ABSTRACT
Progression of prostate cancer has been associated with EGFR and HER2 activation and to tumor-initiating cells contribution toward chemotherapy resistance. We investigated the efficacy of a dual intervention against EGFR and HER2 to deplete the tumor-initiating cells, optimize the chemotherapy management and prevent the progression of castration-resistant prostate cancer (CRPC) cells. Using DU145, PC3, and 22Rv1 CRPC cell lines, biochemical analysis revealed activation of EGFR, HER2, MAPK, and STAT3 in DU145 and 22Rv1, and AKT and SRC in DU145 and PC-3. pSTAT3 nuclear staining was observed in DU145 xenografts and in 12 out of 14 CRPC specimens. The in vivo dual targeting of ErbB receptors with Cetuximab and Trastuzumab combined with chemotherapy caused an effective antitumor response in DU145 xenografted mice displaying STAT3 activation; conversely PC-3 bearing mice experienced tumor relapse. The potentiating of in vivo cytotoxic effect in DU145 model was accompanied by a significant decrease of prostatosphere-forming capacity assessed in vitro on residual tumor cells. Additionally, combined treatment in vitro with Cetuximab, Trastuzumab and chemotherapy negatively affected DU145 and 22Rv1 sphere formation, suggesting the critical function of ErbB receptors for tumor-initiating cells proliferation; no effect on PC-3 clonogenic potential was observed, indicating that other receptors than EGFR and HER2 may sustain PC3 tumor-initiating cells. These findings provided the preclinical evidence that the dual inhibition of EGFR and HER2 by targeting tumor-initiating cells may improve the efficacy of the current chemotherapy regimen, bringing benefits especially to castration-resistant patients with activated STAT3, and preventing disease progression.
Introduction
Prostate cancer (PC) is among the most common cancer diagnosis and the second-leading cause of cancer-related deaths in men.Citation1,Citation2 The standard care for clinically localized PC includes surgery, anti-hormonal therapies, and radiotherapy. By contrast, the advanced and metastatic PC forms are mainly treated with androgen deprivation therapy (ADT). Despite an early response to ADT, the majority of patients generally develop castration-resistant prostate cancers (CRPC) within few years. Despite docetaxel (taxotere)-based chemotherapy confers a survival benefit in CRPC patients,Citation3 median overall survival (OS) for patients affected by metastatic CRPC is less than 20 months supporting the need to identify events crucial in tumor progression.
Among the mechanisms supporting the progression of CRPCs, several studies have indicated the aberrant expression and activation of ErbB1 (EGFR) and ErbB2 (HER2) receptors.Citation4–Citation10 The physiologic significance of HER2 in CRPC is also corroborated by studies showing an increased HER2 expression or activity in CRPC clinical samplesCitation11-Citation13 and the positive association between the expression of the receptor and the aggressiveness of primary untreated PC.Citation14 Similarly, the overexpression and activation of EGFR during the CRPC developmentCitation15 and the disease progressionCitation16 has been reported.
The theoretical cancer stem cell (CSC) model postulates a hierarchical organization of human tumors, in which only a subset of tumor cells is endowed with tumor-initiating and long-term tumor propagating activity. These cells exhibit phenotypic and functional features typical of normal stem cells and are involved in tumor initiation, metastatic spreading, and drug resistance,Citation17 providing the basis for disease relapse following initial tumor response to the standard therapeutic regimen.
EGFR and HER2 module plays pivotal functions in the rapid clonal expansion of progenitor cells derived from CSCs.Citation18 This scenario is especially relevant to solid tumors. Opportunities for drug interventions against EGFR and HER2 could precisely and timely eliminate very small populations of tumor-initiating cells and block tumor progression. Accordingly, the use of selective inhibitors against EGFR and Hedgehog increased the cytotoxicity of Taxotere-based chemotherapy in CRPC, thus reducing disease relapse.Citation19
In the present study, we aimed to investigate the role of EGFR and HER2 in sustaining tumor-initiating cells in three CRPC cell lines DU145, PC-3 and 22Rv1 responsive to docetaxel. We also evaluated whether co-targeting EGFR and HER2 in combination with docetaxel (Taxotere)-based therapy might perturbate the pool of CSCs and enhance the efficacy of the current chemotherapeutic regimen.
Results
EGFR and HER2 are expressed and activated in hormone-independent prostate carcinoma cells
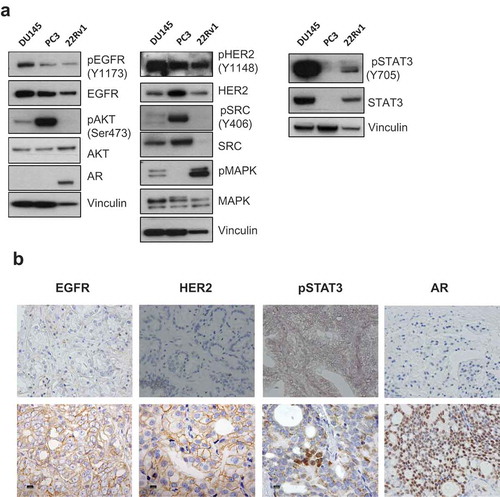
As the first step to explore the involvement of EGFR and HER2 in CRPC progression, the expression and activation status of EGFR and HER2 and of key molecules downstream of the two receptors were investigated by Western blot in three-CRPC cell lines: DU145 and PC-3 both lacking Androgen Receptor (AR) expression and 22Rv1 expressing AR. As shown in , all cell lines expressed activated EGFR and HER2 and in keeping with their activation, MAPK, and STAT3 were almost exclusively activated in DU145 and 22Rv1, while AKT and SRC were phosphorylated in DU145 and PC-3 (). IHC analysis of formalin-fixed paraffin-embedded (FFPE) blocks from 14 hormone-independent prostate carcinomas all expressing AR showed a focal staining of pSTAT3 in 12 cases. Moreover, the expression of EGFR and HER2 in pSTAT3-positive tumors was detectable in 6 out of 10 specimens in which analysis of ErbB receptors was possible ( and Supplementary Table 1). Normal prostate counterpart of CRPCs showed AR and EGFR positivity and lack of HER2 and pSTAT3 expression (Supplementary Figure 1).
Figure 1. EGFR and HER2 are expressed and functional in prostate cancer cell lines and human tumors. (a) The expression levels and the activated (phosphorylated) status of EGFR and HER2 and downstream molecules of signaling pathways, AKT, MAPK, STAT3 and SRC, in DU145, PC-3 and 22Rv1 cells evaluated by Western blot; β-actin and vinculin were used as loading protein controls (data are representative of 2 independent experiments). (b) Representative IHC images of FFPE human malignant prostate tissue specimen probed with an anti-HER2, anti-EGFR, anti-pSTAT3 or anti-AR antibody. All sections were analyzed under a microscope and immunoreactivity was evaluated by dark brown staining. Upper panels: negative staining, lower panels: positive staining. Scale bar: 10 µm.
Based on evidence indicating that EGF-induced STAT3 phosphorylation promotes prostate cancer cell progression through TWIST1 expression and prostate cancer cell epithelial/mesenchimal transitionCitation20 and our previous results showing that EGFR through STAT3 sustains tumor-initiating cell proliferation in squamous cell carcinomas,Citation21 we speculated that EGFR-HER2 module could sustain the progression of CRPC cells by maintaining tumor stemness through STAT3 activation.
