ABSTRACT
In triple-negative breast cancer (TNBC), the lack of therapeutic markers and effective targeted therapies result in an incurable metastatic disease associated with a poor prognosis. Crosstalks within the tumor microenvironment (TME), including those between cancer and stromal cells, affect the tumor heterogeneity, growth, and metastasis. Previously, we have demonstrated that IL-6, IL-8, and CCL5 play a significant role in TNBC growth and metastasis. In this study, we performed a systematic analysis of cytokine factors secreted from four stromal components (fibroblasts, macrophages, lymphatic endothelial cells, and blood microvascular endothelial cells) induced by four TNBC cell types. Through bioinformatic analysis, we selected putative candidates of secreted factors from stromal cells, which are involved in EMT activity, cell proliferation, metabolism, and matrisome pathways. Among the candidates, LCN2, GM-CSF, CST3, IL-6, IL-8, and CHI3L1 are ranked highly. Significantly, Lipocalin-2 (LCN2) is upregulated in the crosstalk of stromal cells and four different TNBC cells. We validated the increase of LCN2 secreted from four stromal cells induced by TNBC cells. Using a specific LCN2 antibody, we observed the inhibition of TNBC cell growth and migration. Taken together, these results propose secreted factors as molecular targets to treat TNBC progression via crosstalk with stromal components.
Introduction
Triple-negative breast cancer (TNBC) lacks the expression of three key therapeutic markers: ER, PR, and HER2. The lack of therapeutic markers limits using targeted therapies in TNBC and leads to poorer outcomes compared to other types of breast cancer; chemotherapy remains the primary treatment for TNBC patients.Citation1 Numerous clinical trials are being conducted involving drug development in TNBC looking at chemotherapy, molecular targeted therapies such as PARP, P13 K inhibitors, MEK inhibitors, CAR-T cells, immune checkpoint inhibitor antibodies as standalone drug options, and also these treatments in combination.Citation2
The tumor microenvironment (TME) comprising cancer-associated fibroblasts (CAF), tumor-associated macrophages (TAM) and other immune cells, and blood and lymphatic vascular networks contributes to tumor heterogeneity, growth, metastasis, and drug resistance; these factors lead to poor prognosis in most cancers.Citation3-Citation16 Recent findings suggest that secreted factors in the TNBC microenvironment play a critical role in breast cancer progression and metastasis.Citation17 The interaction between secreted factors and their receptors contribute to the process of cancer in the TME, including fibroblasts, macrophages, endothelial cells, bone marrow-derived stem cells, and infiltrating leukocytes.Citation18-Citation20 These factors are involved in cancer metastasis in accordance with the “seed and soil” hypothesis.Citation21 Furthermore, TME alters responses to chemotherapy in cancer, and it has been considered as a factor for a successful treatment strategy.Citation22 Several mechanisms were discovered that regulate the secreted factors in a crosstalk between cancer and TME,Citation23-Citation25 but unified therapeutic strategy is yet to be revealed.
We have previously shown that the secreted factors such as CCL5, IL-6, and IL-8, and angiogenic factors play an important role in TNBC tumor growth and metastasis via crosstalk between cancer cells and stromal components.Citation26-Citation31 Here, in search of novel secreted factors in the crosstalk between TNBC cells and stromal cells, we performed the cytokine antibody arrays on the secretomes from 16 different combinations of cells: 4 different TNBC cell types co-cultured with 4 different stromal cells (lymphatic endothelial cells (LEC), microvascular endothelial cells (MEC), fibroblasts, and macrophages). We corroborate the cellular and molecular roles of top secreted factors obtained in our in vitro experiments through bioinformatic analysis of RNA-sequencing data for clinical samples from TNBC patients in The Cancer Genome Atlas (TCGA). Based on our results, we propose several secreted factors in the crosstalk between TNBC cells and stromal cells as potential therapeutic targets.
