25
Hemoglobin estimation assay
The Drabkin method was used to assess hemoglobin level in tumor cell Matrigel plugs. In brief, tumor cell Matrigel plugs were immediately put into 5 ml of Drabkin’s solution (containing 200 mg potassium ferricyanide, 50 mg potassium cyanide, 140 mg potassium dihydrogen phosphate, and 1 ml nonionic detergent in 1 liter of ddH2O) and mixed thoroughly and measured using a spectrophotometer (maker, city, state) at 540 nm. The hemoglobin concentration (mg/mL) in each group was estimated against the standard calibration curve of hemoglobin and the relative hemoglobin content was the hemoglobin level (mg) divided by the volume of each Matrigel plug (the mean ± s.d.).
In vivo Matrigel plug angiogenesis and lung metastasis assays
The animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of The Second Affiliated Hospital of Nanjing Medical University (Nanjing, China). In brief, 18 BALB/c male mice aged 3 to 4 weeks were obtained from Shanghai Experimental Animal Center (Chinese Academy of Sciences, Shanghai, China) and randomly divided into six groups, i.e., SW1990-141M-NC, SW1990-141M, SW1990-141M-oeTM4SF1, BxPc-3-141I-NC, BxPc-3-141I, and BxPc-3-141I-siTM4SF1. These cells were resuspended at a density of 5 × 107 cells/ml in serum-free medium, and 0.1 mL of cell aliquots (approximately 5 × 106 cells) was mixed with 0.4 ml Matrigel and then injected bilaterally into the flanks of each mouse (100 µl of cell mixture per injection). The Matrigel plugs were removed 15 days after cell injection and measured for hemoglobin content using Drabkin’s reagent (Cat# D5941; Sigma Chemicals, St Louis, MO, USA). The data were summarized as the mean ± s.d. of replicate experiments.
For the pancreatic cancer lung metastasis model, we purchased additional 18 BALB/c male mice with eighteen that were 3 to 4 weeks of age and randomly divided them into these six groups. We injected 100 µl of tumor cell suspension into the tail veins of these mice. The mice were sacrificed 15 days after tumor cell injection, and the mouse lungs were resected and evaluated for lung nodules of tumor metastases macroscopically and microscopically.
Statistical analysis
All experimental data were statistically analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA), i.e., the association of TM4SF1 and miR-141 expression with the clinicopathological features was assessed using the χ2 or Fisher’s exact test, while Pearson’s correlation coefficient test was used to determine the association between TM4SF1 and miR-141 expression in pancreatic cancer tissues. The Kaplan-Meier curves and the log rank test were used to assess the association of TM4SF1 or miR-141 expression with overall survival of pancreatic cancer patients. Student’s t-test was performed to assess the association between the two groups for in vitro data. Statistical significance of our data was defined as P < .05.
Results
Upregulated TM4SF1 but lost miR-141 expression in pancreatic cancer tissues and cell lines
In this study, we performed immunohistochemistry, qRT-PCR, and Western blot to assess TM4SF1 expression in pancreatic cancer tissue samples and also compared TM4SF1 expression in the two gene expression omnibus (GEO) profile datasets. We found that TM4SF1 expression was up-regulated in 118 and 45 cases of pancreatic cancer compared with that of 13 and 45 cases of normal tissue samples of the two GEO profile datasets (P < .001; ). We then assessed TM4SF1 expression using Western blot and qRT-PCR in three paired pancreatic cancer vs. matched normal tissues and found that TM4SF1 expression was higher than that in normal tissues (). Furthermore, pancreatic cancer cells (SW1990, PANC-1, BxPC-3 and CFPAC-1) and HUVECs also highly expressed TM4SF1 with different degrees compared to that of human pancreatic duct epithelial HPDE cells ( and Supplement 1). In addition, our immunohistochemistry further confirmed the overexpression of TM4SF1 expression protein in pancreatic cancer tissues compared with that of the normal tissue samples ()).