Combinatorial treatment of human prostate xenografted tumors with taxotere, cetuximab, and trastuzumab overcomes tumor relapse
To investigate the blockade of EGFR and HER2 in eradicating tumor mass by inhibition of tumor-initiating cells, we performed in vivo experiments on male SCID mice xenografted with established subcutaneous DU145 or PC-3 tumors. In keeping with in vitroresults shown in , PC-3 and DU145 cells grown in SCID mice showed IHC staining of EGFR and HER2, and focal nuclear labeling of pSTAT3 was observed only in DU145 xenografts (). When tumor volume reached the size of ~ 100 mm3 (day 22 in DU145 and day 15 in PC-3 xenografts), mice were randomized in 8 groups (4–5 mice/group) and treated i.p. with Trastuzumab, a specific monoclonal antibody against HER2 (10mg/Kg) or Cetuximab, a specific monoclonal antibody against EGFR (1mg/Kg) administered alone or in combination, i.v. with Taxotere (6.66mg/Kg) given alone or in combination with Trastuzumab or Cetuximab or both; a control group was treated with saline solution. Treatments were repeated on days 30, 36, 44 and 50 in DU145, on days 21, 29, 37 and 43 in PC-3 and were all well tolerated. As shown in and , in DU145 model, tumors of mice treated with saline solution or Trastuzumab grew progressively, while treatment with Cetuximab, the bi-combination Trastuzumab plus Cetuximab and Taxotere alone induced a significant tumor regression as compared to saline-treated mice, but tumor regrowth was observed in taxotere-treated mice from the end of treatment (day 56). The addition of Trastuzumab or Cetuximab to Taxotere-based therapy resulted in a considerable tumor growth inhibition during treatment, but tumor regrowth was observed from day 80 in mice treated with Taxotere plus Trastuzumab, and from day 136 in mice treated with Taxotere plus Cetuximab. Intriguingly, treatment with the triple combination Taxotere, Trastuzumab and Cetuximab markedly inhibited tumor growth (p = .0052 versus Taxotere alone), and at the end of the experiment (day 200) 4 out 5 mice (80%) resolved their tumor and became tumor-free, whereas 1 out of 5 mice (20%) was with a barely palpable tumor.
Figure 2. Antitumor activity of Taxotere, Trastuzumab, and Cetuximab in DU145 and PC-3 xenografted models. (a) Representative IHC image of FFPE DU145 and PC-3 xenografts probed with an anti-HER2, anti-EGFR or anti-pSTAT3 antibody. Scale bar: 50 µm. (b) Male SCID mice bearing established (100mm3) DU145 or PC-3 xenograft models were treated with Trastuzumab (10mg/Kg) i.p., Cetuximab (1mg/mouse) i.p., Taxotere (6.66mg/Kg) i.v., given alone, in dual or triple combination; control mice were treated with saline. Treatments were administered weekly for 5 consecutive weeks (arrow). Mean tumor volumes ± SD are shown (n = 4–5). (c) DU145 and PC-3 tumor volumes 1 week after the end of treatments (at day 57 and day 50, respectively). ** p < .01, ***p < .001 by unpaired t-test.
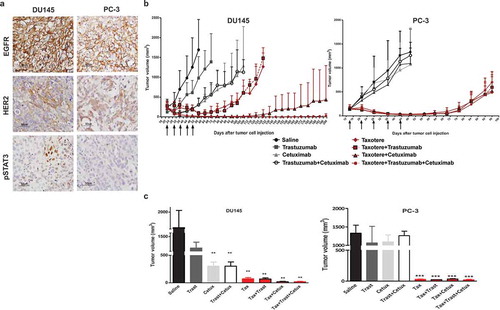
In sharp contrast, in PC-3 model lacking STAT3 activation as already reported,Citation22 treatment with Trastuzumab or Cetuximab given alone or in combination did not inhibit the tumor progression. As expected, tumors were sensitive to Taxotere resulting in a marked tumor regression. However, at the end of treatment mice experienced the tumor regrowth. Despite the addition of Trastuzumab, or Cetuximab or both to chemotherapy, mice experienced an immediate tumor relapse ( and ).
Hence, these results indicated that although both DU145 and PC-3 models express active EGFR and HER2, the simultaneous use of Cetuximab and Trastuzumab significantly improved the outcome of the current conventional Taxotere-based monotherapy only in DU145 models that displayed activation of STAT3 ( and ). Moreover, the overcoming of tumor regrowth that normally occurs at the end of drugs’ treatment, observed with the triple combination of drugs was suggestive of the efficacy of a target-therapy against EGFR and HER2 to eliminate the tumor-initiating cells and completely eradicate the tumor in these xenografts.
The in vivo treatment with taxotere, trastuzumab, and cetuximab inhibits DU145 tumor-initiating cells
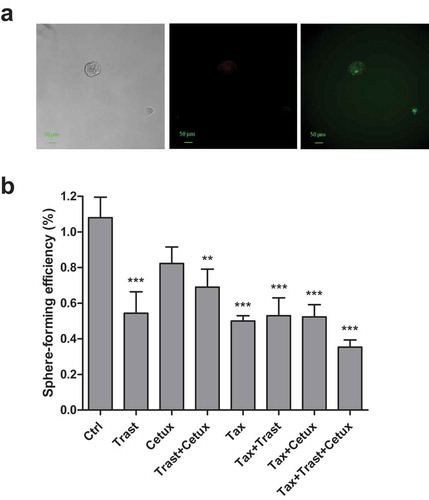
The capacity to form prostatospheres is a marker of cell stemness and correlates to tumor-initiating capacity, thus representing a reliable approach to carry out in vitro evaluation of tumor-initiating cells. At this regard, to demonstrate that co-targeting EGFR and HER2 in vivo in combination with Taxotere could have ameliorated the effects of standard chemotherapy through the selective inhibition of tumor-initiating cell subset, we tested the ability of alive tumor cells isolated from residual tumors harvested 24 hr after the last treatment in vivo to form prostatospheres in vitro. The average volume of harvested tumors ranged from >1500 mm3 in saline group to 20 mm3 in Taxotere plus Cetuximab and Taxotere plus Cetuximab and Trastuzumab groups. As shown in , the triple combination Taxotere plus Trastuzumab and Cetuximab led to a significant decrease of sphere-forming efficiency (SFE) as compared to Taxotere alone and saline-treated tumors (4- and 7.1-fold; p < .05 and p < .001, respectively) Trastuzumab and Cetuximab alone or in combination resulted to important decrease of prostatosphere formation as compared to saline treated cells (2.5-, 2.5- and 4.9-fold; p < .001, p < .01, respectively). These findings suggest that either the complete tumor regression observed in vivo or the significant prostatosphere-forming efficiency inhibition in vitro upon treatment with the triple drug combination can be explained through the elimination of either the tumor bulk or the tumor-initiating cell sub-population. Moreover, the strong decrease of prostatosphere-forming efficiency assessed ex vivo strongly supports the hypothesis of a pivotal role by EGFR and HER2 in the maintenance of tumor-initiating cells that reportedly occurs during cytotoxic treatment.Citation23 No different cell growth of cells derived from xenografts in adherent condition as evaluated by MTT (data not shown) indicated that the differences in SFE efficiencies could depend on the reduction of tumor-initiating cell sub-population upon treatments impairing EGFR and/or HER2 signaling.