Results
Cytokine analysis of the conditioned media from stromal cells in crosstalk with TNBC cells
Previously, we have found that crosstalks between the MDA-MB-231 cells and the stromal cells such as LEC, fibroblasts, and macrophages in the pre-metastatic niches occur through their respective secretomes; specifically, secreted factors CCL5, IL-6, and IL-8 were found critical for metastasis.Citation28,Citation29,Citation32-Citation34 In order to discover a profile of secreted factors in crosstalk with TNBC cell subtypes described in Lehmann et al.,Citation35 we utilized MDA-MB-231 (mesenchymal-like), SUM159 (mesenchymal-like), SUM149 (basal-like: BL2), and MDA-MB-468 (basal-like; BL1) cells and four different stromal cells including lymphatic endothelial cells (LEC), microvascular endothelial cells (MEC), normal fibroblasts (F), and M2-type macrophages (MΦ). We collected three different types of secretomes; (1) TCM: conditioned media of TNBC cell, (2) (SFM-stromal cell)CM: conditioned media of stromal cell induced by serum-free media (SFM), and (3) (TCM-stromal cell)CM: conditioned media of stromal cell induced by TCM of TNBC cells. The first two secretomes served as the baseline that allowed us to determine factors secreted after induction. The last secretome represented the secretomes resulting from crosstalk between the TNBC cells and stromal cells. The various cell types in the tumor co-exist; therefore, it is difficult to determine which cell types are secreting the factors of interest. Here we applied the secretome from one cell type on another to allow conditioning, removing the added secretome, and collecting the new induced secretome into serum-free media. We hypothesized that this method allowed unequivocal identification of the cell type secreting a particular factor as we have shown with CCL5, IL-6, and IL-8 induction from LEC, fibroblasts, and macrophages induced with TNBC TCM in our previous work.Citation28,Citation29,Citation32-Citation34
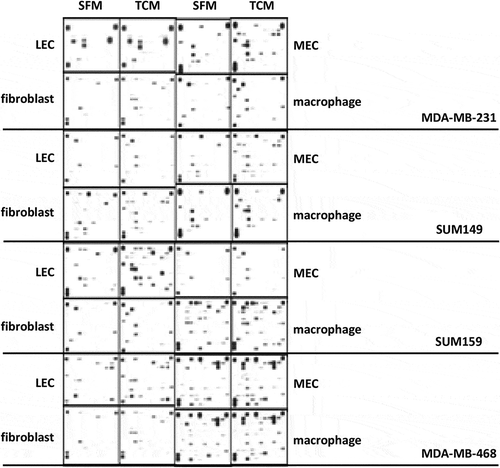
Using four secretomes (conditioned media) from four stromal cells induced by four TNBC cells (TCM-Stroma)CM (), we performed human cytokine arrays from R&D Systems (Proteome Profiler™ Human XL Cytokine Array Kit) to target 105 human cytokines simultaneously. The array contains antibodies against selected cytokines, chemokines, and growth factors. Arrays were run in duplicate using protocols from R&D Systems, for a total of 16 different combinations with 4 different TNBC cells and 4 different stromal cells ().
Figure 1. Schematic diagram of experimental procedure to collect the conditioned media from stromal cells in crosstalk with TNBC cells. Stromal cells were cultured with TCM for 3 d and the conditioned media from the stromal cells were saved to perform the cytokine array.
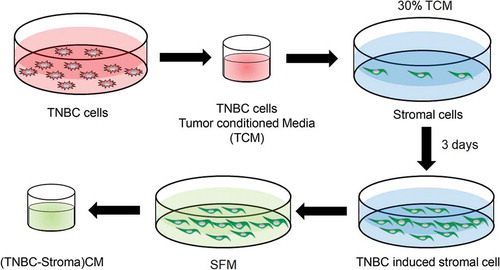
Figure 2. Cytokine analysis. The relative amounts of cytokines present in the conditioned media from stromal cells cultured with serum-free media (SFM) containing 2% serum or tumor condition media (TCM) of TNBC cells were visualized using a human cytokine antibody array (Proteome Profiler Human XL Cytokine Array Kit with 105 target proteins, R&D Systems).