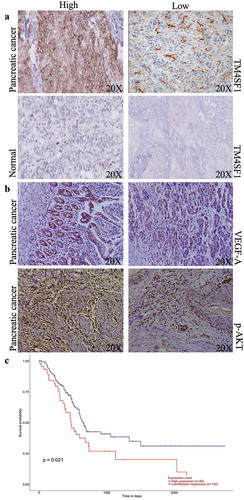
Figure 2. TM4SF1 expression and association with poor overall survival of pancreatic cancer patients. (a) Immunohistochemistry. Representative images of TM4SF1 expression in pancreatic cancer vs. normal tissue samples. (b) Immunohistochemistry. Representative images of VEGF-A and p-AKT expression in patients with high and low TM4SF1 expressing pancreatic cancer. (c) The Kaplan-Meier curves and the log rank analysis of 177 pancreatic cancer patients stratified by high and low expression of TM4SF1 in the TCGA profile dataset.
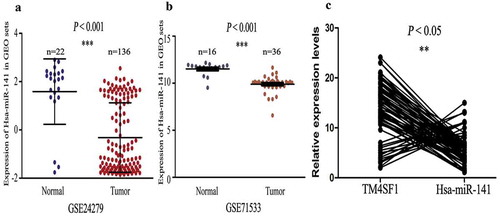
Furthermore, we also analyzed miR-141 expression in pancreatic cancer and normal tissue samples and cell lines using the GEO profiles datasets. Our data showed that levels of hsa-miR-141 were reduced in pancreatic cancer samples (136 and 36 cases) compared with normal tissues (22 and 16 cases; P < .001; ). Additionally, in our 90 cases of pancreatic cancer tissues, hsa-miR-141 expression was reduced and inversely associated with TM4SF1 expression (P < .05; )).
Figure 3. Down-regulation of hsa-miR-141 expression, and inverse correlation with TM4SF1 in pancreatic cancer tissues. (a) and (b) The level of hsa-miR-141 was downregulated in 136 and 36 pancreatic cancer tissues samples (Tumor) compared with that of 22 and 16 normal tissue (Normal) in the GEO profile datasets. (c) Association between miR-141 and TM4SF1 expression in our 90 cases of pancreatic cancer tissue samples. The data show that hsa-miR-141 expression was inversely associated with TM4SF1 expression in pancreatic cancer tissue samples.
Association of TM4SF1 expression with advanced clinicopathological features and poor survival of pancreatic cancer patients
We then associated TM4SF1 and miR-141 expression with the clinicopathological features and survival of pancreatic cancer patients and found that TM4SF1 expression was associated with tobacco smoke (P = .0006), diabetes (P = .017), tumor size (P = .037), clinical stage (P = .033), T stage (P = .002), lymph node metastasis (P = .0014), distant tumor metastasis (P = .046), tumor cell venous invasion (P = .046), and lymphatic invasion (P = .038) (). Moreover, TM4SF1 expression was also associated with poor overall survival of pancreatic cancer patients using the published profiles of the TCGA dataset (P = .021; )).
Furthermore, a reduced miR-141 level was associated with TM4SF1 expression and advanced clinicopathological features such as poor survival in pancreatic cancer patients ().