Figure 3. Effects of in vivo Trastuzumab, Cetuximab, and Taxotere treatment on prostatosphere-forming efficiency in vitro. (a) DU145 xenografted mice were treated with Trastuzumab (10mg/kg) i.p., Cetuximab (1mg/mouse) i.p., and Taxotere (6.66mg/kg) i.v. given alone or in combination, or left untreated. Drugs were administered weekly for 5 consecutive weeks. 24 h after the last treatment, mice were sacrificed, residual tumors excised, tumor cells isolated and plated in vitro under the serum-free condition to form prostatospheres. Sphere-forming efficiency, calculated as the ratio between the number of spheres counted in each well and the number of single cells seeded each well, was evaluated after 96 h. Results (mean values ± S.D.) from triplicates for each treatment condition are shown. §§ p < .01; §§§ p < .001 against untreated tumor cells; *p < .05 against Taxotere-treated cells by Bonferroni’s Multiple comparison test. Abbreviations: Cetux = Cetuximab; Trast = Trastuzumab; Taxo = Taxotere. (b) Fluorescence microscopy images from DU145 and PC-3 cells labeled with PKH-67 (green) and PKH-26 (red) dyes, and plated under prostatosphere-forming conditions. After 4 days the capability to form prostatospheres from a single cell and characterized by a homogeneous staining for PKH-67 or PKH-26 dye was evaluated.
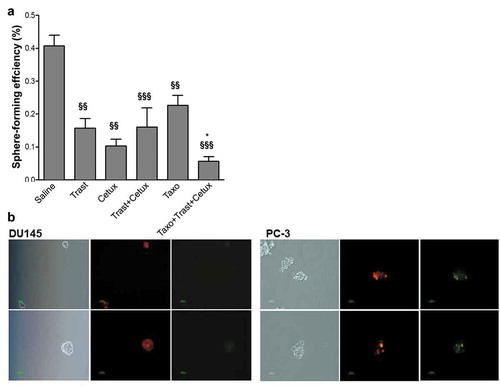
PC-3 cells obtained from different xenograft groups, including saline one, did not form prostatospheres suggesting that evaluation of tumor-initiating cells cannot be performed by sphere methodology. Accordingly, further analyses performed on PC-3 cells grown in vitro confirmed this hypothesis. Indeed, PC-3 cells stained with PKH26 or PKH67 dyes before seeding in nondifferentiating culture conditions, did not form individual spheres homogeneously stained with a single dye, as was the case of DU145 cells (), confirming the impossibility of using sphere-forming assay to investigate tumor-initiating cells in this PC model.
EGFR and HER2 sustain du145-derived prostatosphere proliferation
To further determine whether EGFR and HER2 were functional for DU145 tumor-initiating cells proliferation, we evaluated the activation of these receptors and downstream molecules in cells grown in monolayer or in sphere-forming conditions. As shown in , EGFR, HER2, and STAT3 resulted more activated in cells grown as spheres other than monolayer. To test the efficacy of targeting selectively EGFR and HER2 with Cetuximab and Trastuzumab, respectively, in inhibiting the prostatosphere formation, single-cell suspensions were grown under prostatosphere-forming or adherent conditions in the presence or not of Trastuzumab, Cetuximab or both, and the prostatosphere-forming efficiency was evaluated 96 h later. Cetuximab treatment (25 or 100µg/ml) significantly inhibited the SFE as compared to untreated cells and no differences were observed according to the dose. Similarly, upon treatment with Trastuzumab, at the doses 25µg/ml, and 100µg/ml the SFE values decreased significantly as compared to untreated cells with a higher inhibition at dose 100µg/ml. When Cetuximab (25µg/ml) was co-administered with Trastuzumab (100µg/ml), the SFE decreased further as compared to Cetuximab alone (25µg/ml) and Trastuzumab alone (100µg/ml) (). In sharp contrast, results from MTT assay revealed no effect on monolayer cell proliferation by Cetuximab and Trastuzumab given alone or in combination (). Together with the sphere-forming efficiency inhibition observed upon treatment with anti-EGFR and anti-HER2 antibodies in vivo (), these results support the crucial role of EGFR and HER2 in tumor-initiating cell proliferation.
Figure 4. Effects of anti-EGFR and anti-HER2 monoclonal antibodies and chemotherapy treatment on prostatosphere-forming efficiency and adherent growth of DU145 cells. (a) The expression levels and the activated (phosphorylated) status of EGFR and HER2 and downstream signaling molecules, AKT, MAPK, STAT3 and SRC, were analyzed in cell line grown in adherent (Ad) and prostatosphere-forming (Sp) conditions by Western blot (one representative of two-independent experiments is shown). β-actin was used as a loading control. (b) Dose–response assay on DU145 cells treated with escalating doses of Cetuximab, Trastuzumab alone or in combination in serum-free medium and then plated under prostatosphere-forming conditions; the prostatosphere-forming efficiency was evaluated 96 h later and calculated as the ratio between the number of spheres/well and the number of single cells seeded in each well. Results (mean values±S.D.) representing one of two independent experiments, from triplicates for every treatment condition are shown. **p < .01; ***p < .001 (against control) by Bonferroni’s Multiple comparison test. Abbreviations: Ctrl = control; Cetux = Cetuximab; Trast = Trastuzumab. (c) Dose–response assay on DU145 cells treated with escalating doses of Cetuximab, Trastuzumab alone or in combination in serum-free medium under monolayer conditions. Results from MTT assay revealed no effect on cell proliferation by Cetuximab and Trastuzumab given alone or in combination. Abbreviations: Ctrl = control; Cetux = Cetuximab; Trast = Trastuzumab. (d) Cells were treated with Trastuzumab (100µg/ml), Cetuximab (25µg/ml), Taxotere (2.5nM) alone or in combination under adherent growth condition for 72 h, then trypsinized and seeded in the serum-free medium; the prostatosphere-forming efficiency was evaluated after 96 h. Results (mean values ± S.E.M) from two independent experiments, from triplicates for every treatment condition are displayed. **p< .01; *** p < .001 versus untreated cells, by Bonferroni’s Multiple comparison test. Abbreviations: Ctrl = control; Cetux = Cetuximab; Trast = Trastuzumab; Taxo = Taxotere.
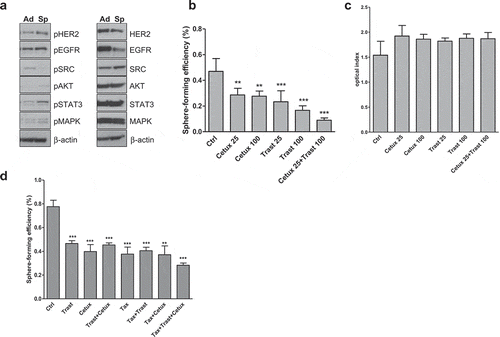
To mimic the effects induced by in vivo treatments, DU145 monolayer cells were treated in vitro with Taxotere (2.5nM), Cetuximab (25µg/ml) and or Trastuzumab (100µg/ml) for 72 h and the effects on prostatosphere-forming efficiency evaluated after 96 h. As shown in , Taxotere alone induced a significant SFE inhibition as compared to untreated cells as similar as that induced by Trastuzumab, Cetuximab given alone or in combination. The addition of Trastuzumab or Cetuximab to Taxotere did not impair the SFE, except for the triple combination of drugs for which the SFE values further declined. In agreement with the SFE inhibition observed ex vivo from in vivo treated-cells, these results support the pivotal role of EGFR and HER2 in sustaining the growth of stem-like cells in DU145 model.