Selection of secreted factors by bioinformatic analysis
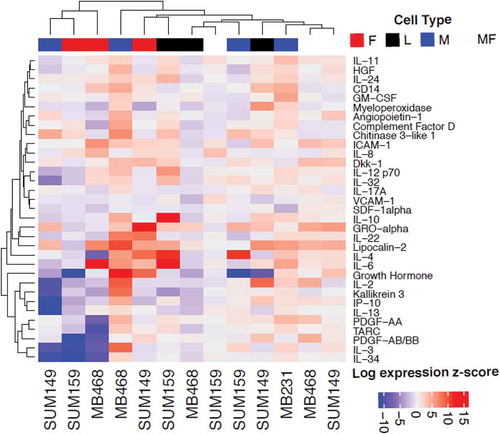
We performed paired analyses to assess differential cytokine expression across four stromal secretomes induced by four TNBC cells ( and Supplementary Table 1). The protein expression of the top ten cytokines for each stromal cell type is summarized in . However, none reached statistical significance ( and Supplementary Figure 1). We performed further analysis of TNBC breast cancer samples from TCGA to assess whether these top cytokines for macrophages are associated with the proportion of macrophages estimated with CIBERSORT (Supplementary Figure 2A and ). Of the cytokine candidates found from the screen, we confirm that two of the top ten are positively correlated with the proportion of M0 macrophages (IL-11 and IL-32), six of the top ten with the proportion of M1 macrophages (ICAM1, IL-10, IL-32, IL-34, IL-6, and VCAM1), and one of the top ten with the proportion of M2 macrophages (CXCL12). Thus, a total of eight out of ten cytokines found in the array are also associated with macrophage cell type proportions in vivo (Supplementary Figure2B). We note that no other cell types were available from CIBERSORT, limiting similar confirmatory analyses. Pathway analysis ( and Supplementary Tables 3 and 4) reveals a significant immune response associated with overexpression of the top ten cytokines for each cell type. Additional pathways indicate that these genes are also associated with EMT activity, cellular growth, metabolism, and matrisome pathways.
Table 1. Top ten putative candidates of secreted factors from stromal cells in crosstalk with TNBC cells
Table 2. Top five candidates of secreted factors from stromal cells in crosstalk with TNBC cells
Differential expression of secreted factors from stromal cells in crosstalk with TNBC cells
Based on the results of bioinformatic analysis and experimental analysis together, we selected and validated the expression of top 21 secreted factor candidates such as LCN2, IL-8, CXCL1, IL-6, CXCL5, DKK-1, CD14, uPAR, IGFBP3, CST3, endoglin, GM-CSF, BAFF, CCL5, ICAM-1, PDGFA, CCL20, MIF, PDGFB, VEGF, and CHI3L1 in stromal cells-induced by TCM of four TNBC cells using a real-time qRT-PCR (). We ranked secreted factors based on the sum of fold change and found that LCN2, GM-CSF, IL-6, and CHI3L1 are highly expressed in LEC and MEC in crosstalk with TNBC cells while upregulation of LCN2, GM-CSF, and IL-6 was shown in fibroblasts and macrophages. The expression of IL-8 and CCL20 was increased in fibroblasts and macrophages and the upregulation of VEGF was shown in MEC and macrophages. In addition, we found that CST3 from LEC, CXCL5 from fibroblasts, and CXCL1 from macrophages are significantly expressed in crosstalk with TNBC cells ().
Figure 5. Real-time quantitative PCR analysis of the candidate secreted factors. The relative mRNA levels of the secreted factors in stromal cells cultured with serum-free media (SFM) containing 2% serum or tumor condition media (TCM) of TNBC cells were measured by a real-time RT-qPCR.
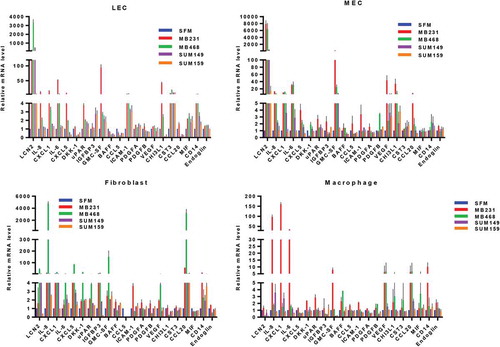
Furthermore, we found that LCN2 was a distinct factor secreted from stromal cells in crosstalk with four TNBC cells. In stromal cells crosstalked with the mesenchymal-like TNBC subtype (MDA-MB-231 and SUM159 cells), LCN2, GM-CSF, and IL-8 were highly expressed, and LCN2 and CST3 were highly expressed in crosstalk with the basal-like subtype (MDA-MB-468 and SUM149 cells). However, we found upregulation of GM-CSF and IL-8 in crosstalk with MDA-MB-231, SUM159, and MDA-MB-468 cells. In addition, we discovered that CXCL1 was upregulated in stromal cells in crosstalk with MDA-MB-231 and MDA-MB-468 cells while the expression of CCL20 and CXCL5 was increased in crosstalk with SUM159 cells. We found the upregulation of CXCL1 (MDA-MB-468), soluble CD14 (SUM149), and VEGF (SUM149) (Supplementary Table 5).