miR-141 suppression of tumor cell angiogenesis in vitro and in vivo
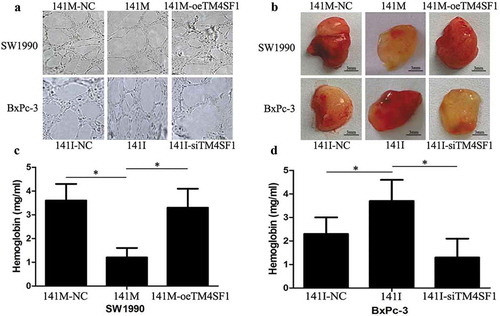
To determine the effects of miR-141 expression and knockdown on the regulation of HUVEC tube formation (angiogenesis potential), we produced the conditioned media from pancreatic cancer cells after infection with lentiviruses carrying miR-141 mimic, inhibitor, or their negative controls and then used them to culture HUVECs for tube formation capacity. Our data showed that conditioned media from SW1990-141M-oeTM4SF1 and BxPc-3-141I cultures promoted HUVEC tube formation, whereas the conditioned media from SW1990-141M and BxPc-3-141I-siTM4SF1 cultures failed to induce the complete network formation in HUVECs ()). These data suggest that hsa-miR-141 suppresses HUVECs tube formation through the regulation of TM4SF1 in vitro. Furthermore, our nude mouse Matrigel plug assay data showed that the subcutaneous injection of SW1990-141M-NC, SW1990-141M, SW1990-141M-oeTM4SF1, BxPc-3-141I-NC, BxPc-3-141I, and BxPc-3-141I-siTM4SF1 cells into nude mice, respectively, showed that only SW1990-141M and BxPc-3-141I-siTM4SF1 cell injection formed significantly fewer vessels compared with those of SW1990-141M-oeTM4SF1 and BxPc-3-141I cell injection ()). In addition, the hemoglobin concentration measured following Matrigel dissolution showed that the SW1990-141M and BxPc-3-141I-siTM4SF1 cell groups had significantly lower levels of hemoglobin (P < .05; ).
Figure 4. Hsa-miR-141 suppression of angiogenesis in vitro and in vivo. (a) Tube formation assay. HUVECs were re-suspended in 100 µl of conditioned media of SW1990-141M-oeTM4SF1, BxPc-3-141I, SW1990-141M, and BxPc-3-141I-siTM4SF1 cultures at a density of 2 × 105 cells per mL and added to 96-well plates precoated with Matrigel and grown for 18 h. Imagines were then taken and analyzed. Each experiment was performed in triplicate and repeated at least once, and the data are expressed as the mean ± s.d. (b) Nude mouse experiments. SW1990-141M-NC, SW1990-141M, SW1990-141M-oeTM4SF1, BxPc-3-141I-NC, BxPc-3-141I, and BxPc-3-141I-siTM4SF1 cells were suspended in Matrigel and DMEM and then subcutaneously injected into the flanks of each mouse (n = 3). At Day 15, tumor cell Matrigel plugs were removed from each mouse and photographed. (c) and (d) Drabkin method to assay hemoglobin level (see details in the methods section). The tumor cell Matrigel plugs were then subjected to the assay to obtain the hemoglobin level. The relative hemoglobin content was the hemoglobin level (mg) divided by the volume of each Matrigel plug (the mean ± s.d.).
miR-141 modulation of VEGF-A and E-cadherin expression by regulating TM4SF1 in vitro and inhibiting tumor cell EMT in vivo
To better understand the underlying molecular events, we infected miR-141 mimic, inhibitor, or their negative controls into pancreatic cancer SW1990 and BxPc-3 cells and then assessed the level of VEGF-A and E-cadherin. Our data showed that SW1990-141M-oeTM4SF1 and BxPc-3-141I cells had an increased expression of VEGF-A protein but a decreased level of E-cadherin, whereas SW1990-141M and BxPc-3-141I-siTM4SF1 cells had a reduced level of VEGF-A and increased E-cadherin at mRNA and protein levels (P < .05; –). These data indicate that miR-141-modulated VEGF-A and E-cadherin expression was through the regulation of TM4SF1 expression and that the modulation of TM4SF1 expression using TM4SF1 shRNA abolished the role of the miR-141 mimic.