Since 22Rv1 cells that express AR along with activated EGFR, HER2, and STAT3 showed the capability to form individual spheres homogeneously stained with PKH26 or PKH67 (), the effect of inhibition of EGFR and HER2 in combination or not with Taxotere was investigated in prostatosphere assay. 22Rv1 monolayer cells were treated in vitro with Taxotere (1.25nM), Cetuximab (25µg/ml) and or Trastuzumab (100µg/ml) for 72 h and the effects on prostatosphere-forming efficiency evaluated after 96 h. Similarly to what observed in DU145 cells, Taxotere, Trastuzumab, and Cetuximab alone induced SFE inhibition as compared to untreated cells and the addition of Trastuzumab or Cetuximab to Taxotere did not further decrease SFE (). As observed in DU145 cells, the triple combination of drugs determined the highest inhibition of prostatosphere growth.
Figure 5. Effects of in vivo Trastuzumab, Cetuximab, and Taxotere treatment on 22Rv1 prostatosphere-forming efficiency in vitro. (a) Fluorescence microscopy images from cells labeled with PKH-67 (green) and PKH-26 (red) dyes, and plated under prostatosphere-forming conditions. After 4 days the capability to form prostatospheres from a single cell and characterized by a homogeneous staining for PKH-67 or PKH-26 dye was evaluated. Scale bar: 50 µm. (b) Cells were treated with Trastuzumab (100µg/ml), Cetuximab (25µg/ml), Taxotere (1.25nM) alone or in combination under adherent growth condition for 72 h, then trypsinized and seeded in serum-free medium; the prostatosphere-forming efficiency was evaluated after 96 h. Results (mean values ± S.D.) from one representative of two-independent experiments, respectively, from triplicates for every treatment condition are displayed. **p< .01; *** p < .001 versus untreated cells, by Bonferroni’s Multiple comparison test. Abbreviations: Ctrl = control; Cetux = Cetuximab; Trast = Trastuzumab; Taxo = Taxotere.
EGFR and HER2 sustain the clonogenic capacity of prostate tumor-initiating cells
Clonogenic growth is also an in vitro indicator of self-renewal of normal and tumor-initiating cells. Therefore, since PC-3 cells did not form in vitro prostatospheres in adequate conditions, we performed experiments aimed to modulate EGFR and HER2 activation pathways in combination or not with Taxotere, and explored the effects on the clonogenic potential of prostate cancer cell lines. DU145, PC-3 and 22Rv1 grown in monolayer conditions were treated for 72 h with Trastuzumab (100μg/ml) or Cetuximab (25μg/ml), given alone or in combination with Taxotere (2.5nM for DU145 and PC3 and 1.25nM for 22Rv1), and then plated in 96-well plates at the seeding density of one cell per well and allowed to grow for 15 days in serum-free medium. displays that in DU145 neither Trastuzumab nor Cetuximab alone did reduce the colony formation (mean±SEM: 25.5%±1.56% for Trastuzumab, 29.7%±1.56% for Cetuximab versus 25.0%±1.04% for untreated cells), whereas the dual combination Trastuzumab and Cetuximab did impair the clonogenic capacity to 19.3%±0.52%. Treating with Taxotere induced a 2.7-fold reduction of clonogenic potential as compared to untreated cells (9.38%±1.05% versus 25.0%±1.04%; p < .001), that further decreased with the combination Taxotere plus Trastuzumab and with Taxotere plus Cetuximab. Interestingly, a remarkable reduction of clonogenic capacity was observed with the triple combination Taxotere plus Trastuzumab and Cetuximab as compared to control (mean±SEM: 2.6%±0.53% versus 25.0%±1.04%; p = <0.0001).
Figure 6. Colony formation of prostate cancer cells. (a) DU145, (b) PC-3 and (c) 22Rv1 were treated with Trastuzumab (100µg/ml), Cetuximab (25µg/ml) alone and in combination, and or with Taxotere (2.5nM and 1.25nM in 22Rv1) for 72 h in adherent conditions. Cells were then trypsinized, plated one cell/well in 96-well plates in serum-free medium. The clonogenic capacity was evaluated after 2 weeks. Results (mean ± S.E.M.) from two independent experiments are represented (*p<.05; **p<.01; ***p < .001 versus control; versus control, by Bonferroni’s Multiple comparison test). Abbreviations: Ctrl = control; Cetux = Cetuximab; Trast = Trastuzumab; Taxo = Taxotere.
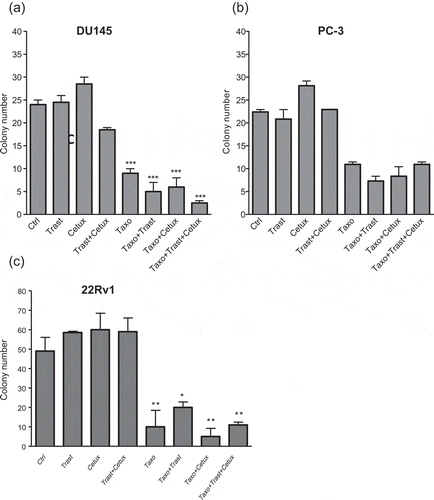
In PC-3 cells () the monoclonal antibodies anti-EGFR and anti-HER2 alone or in combination did not decrease the clonogenic capacity. Although Taxotere treatment induced a 2-fold reduction of clonogenic potential as compared to untreated cells (10.9%±0.52% versus 22.4%±0.52%, p < .0001), the addition of Cetuximab and Trastuzumab to Taxotere-based chemotherapy did not contribute to impair the colony formation, resulting in percentage values of clonogenic potential as similar as in Taxotere alone. Regarding 22Rv1 cells, no reduction of clone formation was observed upon Trastuzumab and Cetuximab alone or in combination, while Taxotere treatment induced a 5-fold reduction of clonogenic potential as compared to untreated cells (10%±6% versus 49%±5%, p < .001). Only the addition of Cetuximab to Taxotere further lowered the clonogenic capacity as compared to untreated cells (5%±3% versus 49%±5%, p < .001) ().
Taken together, these results show that only taxotere heavily impaired the formation of clones in all the three-cell lines, while the blockade of EGFR and HER2 decreased colony formation only in DU145 cells.
Discussion
Previous studies have indicated that the expression and activity of EGFR and HER2 receptors may contribute to the emergence of castration resistance of prostate carcinoma even after chemotherapy treatment. The model based on the presence of cancer stem cells, playing a major role in cancer formation, progression, and chemotherapy resistance,Citation24–Citation26 could provide an explanation for treatment failure and tumor relapse, representing a promising target for therapeutic interventions.
In this study, we used the castration-resistant AR negative prostatic tumor cell lines DU145 and PC-3 and the AR-positive 22Rv1 cell line responsive to docetaxel as it mainly occurs in CRPC patients. Although EGFR and HER2 were expressed in all the three prostate cell lines, the two receptors as well as the downstream kinases resulted differently activated suggesting that EGFR and HER2 may diversely take part in tumor cell safekeeping. Interestingly, the activation of STAT3 observed in vitro mirrored the positivity for pSTAT3 detected in the majority of our prostate tumor samples together with EGFR and HER2, suggesting a role of EGFR/HER2/STAT3 module in CRPC growth.