Decrease of TNBC cell growth and migration by inhibition of LCN2 signaling
The previous studies showed that LCN2 promotes breast cancer progression and metastasis in the crosstalk of stromal components.Citation36-Citation41 To validate the upregulation of secreted LCN2 proteins from the stromal cells incubated with TCM of four TNBC cells, we performed LCN2 ELISA analysis (R&D Systems) and confirmed that the secretion of LCN2 was significantly increased from LEC, MEC, fibroblasts, and macrophages induced by TCM of MDA-MB-231 cells compared to SFM as a control (). These results implied that LCN2 is highly secreted from the stromal cells in crosstalk of TNBC cells and could be the key factor that enhances the TNBC cell growth and migration.
Figure 6. The growth and migration of MDA-MB-231 cells in the crosstalk of stromal cells. (a) ELISA of human LCN2 (R&D Systems) in conditioned media (CM) of LEC, MEC, fibroblasts (f), macrophages (MF) cultured with SFM containing 2% serum (SFM) or TCM of MDA-MB-231 cells. (b) The proliferation and (c) migration of MDA-MB-231 cells in CM of stromal cells cultured with TCM of MDA-MB-231 cells in treatment with anti-LCN2 antibody (50 μg/ml) for 6 d. (*P < .001, n = 3). (SFM-stromal cells)CM: conditioned media from stromal cells cultured with SFM containing 2% serum. (TCM-stromal cells)CM: conditioned media from stromal cells cultured with TCM (tumor-conditioned media) of MDA-MB-231cells.
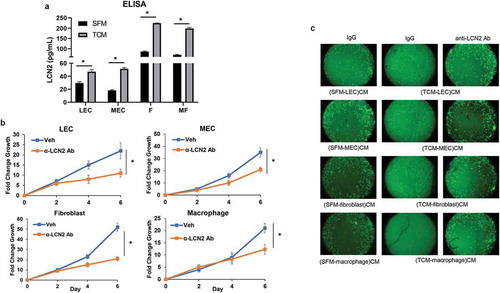
Next, in order to examine if LCN2 is a critical secreted factor in the crosstalk between TNBC cells and stromal cells in the TNBC cell growth and migration, using anti-specific LCN2 antibody (R&D Systems) to neutralize LCN2 proteins, we examined MDA-MB-231 cell proliferation and migration in conditioned media of each stromal cell induced by TCM of MDA-MB-231 cells. The results showed that the neutralization of LCN2 significantly decreased MDA-MB-231 cell growth in each conditioned medium (). Furthermore, the migration of MDA-MB-231 cells was significantly increased in the conditioned media of each stromal cell induced by TCM of MDA-MB-231 cells compared to the conditioned media of stromal cells with serum-free media while the migration was dramatically attenuated in LCN2 neutralized conditioned media (). These results show that secreted LCN2 from stromal cells in the crosstalk of TNBC cells plays a critical role in TNBC cell proliferation and migration.