Figure 5. miR-141 modulation of VEGF-A and E-cadherin expression depending on TM4SF1 expression and inhibition of the EMT and tumor metastasis in vivo. (a) Western blot. SW1990 and BxPc-3 cells were grown and infected with miR-141 mimic (141M), inhibitor (140I), TM4SF1 shRNA, or their negative controls and then subjected to protein extraction and western blot. GAPDH was used as a loading control. (b) and (c) qRT-PCR. These cells were also subjected to qRT-PCR analysis of VEGF-A and E-cadherin mRNA. (d) and (e) Nude mouse tumor cell metastasis assay. These cell lines were injected into the mouse tail vein and at Day 15, the mouse lungs were resected to count the lung metastasis nodules. The graph data were from the mean ± s.d. (n = 3). (f) Western blot of metastatic lung tumor nodules. SW1990-141M-oeTM4SF1 and BxPc-3-141I cells had an increased expression of VEGF-A protein but a decreased level of E-cadherin, whereas SW1990-141M and BxPc-3-141I-siTM4SF1 cells had a reduced level of VEGF-A and increased E-cadherin at mRNA and protein levels. GAPDH was used as a loading control.
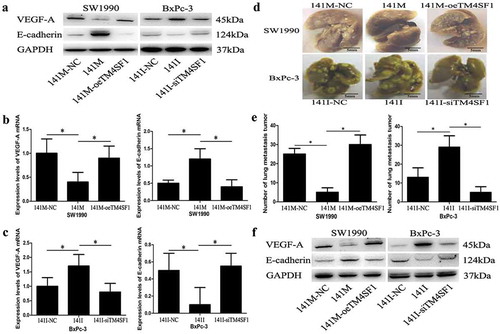
Furthermore, we performed a nude mouse tumor cell metastasis assay and found that the numbers of metastatic lung tumor nodules in the SW1990-141M and BxPc-3-141I-siTM4SF1 cells groups were significantly fewer compared with those of the SW1990-141M-oeTM4SF1 and BxPc-3-141I cells groups (P < .05; Figure 5(d,e)). Moreover, our Western blots of metastatic lung tumor nodules showed that SW1990-141M-oeTM4SF1 and BxPc-3-141I cells had an increase in expression of VEGF-A protein but a decrease in E-cadherin level, whereas SW1990-141M and BxPc-3-141I-siTM4SF1 cells had a reduced level of VEGF-A and increased E-cadherin at mRNA and protein levels ()).
TM4SF1 regulation of AKT signaling in pancreatic cancer cells
After that, we performed the Kyoto Encyclopedia of Genes and Genomes (KEGG) database analysis to explore the important signaling pathways that are involved in TM4SF1-mediated pancreatic cancer progression and found that the AKT signaling pathway may play an important role in it. As shown in ), VEGF-A expression and AKT phosphorylation (p-AKT) were increased in TM4SF1-overexpressed BxPc-3 cells, whereas they were decreased in TM4SF1-knocked down SW1990 cells. Furthermore, the expression of VEGF-A and p-AKT were higher in TM4SF1 highly expressed tissue samples than in the TM4SF1 lowly expressed samples ()). In contrast, E-cadherin expression was decreased in these BxPc-3 cells but increased in these SW1990 cells.
Figure 6. TM4SF1 regulation of VEGF-A and E-cadherin expression through the activation of the AKT signaling pathway. (a) Western blot. SW1990 and BxPc-3 cells were grown and infected with TM4SF1 shRNA or the negative control and then subjected to protein extraction and western blot. The data showed that the knockdown of TM4SF1 expression in SW1990 cells increased E-cadherin but decreased VEGF-A and p-AKT expression, whereas BxPc-3 cells with TM4SF1 cDNA transfection showed opposite effects on the expression of these proteins. (b) Western blot. SW1990 and BxPc-3 cells were grown and infected with TM4SF1 shRNA or the negative control and treated with the Akt activator or inhibitor and then subjected to protein extraction and western blot. The experiment was repeated three times.