To confirm this hypothesis we carried out in vivo experiments on DU145 and PC-3 xenografted models, and we demonstrated that only in DU145 model targeting EGFR and HER2 receptors with Cetuximab and Trastuzumab, respectively, alone and in combination, produced a favorable antitumor activity with the exception for Trastuzumab alone. However, despite the in vivo Trastuzumab treatment did not inhibit the tumor growth, the prostatosphere-forming efficiency from residual tumor cells considerably decreased as compared to saline-treated tumors. The reduced presence of tumor-initiating cells in Trastuzumab-treated mice may depend on their higher sensitivity to Trastuzumab than bulk tumor population and explain the lack of a large tumor debulking as that induced by chemotherapy. The higher expression of EGFR than HER2 in xenografts could be at the base of Cetuximab capability to inhibit in vivo tumor growth by targeting the bulk tumor population in addition to tumor-initiating cells.
Intriguingly, cotreatment with Taxotere, Cetuximab, and Trastuzumab led to a greater and more durable tumor growth inhibition than that observed with the single agents or when administered in dual combination. Therefore, according to the preferential dimerization of EGFR with HER2 as a proposed mechanism associated with the CRPC growthCitation27 our data confirmed the role of EGFR and HER2 in prostate tumor growth and clearly indicated that the inhibition of both the two receptors might represent a rational therapeutic approach for CRPC. Conversely, PC-3 tumors resulted insensitive to both the anti-EGFR and anti-HER2 treatments and, upon an initial response to chemotherapy combined or not with Cetuximab and Trastuzumab, mice experienced an immediate tumor regrowth, suggesting that PC-3 tumor growth is sustained by other receptors than EGFR and HER2 as c-MET that is reportedly relevant in these tumor cellsCitation28 The favorable potentiation of Taxotere anti-tumor activity by Cetuximab and Trastuzumab in DU145 model shown in our in vivo experiment could indicate that the activation of both EGFR and HER2 may have contributed to the proliferation of residual chemotherapy-resistant tumor cells, referred as tumor-initiating cells, favoring the tumor regrowth after chemotherapy debulking effect. This observation would explain the failure of clinical trials using ErbB signaling inhibitors as monotherapy in patients that experienced progression after taxane-based chemotherapy,Citation29–Citation33 highlighting the clinical need of a combination therapy targeting both EGFR and HER2 receptors to achieve an effective therapeutic intervention through the disruption of the tumor-initiating cell pool.
In support of this possibility, we assessed in vitro the prostatosphere-forming ability of cells isolated from residual tumors at the end of treatments. Our results clearly indicated that the in vivo targeting of EGFR and HER2 alone and in combination, and more markedly in association with chemotherapy, led to a decreased prostatosphere-forming efficiency suggestive of an efficient elimination of tumor-initiating cell subset. Our observation providing evidence of the direct involvement of EGFR and HER2 in the proliferation of DU145 tumor-initiating cells is consistent with the contribution attributed to EGFR and HER2 module in sustaining the rapid clonal expansion of progenitor cells derived from CSCs.Citation18 With regard to this, EGFR reportedly promotes survival of prostate tumor-initiating cells and circulating tumor cells that metastasize, while HER2 supports the growth of prostate cancer cells once they are established at the metastatic site.Citation34 Conversely, in vitro clonogenic assay of PC3 cells showed that targeting of EGFR and HER2 did not improve the therapeutic effect of taxotere consistently with in vivo data, supporting that tumor-initiating cells do not exclusively depend on EGFR and HER2 in this cell line and other receptors (e.g. cMET) could be responsible for cancer stem cell properties and therapy resistance.Citation28
It is important to note that contrary to what observed in sphere formation assay in which the blockade of EGFR and HER2 alone or in combination significantly inhibited the SFE in DU145 and 22Rv1 cells, in clonogenic assay only taxotere significantly impaired the growth of all the three cell lines, supporting that initiating and tumor bulk cells mighty equally give rise to clones.
Additional evidences sustaining the functional role of EGFR/HER2/STAT3 in DU145 tumor-initiating cell proliferation were the biochemical analysis on cells grown in prostatosphere-forming and monolayer conditions that revealed higher levels of these activated molecules in the former than in latter cellular growth condition. Consistently, the dose-dependent inhibitory effect induced by the in vitro treatment with Cetuximab and Trastuzumab on prostatosphere-forming capability and not on adherent growth () confirmed the contribution of ErbB signaling in the biology of prostate tumor-initiating cells. Furthermore, the inhibitory effects on the prostatosphere-forming ability induced by the triple combination of Taxotere, Trastuzumab, and Cetuximab administered on DU145 cells grown in adherent conditions, resembling the in vivo tumor bulk condition, and then grown under undifferentiating conditions, extended the role of EGFR and HER2 signaling in the proliferation of chemotherapy-resistant cells. These in vitro results are reminiscence of the effects observed on prostatosphere-forming assay performed on residual tumors treated in vivo with the combo-therapy (). Similarly, the inhibitory effects observed upon treatment with Taxotere and, more conspicuously, with the addition of Cetuximab and Trastuzumab in experiments evaluating the effects of combo-therapy on the clonogenic potential of DU145 further pointed to the role of EGFR and HER2 on the stemness-related properties of DU145 model. Since Taxotere per se significantly impaired the clonogenic potential of tumor bulk population enriched in progenitor cells, and the addition of Cetuximab and Trastuzumab strongly negatively regulated the clonogenic capacity, it is conceivable that the activation of EGFR and HER2 signaling may be an effort to rescue from the chemotherapy-mediated tumor debulking.
The low activity of Trastuzumab as a single agent in reducing tumor growth of DU145 xenografts is likely due to the fact that these cells are not addicted to HER2 oncogene to grow. Thus, HER2 inhibition by Trastuzumab is not sufficient to stop tumor proliferation, as also observed in in vitro experiments, and/or to induce apoptosis of tumor cells. The in vitro SFE results, instead, highlight the relevance of HER2 in stem cell maintenance/proliferation as already demonstrated.Citation35 Citation36 Stem cells need HER2 to enter the cell cycle to form sphere in vitro. Thus, stem cells derived from trastuzumab treated tumors are inhibited in forming prostatosphere because they lack HER2 activity. It is also possible that trastuzumab treatment in vivo reduced the number of stem cells that are dependent on HER2 and thus the reduced number of ex vivo prostatospheres could derive by a lower amount of residual stem cells in tumors of mice treated with trastuzumab.
Interestingly, SFE evaluations performed in AR-positive 22Rv1 cells showed that similarly to what observed in DU145 cells, Taxotere, Trastuzumab, and Cetuximab induced a significant inhibition of sphere formation and the triple combination of drugs further improved the effect. Thus, despite the presence of AR in this CRPC cells, EGFR and HER2 likely play a relevant role in sustaining tumor-initiating cells. Since AR negatively regulates STAT3,Citation37 pSTAT3-positive cells in human CRPCs are potentially those with low AR levels and sustained by EGFR/HER2/STAT3 axis leading to stemness features. Although we are aware that our CRPC cohort is small and that a new large case series deserves to be further investigated, in the analyzed specimens, all expressing AR, pSTAT3 staining always resulted focal and pSTAT3 positivity did not exceed 20% of tumor cells, supporting the stemness survival through EGFR/HER2/STAT3 axis.
Considering the tremendous efficacy of anti-EGFR -HER2 therapy in combination with Taxotere in CRPC cells with activated STAT3, analysis of pSTAT3 as well as EGFR and HER2 in human tumor specimens may represent a valid approach to identify CRPC patients who benefit from the addition of Cetuximab and Trastuzumab to chemotherapy protocol.
Although the ErbB-specific therapeutics we used in this study previously showed a low benefit in the clinical setting,Citation38–Citation40 our results showing the crucial role of EGFR and HER2 in the proliferation and response to chemotherapy insult of prostate tumor-initiating cells, suggest the usefulness in targeting selectively EGFR and HER2 with Cetuximab and Trastuzumab in combination with Taxotere-based chemotherapy for eradicating either bulk tumor cell population or the subset of tumor-initiating cells and improving the clinical response to the current chemotherapy regimen.
Eventually, it should be taken into account that STAT3 is also downstream of the activated cytokine receptors (e.g. IL6/IL6R) in prostate cancer cellsCitation41 and may positively control IL6 production as reported in fibroblasts.Citation42 Therefore, the involvement of cytokine and growth factor receptors in maintaining prostate cancer stemness deserves to be further investigated.
Materials and methods
Cell lines and culture
The castration-resistant human prostate cancer cell lines DU145, PC-3 and 22Rv1 were obtained from the American Type Culture Collection (ATCC® HTB-81, ATCC® CRL1435 and ATCC® CRL-2505, respectively). Cell lines were maintained in RPMI 1640 medium (Lonza, catalog number BE12-702F) supplemented with 10% (v/v) FBS (Gibco by Life Technologies, catalog number 16000–044) in a humidified 5% CO2 atmosphere at 37°C. Cell lines were routinely tested for mycoplasma and cellular contamination or misidentification using STR analysis (DNA analysis).
Patients
Samples from 14 human hormone-independent prostate carcinomas obtained from patients diagnosed in 2002–2003 were collected in our institute (Fondazione IRCCS Istituto Nazionale dei Tumori). All patients gave written consent for the use of their biological materials for future investigations and research purposes, and the presented analysis was approved by the Ethics Committee of Fondazione IRCCS Istituto Nazionale dei Tumori (INT). All data were analyzed anonymously, and all experiments complied with the Declaration of Helsinki.
Drugs
Taxotere, Trastuzumab and Cetuximab were purchased from Sanofi Aventis (Italy, EU), Genentech/Roche (Basel, Switzerland) and Merck Serono (Darmstadt, Germany, EU), respectively.
Western blot analysis and antibodies
Cells were harvested, washed with ice-cold PBS and solubilized with lysis buffer (50 mM Tris-HCl, pH 6.8, 150 mM NaCl, 0.5% Triton-X100, 0.5% ß−octoglucoside, 10% glycerol, 10 mM iodoacetamide, 10 mM NEM, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 2 mM PMSF, 1 mM benzamidine, 10 mM NaF, and 2 mM sodium orthovanadate). Whole-cell lysates were heated, electrophoresed on NuPage precast 4-12% polyacrylamide gels (Invitrogen, catalog number NP0335BOX), and transferred to PVDF membranes (Merck Millipore, catalog number IPVH00010). The membranes were blocked in TBS containing 0.1% Triton X-100 and 5% skim milk powder for 1 h at RT, and incubated with the appropriate primary antibodies in Tris Buffer solution containing 0.1% Tween-20 (TBS-T) and 3% skim milk powder with gentle shaking at 4°C overnight. After hybridization with horseradish peroxidase-conjugated secondary antibody, Western blots were developed using the enhanced chemiluminescence method (GE Healthcare Bio-Sciences, catalog number RPN2108). Blotting analysis was performed using the following antibodies: rabbit anti-human EGFR (clone sc-1005; 1:1000), rabbit anti-human STAT3 (1:200), mouse anti-human phospho-STAT3 antibody (Y705); (clone B-7, 1:1000), rabbit polyclonal anti-human phospho-HER2 p-Neu (Tyr1248) (0.5 mg/ml), rabbit polyclonal anti-human phospho- EGFR (Tyr 845) (1:200) from Santa Cruz Biotechnology; mouse anti-human HER2 (clone Ab3; 1:300) from Calbiochem; mouse anti-human SRC (clone GD11; 1:300), rabbit polyclonal anti-human AKT (1:1000), rabbit polyclonal anti-human phospho-p44/42 MAPK (Erk1/2; Thr201/Tyr204) (1:2000), rabbit polyclonal anti-human p44/42 MAPK (Erk1/2; 1:1000), rabbit monoclonal anti-human phospho-AKT (Ser473) (clone D9E; 1:1000) and rabbit polyclonal anti-human phospho-SRC (Tyr416) (1:1000) from Cell Signaling Technology, mouse monoclonal anti-human phospho-EGFR (Tyr1173) (clone 9H2; 1:200) from Nanotools, monoclonal anti-human actin (clone AC-40; 0.4 μg/ml) and anti-human vinculin (clone hVIN-1; 1:1000) from Sigma Aldrich. Equal sample loading was confirmed by measuring amounts of β-actin and vinculin. Autoradiographic signals were measured using a Bio-Rad scanning densitometer (Bio-Rad; ChemiDoc/XRS, Bio-Rad). Data were acquired using ImageJ software and densitometry was performed in Quantity One 4.6.6 (Bio-Rad, Hercules, CA).
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tumors were sectioned at 4 µm, dewaxed, hydrated, and stained with hematoxylin and eosin or processed for IHC with: rabbit polyclonal anti-human HER2 (1:150, DAKO) after antigen retrieval with citrate buffer, pH 6.0, at 95°C for 6 min, rabbit monoclonal anti-phosphoSTAT3 (clone D3A7, 1:400, Cell Signaling Technology) after antigen retrieval with EDTA 1mM, pH 8.0 at 95°C for 15 min, with mouse monoclonal anti-EGFR (clone E30; 1:25, DAKO) upon treatment with proteinase K at 37°C for 5 min, and with mouse monoclonal anti-AR (clone AR441; 1:25, DAKO) after antigen retrieval with PTLink EDTA (DAKO) 15 min at 96°C.
In vivo experiments
Experimental protocols used for animal studies were approved by the Ethics Committee for Animal Experimentation of INT and the Ministry of Health in accordance with Italian guidelines.Citation43 Six-week-old male SCID mice from Charles River s.r.l., Calco, Italy, EU) were housed in our facilities at constant temperature and humidity with food and water given ad libitum. Mice were xenografted with 5 × 106 cells/mouse of DU145 or PC-3 injected subcutaneously (s.c.) in the right flank. In presence of palpable tumors (mean tumor volume = 100 mm3), mice were randomly divided in 8 groups and treated intraperitoneally (i.p) with Trastuzumab (10mg/kg), Cetuximab (1mg/mouse) administered alone or in combination, and intravenously (i.v.) with Taxotere (6.66mg/kg) injected alone or in combination with Trastuzumab or Cetuximab or with both of them. Control mice were treated with saline solution. Drugs were administered weekly for 5 weeks. Mice were monitored once a week for tumor size and body weight. Tumors were measured with calipers and tumor volume calculated as (D × d2)/2, where D and d represent the longest and shortest diameters, respectively.