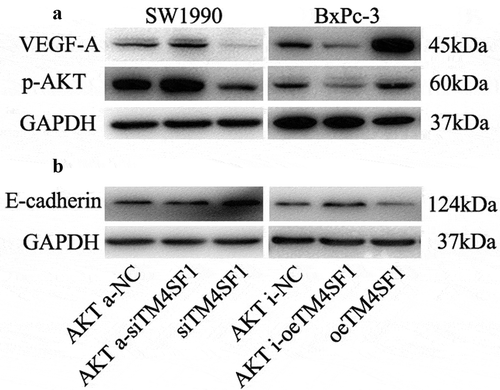
To further delineate whether the TM4SF1 regulation of VEGF-A and E-cadherin expression was through AKT pathway activation, we treated these SW1990 or BxPc-3 cells with an AKT activator (AKTa), inhibitor (AKTi), or the negative control (NC). Our data showed that the expression of p-AKT and VEGF-A was increased but E-cadherin was decreased in SW1990 cells, whereas the expression of p-AKT and VEGF-A was decreased but E-cadherin was increased in BxPc-3 cells ()). These results suggest that VEGF-A and E-cadherin expression was regulated by TM4SF1 through the AKT signaling pathway.
Discussion
Pancreatic cancer progression is involved in angiogenesis during early metastasis; thus, in the current study, we investigated the miR-141-targeting TM4SF1 downregulation in pancreatic cancer tissues for association with clinicopathological data and patient survival, as well as their role in regulating pancreatic cancer epithelial-mesenchymal transition (EMT) and angiogenesis in vitro and in nude mice. We found that TM4SF1 expression was upregulated but miR-141 expression was reduced in pancreatic cancer tissues, the aberrant expression of which was associated with advanced pancreatic cancers and poor overall survival. Moreover, the level of miR-141 was inversely associated with TM4SF1 expression in pancreatic cancer tissues, and miR-141 targeted and reduced TM4SF1 expression in pancreatic cancer cells. The expression of miR-141 inhibited pancreatic cancer cell EMT in vitro and Matrigel plug angiogenesis and lung metastasis in nude mice. In addition, miR-141-reduced TM4SF1 expression was able to induce E-cadherin expression and reduce VEGF-A expression by regulating the AKT signaling activity. Our current study demonstrated that upregulated TM4SF1 and lost miR-141 expression was associated with advanced clinicopathological features and poor prognosis of pancreatic cancer. Upregulated miR-141 expression but reduced TM4SF1 expression could modulate VEGF-A and E-cadherin expression to promote pancreatic cancer cell EMT and angiogenesis through the AKT signaling pathway. Future study will investigate whether targeting miR-141 and TM4SF1 can serve as a potential therapeutic strategy in controlling pancreatic cancer.
Indeed, TM4SF1 was identified as a L6 tumor cell surface antigen through screening approximately 10,000 monoclonal antibodies in human lung carcinoma cells and demonstrated to have a high level of tumor selectivity;Citation16 additionally, in 1992, the full length of TM4SF1 cDNA was cloned, and the data showed that TM4SF1 protein contains three predicted NH2-terminal hydrophobic transmembrane regions, two potential N-linked glycosylation sites, and a COOH-terminal hydrophobic transmembrane region; thus, TM4SF1 was classified as a member of the transmembrane 4 superfamily and TM4SF1 expression has been implicated for cell growth.Citation17 Previous studies showed TM4SF1 overexpression in a number of different human cancersCitation16-Citation18 and promoted their migration and invasionCitation26-Citation29 and resistance to gemcitabineCitation30 as well as predicted the poor prognosis of pancreatic adenocarcinoma or glioma.Citation28,Citation31 In esophageal cancer, TM4SF1 promoted tumor stem-like cell self-renewal, the expression of which was regulated by miR-141,Citation32 while our previous study revealed that miR-141 could directly target TM4SF1 detected by using the Luciferase reporter assay and reduced TM4SF1 expression and in turn suppressed the migration and invasion of pancreatic cancer cells in vitro.Citation20 Our current study further confirmed TM4SF1 overexpression and the reverse association with miR-141 in pancreatic cancer tissues, and we also found that the aberrant expression of these two genes was associated with pancreatic cancer progression and poor prognosis of pancreatic cancer patients.