Prostatosphere formation assay
DU145 and 22Rv1 monolayer cells were enzymatically dissociated in trypsin/EDTA and then plated (3 × 103/well) in 6-well low attachment plates (Corning, catalog number 3471). DU145 cells were grown in a serum-free mammary epithelium basal medium (MEBM) (Lonza, catalog number CC-3151) supplemented with the human epidermal growth factor (EGF) (20ng/ml) (Peprotech, catalog number 100–47), human FGF-basic (b-FGF) (20ng/ml) (Peprotech, catalog number 100-18B), B27 supplement (Gibco by Life Technology Ltd, catalog number A3582801), and heparin (3000 U.I./ml) (PharmaTex, catalog number B01AB01). 22Rv1 were grown in complete MammoCult medium (StemCell Technologies) SFE was calculated as the number of spheres formed in 4 days divided by the number of single cells seeded and expressed as a percentage (± SD).
The SFE was also evaluated on tumor cells isolated from the residual tumors at the end of in vivotreatments. Tumors were excised and then enzymatically desegregated with 250 U Collagenase IV/mg of tumor (Worthington Biochemical Corporation, catalog CLSPA) for 1 h at 37°C. After two washes, the same number of viable cells recovered from residual tumors upon different treatments were seeded under sphere-forming conditions as above described.
PKH-67 and PKH-26 assay
DU145, PC-3, and 22Rv1 cell lines were dissociated in trypsin/EDTA, resuspended in serum-free medium and stained for 5 min at 37°C with PKH-67 or PKH-26 dyes (1:500) (Sigma-Aldrich, catalog PKM67-1KT and PKM26GL-1KT, respectively), blocked with FBS, washed twice with complete medium and twice with serum-free medium, and then seeded by mixing PKH-67 and PKH-26-labeled cells (ratio 1:1) to obtain prostatospheres. Images were analyzed after 4 days on a fluorescence microscopy.
Clonogenic assay
To examine the effects on the clonogenic potential of DU145, PC-3 and 22Rv1 cells by Taxotere, Trastuzumab and Cetuximab administered as single agents or in combination, cells were plated on 6-well plates and treated with Taxotere (2.5nM or 1.25nM), Trastuzumab (100μg/ml) and Cetuximab (25μg/ml). After 72 h, cells were trypsinized and single cells plated in 200 µl of serum-free medium in 96-well plates. The colony number was assessed after 2 weeks. The clonogenic capacity was calculated as the number of colonies grown divided by the number of wells seeded at single cell-dilution. The mean±SEM of two biological replicates was calculated.
In vitro experiments
The effects of Trastuzumab and Cetuximab on SFE were evaluated in dose–response experiments performed on DU145 cells. To this aim, 106 cells were suspended in serum-free medium in presence of Trastuzumab (25–100 μg/ml), Cetuximab (25–100 μg/ml) or both of them and incubated for 2 h at 37°C. Then, cells were divided in two aliquots. The first one was seeded to grow as spheres and analyzed for SFE after 4 days. The second one was analyzed for adhesion growth ability using MTT colorimetric assay after 4-days.
The effects induced by Taxotere, Trastuzumab and Cetuximab, administered alone or in combination, on the SFE were evaluated on DU145 and 22Rv1 cells. Cells were seeded on 6-well plates (1.8x105/well) in a total volume of 2.0 ml of culture medium. After 4 h, Taxotere (2.5nM or 1.25nM), Trastuzumab (100μg/ml) and Cetuximab (25μg/ml), alone or in combination, were added to the culture medium. After 72 h, cells were enzymatically dissociated with trypsin/EDTA and plated in the absence of drugs to grow as spheres. SFE was evaluated 96 h later.
Statistical analysis
Data were expressed as the mean±S.D. and mean±S.E.M. and analyzed for statistical significance using Students’ t-test (unpaired and two-tailed) and one-way analysis of variance (Anova). Post hoc tests were performed by Bonferroni’s multiple comparison test. All analyses were done using GraphPad Prism Software (GraphPad Prism Software Inc., San Diego, CA, USA). A p value <.05 was considered to be significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (377.3 KB)Acknowledgments
The authors thank Mrs. Laura Mameli for secretarial assistance and the Microscopy Facility of the Fondazione IRCCS Istituto Nazionale dei Tumori, Milan.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan;62(1):10–29. doi:10.3322/caac.20138.
- Williams H, Powell IJ. Epidemiology, pathology, and genetics of prostate cancer among African Americans compared with other ethnicities. Methods Mol Biol. 2009;472:439–453. doi:10.1007/978-1-60327-492-0_21.
- Altavilla A, Iacovelli R, Procopio G, Alesini D, Risi E, Campenni GM, Palazzo A, Cortesi E. Medical strategies for treatment of castration resistant prostate cancer (CRPC) docetaxel resistant. Cancer Biol Ther. 2012 Sep;13(11):1001–1008. doi:10.4161/cbt.21188.
- DeHaan AM, Wolters NM, Keller ET, Ignatoski KM. EGFR ligand switch in late stage prostate cancer contributes to changes in cell signaling and bone remodeling. Prostate. 2009 Apr 1;69(5):528–537. doi:10.1002/pros.v69:5.
- Traish AM, Morgentaler A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: a potential molecular switch for tumour growth. Br J Cancer. 2009 Dec 15;101(12):1949–1956. doi:10.1038/sj.bjc.6605376.
- Berger R, Lin DI, Nieto M, Sicinska E, Garraway LA, Adams H, Signoretti S, Hahn WC, Loda M. Androgen-dependent regulation of Her-2/neu in prostate cancer cells. Cancer Res. 2006 Jun 1;66(11):5723–5728. doi:10.1158/0008-5472.CAN-05-3928.
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999 Mar;5(3):280–285. doi:10.1038/6495.
- Gregory CW, Whang YE, McCall W, Fei X, Liu Y, Ponguta LA, French FS, Wilson EM, Earp HS III. Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin Cancer Res. 2005 Mar 1;11(5):1704–1712. doi:10.1158/1078-0432.CCR-04-1158.
- Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000 Dec 15;60(24):6841–6845.
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999 May 11;96(10):5458–5463. doi:10.1073/pnas.96.10.5458.
- Shi Y, Brands FH, Chatterjee S, Feng AC, Groshen S, Schewe J, Lieskovsky G, Cote RJ. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001 Oct;166(4):1514–1519. doi:10.1016/S0022-5347(05)65822-3.
- Signoretti S, Montironi R, Manola J, Altimari A, Tam C, Bubley G, Balk S, Thomas G, Kaplan I, Hlatky L, et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J Natl Cancer Inst. 2000 Dec 6;92(23):1918–1925. doi:10.1093/jnci/92.23.1918.
- Morris MJ, Reuter VE, Kelly WK, Slovin SF, Kenneson K, Verbel D, Osman I, Scher HI. HER-2 profiling and targeting in prostate carcinoma. Cancer. 2002 Feb 15;94(4):980–986. doi:10.1002/(ISSN)1097-0142.
- Neto AS, Tobias-Machado M, Wroclawski ML, Fonseca FL, Teixeira GK, Amarante RD, Wroclawski ER, Del GA. Her-2/neu expression in prostate adenocarcinoma: a systematic review and meta-analysis. J Urol. 2010 Sep;184(3):842–850. doi:10.1016/j.juro.2010.04.077.
- Shah RB, Ghosh D, Elder JT. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate. 2006 Sep 15;66(13):1437–1444. doi:10.1002/(ISSN)1097-0045.
- Mimeault M, Batra SK. Recent advances on multiple tumorigenic cascades involved in prostatic cancer progression and targeting therapies. Carcinogenesis. 2006 Jan;27(1):1–22. doi:10.1093/carcin/bgi229.
- Chen X, Rycaj K, Liu X, Tang DG. New insights into prostate cancer stem cells. Cell Cycle. 2013 Feb 15;12(4):579–586. doi:10.4161/cc.23721.
- Schneider MR, Yarden Y. The EGFR-HER2 module: a stem cell approach to understanding a prime target and driver of solid tumors. Oncogene. 2016 Jun 9;35(23):2949–2960. doi:10.1038/onc.2015.372.
- Mimeault M, Johansson SL, Vankatraman G, Moore E, Henichart JP, Depreux P, Lin MF, Batra SK. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007 Mar;6(3):967–978. doi:10.1158/1535-7163.MCT-06-0648.
- Cho KH, Choi MJ, Jeong KJ, Kim JJ, Hwang MH, Shin SC, Park CG, Lee HY. A ROS/STAT3/HIF-1alpha signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014 May;74(5):528–536. doi:10.1002/pros.22776.
- Ripamonti F, Albano L, Rossini A, Borrelli S, Fabris S, Mantovani R, Neri A, Balsari A, Magnifico A, Tagliabue E. EGFR through STAT3 modulates deltaN63alpha expression to sustain tumor-initiating cell proliferation in squamous cell carcinomas. J Cell Physiol. 2013;228:871–878. doi:10.1002/jcp.24238.
- Pencik J, Schlederer M, Gruber W, Unger C, Walker SM, Chalaris A, Marie IJ, Hassler MR, Javaheri T, Aksoy O, et al. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat Commun. 2015 Jul 22;6:7736. doi:10.1038/ncomms8736.:7736.
- Pisco AO, Huang S. Non-genetic cancer cell plasticity and therapy-induced stemness in tumour relapse: ‘What does not kill me strengthens me’. Br J Cancer. 2015 May 26;112(11):1725–1732. doi:10.1038/bjc.2015.146.
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006 Mar 16;25(12):1696–1708. doi:10.1038/sj.onc.1209327.
- Rybak AP, He L, Kapoor A, Cutz JC, Tang D. Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim Biophys Acta. 2011 May;1813(5):683–694. doi:10.1016/j.bbamcr.2011.01.018.
- Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, del I, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012 Sep 11;22(3):373–388. doi:10.1016/j.ccr.2012.07.016.
- Mellinghoff IK, Tran C, Sawyers CL. Growth inhibitory effects of the dual ErbB1/ErbB2 tyrosine kinase inhibitor PKI-166 on human prostate cancer xenografts. Cancer Res. 2002 Sep 15;62(18):5254–5259.
- Yin B, Liu Z, Wang Y, Wang X, Liu W, Yu P, Duan X, Liu C, Chen Y, Zhang Y, et al. RON and c-Met facilitate metastasis through the ERK signaling pathway in prostate cancer cells. Oncol Rep. 2017 Jun;37(6):3209–3218. doi:10.3892/or.2017.5585.
- Lara PN Jr., Chee KG, Longmate J, Ruel C, Meyers FJ, Gray CR, Edwards RG, Gumerlock PH, Twardowski P, Doroshow JH, et al. Trastuzumab plus docetaxel in HER-2/neu-positive prostate carcinoma: final results from the California cancer consortium screening and phase II trial. Cancer. 2004 May 15;100(10):2125–2131. doi:10.1002/(ISSN)1097-0142.
- Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005 Apr 10;23(11):2534–2543. doi:10.1200/JCO.2005.03.184.
- Agus DB, Sweeney CJ, Morris MJ, Mendelson DS, McNeel DG, Ahmann FR, Wang J, Derynck MK, Ng K, Lyons B, et al. Efficacy and safety of single-agent pertuzumab (rhuMAb 2C4), a human epidermal growth factor receptor dimerization inhibitor, in castration-resistant prostate cancer after progression from taxane-based therapy. J Clin Oncol. 2007 Feb;25:675–681. doi:10.1200/JCO.2006.07.0649.
- Blackledge G. Growth factor receptor tyrosine kinase inhibitors; clinical development and potential for prostate cancer therapy. J Urol. 2003 Dec;170(6 Pt 2):S77–S83. doi:10.1097/01.ju.0000095022.80033.d3.
- Gross M, Higano C, Pantuck A, Castellanos O, Green E, Nguyen K, Agus DB. A phase II trial of docetaxel and erlotinib as first-line therapy for elderly patients with androgen-independent prostate cancer. BMC Cancer. 2007 Jul 27;7:142–147. doi:10.1186/1471-2407-7-142.
- Day KC, Lorenzatti HG, Kozminsky M, Dawsey SJ, Paul A, Broses LJ, Shah R, Kunja LP, Hall C, Palanisamy N, et al. HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 2017 Jan 1;77(1):74–85. doi:10.1158/0008-5472.CAN-16-1656.
- Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, Sozzi G, Fontanella E, Ménard S, Tagliabue E. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to Trastuzumab. Clin Cancer Res. 2009;15:2010–2021. doi:10.1158/1078-0432.CCR-08-1327.
- Korkaya H, Wicha MS. HER2 and breast cancer stem cells: more than meets the eye. Cancer Res. 2013 Jun 15;73(12):3489–3493. doi:10.1158/0008-5472.CAN-13-0260.
- Schroeder A, Herrmann A, Cherryholmes G, Kowolik C, Buettner R, Pal S, Yu H, Muller-Newen G, Jove R. Loss of androgen receptor expression promotes a stem-like cell phenotype in prostate cancer through STAT3 signaling. Cancer Res. 2014 Feb 15;74(4):1227–1237. doi:10.1158/0008-5472.CAN-13-0594.
- Cathomas R, Rothermundt C, Klingbiel D, Bubendorf L, Jaggi R, Betticher DC, Brauchli P, Cotting D, Droege C, Winterhalder R, et al. Efficacy of cetuximab in metastatic castration-resistant prostate cancer might depend on EGFR and PTEN expression: results from a phase II trial (SAKK 08/07). Clin Cancer Res. 2012 Nov 1;18(21):6049–6057. doi:10.1158/1078-0432.CCR-12-2219.
- Nabhan C, Lestingi TM, Galvez A, Tolzien K, Kelby SK, Tsarwhas D, Newman S, Bitran JD. Erlotinib has moderate single-agent activity in chemotherapy-naive castration-resistant prostate cancer: final results of a phase II trial. Urology. 2009 Sep;74(3):665–671. doi:10.1016/j.urology.2009.05.016.
- Liu G, Chen YH, Kolesar J, Huang W, Dipaola R, Pins M, Carducci M, Stein M, Bubley GJ, Wilding G. Eastern cooperative oncology group phase II trial of lapatinib in men with biochemically relapsed, androgen dependent prostate cancer. Urol Oncol. 2013 Feb;31(2):211–218. doi:10.1016/j.urolonc.2011.01.002.
- Bishop JL, Thaper D, Zoubeidi A. The multifaceted roles of STAT3 signaling in the progression of prostate cancer. Cancers (Basel). 2014 Apr 9;6(2):829–859. doi:10.3390/cancers6020829.
- Nguyen HN, Noss EH, Mizoguchi F, Huppertz C, Wei KS, Watts GFM, Brenner MB. Autocrine loop involving IL-6 family member LIF, LIF receptor, and STAT4 drives sustained fibroblast production of inflammatory mediators. Immunity. 2017 Feb 21;46(2):220–232. doi:10.1016/j.immuni.2017.01.004.
- Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010 May 25;102(11):1555–1577. doi:10.1038/sj.bjc.6605642.