Furthermore, during cancer progression, tumor cells will undergo the EMT to gain mobility capacity by losing cell-cell adhesion and invasion across the basement membrane and into and out of the blood circulation, and then forming metastasized secondary tumor lesions.Citation33–Citation36 Thus, reversing the tumor cell EMT program becomes a useful strategy for future translation to cancer therapy in the clinic.Citation37 Furthermore, tumor cell progression also requires angiogenesis, while the expression of VEGF-A or its receptors has been important in tumor angiogenesis.Citation14 In our current study, we found that lost miR-141 expression led to the upregulation of TM4SF1 in pancreatic cancer tissues and cells, resulting in tumor cell EMT (assayed for the downregulation of E-cadherin) and angiogenesis (assayed for increased VEGF-A expression). Indeed, a previous study demonstrated a proof-of-concept that TM4SF1-targeting antibody-drug conjugates possessed anticancer activity in targeting both tumor cells and tumor vasculature.Citation38 Thus, such a potential target could have anti-angiogenesis and anti-EMT properties.Citation14,Citation15 Furthermore, our previous study revealed that TM4SF1 was targeted by miR-141, which was confirmed using the bioinformatics algorithms and dual-luciferase reporter assay.Citation20 In our current study, we confirmed their association in pancreatic cancer tissues and showed that the overexpression of miR-141 significantly suppressed HUVEC tube formation and nude mouse Matrigel plug angiogenesis and lung metastasis of pancreatic cancer cells. Our current data, therefore, indicate that miR-141 suppressed pancreatic cancer angiogenesis and EMT by directly targeting and inhibiting TM4SF1 expression, suggesting that the manipulation of this gene pathway could be further evaluated as a therapeutic approach for pancreatic cancer. In addition, our current study showed that the effects of TM4SF1 on upregulating VEGF-A and downregulating E-cadherin expression in pancreatic cancer cells were mediated by the activation of the AKT signaling pathway. The Akt pathway genes regulate cell survival and metabolism and promote cell cycle progression.Citation39,Citation40 Nevertheless, further studies are needed to disclose how TM4SF1 regulates Akt activation and the downstream signaling to promote pancreatic cancer cell EMT and angiogenesis.
However, our current study has some limitations; for example, our own pancreatic cancer cases did not have follow-up data for association with prognosis analysis, and we did not assess pancreatic cancer cell migration and invasion after manipulating TM4SF1 and/or miR-141 expression. Furthermore, we just utilized a single cell line each (overexpression and knockdown, respectively) for our experiments to confirm the role of TM4SF1 in pancreatic cancer; thus, further study is needed to verify its in vivo role. In addition, the AKT signaling pathway was first predicted by the KEGG database analysis and our experiments only verified such a prediction.
Conclusion
In conclusion, our current study demonstrated thatTM4SF1 was overexpressed but miR-141 was downregulated in pancreatic cancer tissues, which was associated with advanced clinicopathological features and poor patient survival. Our research revealed that miR-141 targeted TM4SF1 in regulating pancreatic cancer cell angiogenesis and EMT by the activation of the AKT signaling pathway, suggesting that the miR-141/TM4SF1/AKT axis may be a therapeutic target for pancreatic cancer.
Abbreviations
| TM4SF1: | = | Transmembrane-4-L-six-family-1 |
| EMT: | = | Epithelial-mesenchymal transition |
| qRT-PCR: | = | Quantitative real time polymerase chain reaction |
| VEGF-A: | = | Vascular endothelial growth factor-A |
| HUVECs: | = | Human umbilical vein endothelial cells |
| DMEM: | = | Dulbecco’s Modified Eagle’s Medium |
| FBS: | = | Fetal bovine serum |
| DMSO: | = | Dimethyl sulfoxide |
| CM: | = | Conditioned media |
| IACUC: | = | Institutional Animal Care and Use Committee |
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests in this study.
Authors’ Contributions
DX and FY were responsible for in vitro and in vivo experiments, respectively. KW, KZ and XX conducted chip data mining and data statistics, respectively. YA, XL and XHZ performed clinical sample testing and immunohistochemistry. JX, FX, XJY and LJX collected the clinical samples.
Contents
Upregulated TM4SF1 and lost miR-141 expression were associated with advanced clinicopathological features and poor survival of pancreatic cancer patients. Lost miR-141 expression but induced TM4SF1 expression promote pancreatic cancer cell EMT and angiogenesis via the AKT signaling pathway.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Gaochun People’s Hospital. All participants provided written informed consent form before enrollment into this study.
Supplemental Material
Download JPEG Image (33.9 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet (London, England). 2012;380:2095–2128. doi:10.1016/S0140-6736(12)61728-0.
- Organization WH. World cancer report 2014. Pancreatic Cancer. 2014:413–421.
- Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476.
- Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348.
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. doi:10.1056/NEJMra1404198.
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186.
- Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi:10.1053/sonc.2002.37264.
- Ellis LM, Rosen L, Gordon MS. Overview of anti-vegf therapy and angiogenesis. Part 1: angiogenesis inhibition in solid tumor malignancies. Clin Adv Hematol Oncol. 2006;4(suppl):1–10; quz 11-2.
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase iii clinical trials on anti-vegf therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi:10.1056/NEJMoa032691.
- Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. Emt: present and future in clinical oncology. Mol Oncol. 2017;11:718–738.
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166:21–45.
- Zukauskas A, Merley A, Li D, Ang LH, Sciuto TE, Salman S, Dvorak AM, Dvorak HF, Jaminet SC. Tm4sf1: a tetraspanin-like protein necessary for nanopodia formation and endothelial cell migration. Angiogenesis. 2011;14:345–354.
- Lin CI, Merley A, Sciuto TE, Li D, Dvorak AM, Melero-Martin JM, Dvorak HF, Jaminet SC. Tm4sf1: a new vascular therapeutic target in cancer. Angiogenesis. 2014;17:897–907.
- Park YR, Kim SL, Lee MR, Seo SY, Lee JH, Kim SH, Kim IH, Lee SO, Lee ST, Kim SW, et al. Microrna-30a-5p (mir-30a) regulates cell motility and emt by directly targeting oncogenic TM4SF1 in colorectal cancer. J Cancer Res Clin Oncol. 2017;143:1915–1927. doi:10.1007/s00432-017-2440-4.
- Hellstrom I, Horn D, Linsley P, Brown JP, Brankovan V, Hellström KE. Monoclonal mouse antibodies raised against human lung carcinoma. Cancer Res. 1986;46:3917–3923.
- Marken JS, Schieven GL, Hellstrom I, Hellström KE, Aruffo A. Cloning and expression of the tumor-associated antigen l6. Proc Natl Acad Sci U S A. 1992;89:3503–3507.
- Kao YR, Shih JY, Wen WC, Ko Y-P, Chen B-M, Chan Y-L, Chu Y-W, Yang P-C, Wu C-W, Roffler SR, et al. Tumor-associated antigen l6 and the invasion of human lung cancer cells. Clin Cancer Res. 2003;9:2807–2816.
- Lin CI, Lau CY, Li D, Jaminet SC. et al. Nanopodia–thin, fragile membrane projections with roles in cell movement and intercellular interactions. J Visualized Exp. 2014;86.
- Xu L, Li Q, Xu D, Wang Q, An Y, Du Q, Zhang J, Zhu Y, Miao Y. Hsa-mir-141 downregulates TM4SF1 to inhibit pancreatic cancer cell invasion and migration. Int J Oncol. 2014;44:459–466.
- Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi:10.1016/S0092-8674(04)00045-5.
- Hruban R, Klöppel G, Boffetta P. Ductal adenocarcinoma of the pancreas. In: The International Agency for Research on Cancer, Carneiro F, Hruban RH, Theise ND: Who Classification of Tumours of the Digestive System. Lyon (France): World Health Organization; 2010. p. 281–291.
- Edge S, Byrd D, Compton C. Exocrine and endocrine pancreas. Ajcc cancer staging manual. 7th ed. New York, NY: Springer; 2010. p. 241–248.
- Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A, Aas T, Otte AP, Akslen LA. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12:1168–1174. doi:10.1158/1078-0432.CCR-05-1533.
- Eccles SA, Court W, Patterson L, Sanderson S. In vitro assays for endothelial cell functions related to angiogenesis: proliferation, motility, tubular differentiation, and proteolysis. Methods Mol Biol. 2009;467:159–181.
- Allioli N, Vincent S, Vlaeminck-Guillem V, Decaussin‐Petrucci M, Ragage F, Ruffion A, Samarut J. Tm4sf1, a novel primary androgen receptor target gene over-expressed in human prostate cancer and involved in cell migration. Prostate. 2011;71:1239–1250.
- Cao J, Yang JC, Ramachandran V, Arumugam T, Deng DF, Li ZS, Xu LM, Logsdon CD. TM4SF1 regulates pancreatic cancer migration and invasion in vitro and in vivo. Cell Physiol Biochem. 2016;39:740–750.
- Zheng B, Ohuchida K, Cui L, Zhao M, Shindo K, Fujiwara K, Manabe T, Torata N, Moriyama T, Miyasaka Y, et al. TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma is involved in migration and invasion of cancer cells. Int J Oncol. 2015;47:490–498. doi:10.3892/ijo.2015.3022.
- Sun Y, Xu Y, Xu J, Lu D, Wang J. Role of TM4SF1 in regulating breast cancer cell migration and apoptosis through pi3k/AKT/mtor pathway. Int J Clin Exp Pathol. 2015;8:9081–9088.
- Cao J, Yang J, Ramachandran V, Arumugam T, Deng D, Li Z, Xu L, Logsdon CD. TM4SF1 promotes gemcitabine resistance of pancreatic cancer in vitro and in vivo. PLoS One. 2015;10:e0144969.
- Wang P, Bao W, Zhang G, Cui H, Shi G. Transmembrane-4-l-six-family-1, a potential predictor for poor prognosis, overexpressed in human glioma. Neuroreport. 2015;26:455–461.
- Xue L, Yu X, Jiang X, Deng X, Mao L, Guo L, Fan J, Fan Q, Wang L, Lu S-H, et al. TM4SF1 promotes the self-renewal of esophageal cancer stem-like cells and is regulated by mir-141. Oncotarget. 2017;8:19274–19284. doi:10.18632/oncotarget.13866.
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi:10.1126/science.1203543.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi:10.1016/S0092-8674(00)81683-9.
- Ye X, Weinberg RA. Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 2015;25:675–686. doi:10.1016/j.tcb.2015.07.012.
- Jung HY, Fattet L, Yang J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clin Cancer Res. 2015;21:962–968. doi:10.1158/1078-0432.CCR-13-3173.
- Visintin A, Knowlton K, Tyminski E, Lin C-I, Zheng X, Marquette K, Jain S, Tchistiakova L, Li D, O’Donnell CJ, et al. Novel anti-TM4SF1 antibody-drug conjugates with activity against tumor cells and tumor vasculature. Mol Cancer Ther. 2015;14:1868–1876. doi:10.1158/1535-7163.MCT-15-0188.
- Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, Gartel A, Hay N. Activation of AKT/protein kinase b overcomes a g(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol. 2002;22:7831–7841.
- Song G, Ouyang G, Bao S. The activation of AKT/pkb signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi:10.1111/jcmm.2005.9.issue-1.