Discussion
Seeking critical targets for inhibiting metastasis and increasing survival of patients with TNBC, the breast cancer type that is highly metastatic and lacks effective therapies is highly significant. Almost all cancer drugs currently in use, with the exception of anti-angiogenic therapies and immunotherapies, target tumor cells. These strategies have only been moderately successful for the most part. It is becoming increasingly clear that a successful strategy should target the extensive interactions between the tumor stroma and the cancer cells. Many studies reported that the secretomes in the crosstalk between TNBC and stromal components promote TNBC tumor growth and metastasis. In our previous studies, we identified critical secreted factors (CCL5, IL-6, and IL-8) in these interactions and then targeted them with a combination of repurposed drugs.Citation27,Citation28,Citation30 Maraviroc, an FDA-approved antiretroviral drug, an inhibitor of CCR5, dramatically inhibited TNBC metastasis and showed synergistic inhibition of tumor growth and metastasis in combination with an anti-IL-6 receptor antibody, tocilizumab, indicated for the treatment of rheumatoid arthritis, confirming the importance of CCL5 and IL-6 secreted by LEC in response to the TNBC secretome. Furthermore, reparixin, an inhibitor of CXCR1/CXCR2 with anti-neoplastic activity, significantly inhibited TNBC growth and metastasis. In order to discover novel secreted factors to target crosstalk of TNBC cells and tumor stromal cells, here we examined 16 different combinations of secretomes from four stromal cells (LEC, MEC, fibroblasts, and M2-type macrophages differentiated from PBMC) in the crosstalk of four TNBC cell types (MDA-MB-231, SUM159, MDA-MB-468, and SUM149).
Although many co-culture methods have been used to examine the interaction of tumor cells and stromal cells, the various cell types in the tumor co-exist so it is not easy to determine which cell types are secreting the factors of interest. In order to collect and separate an individual secretome from each stromal cell in crosstalk with each TNBC cell, we applied the secretome from one cell type on another to allow conditioning, removing the added secretome, and collecting the new induced secretome into serum-free media. This method allowed unequivocal identification of the cell type secreting a particular secreted factor. The uninduced stromal cell secretome has been used as a control (). Although MS (mass spectrometry) proteomics has been utilized to discover and validate novel biomarkers, there is a major limitation of sensitivity to analyze complex samples such as body fluids with a huge dynamic range of expression. Alternatively, antibody-based technologies with high sensitivity have been used to overcome the limitation of MS (39). We utilized human cytokine arrays from R&D Systems (Proteome Profiler™ Human XL Cytokine Array Kit) to target selected 105 human cytokines, chemokines, and growth factors simultaneously ().
The bioinformatic analysis enables the ranking of cytokines associated with conditioning via each of the cell types. Both the limited number of replicates and batch effects between arrays limit any of these cytokines from reaching statistical significance. Still, we found eight of the top ten cytokines associated with macrophages in vitro were positively correlated with macrophage proportions in TNBC breast tumor samples from TCGA. Additional pathway analysis on TCGA samples also associates all of the top-ranked cytokines for each cell type with immune activity in vivo. This provides proof of principle for the co-culturing approach, warranting future studies on larger sample cohorts to further delineate the impact of different stromal compositions on secreted factors and immune activity in cancer.
Through the bioinformatic analysis, we have selected 21 secreted factor candidates to validate by a real-time RT-qPCR. We discovered that LCN2, GM-CSF, IL-6, IL-8, and CST3 were upregulated in stromal cells induced by TCM of TNBC cells (). Although GM-CSF, IL-6, and IL-8 were well-known targets involved in various cancersCitation42-Citation44 and thus their identification can be regarded as a validation of the methodology, the functions of LCN2 and CST3 in cancer are not yet clear. Secreted Lipocalin-2 (LCN2)/neutrophil gelatinase-associated lipocalin (NGAL) from various cell types is known as a biomarker of inflammation and cancer.Citation45,Citation46 The expression of LCN2 is regulated by DNA methylation in bladder cancer and breast cancer.Citation47,Citation48 The combination of LCN2 and TIMP1 is a serum marker to detect familial pancreatic cancer.Citation49 HIC1, a tumor suppressor gene negatively regulates LCN2 expression and the HIC1-LCN2 axis could serve as a prognostic biomarker in TNBC.Citation50 For a therapeutic approach, the ICAM-1-targeted, LCN2 siRNA-encapsulating liposome (ICAM-LCN2-LP) was engineered and significantly inhibited TNBC progression.Citation51 Recently, in a breast cancer orthotopic mouse xenograft model, the CRISPR-mediated knockout of LCN2 tumor growth rate was significantly decreased.Citation52 In addition, LCN2 selectively expressed in the metastatic Py230 cells and the elevation of LCN2 was significantly associated with poor breast cancer patient survival. LCN2 was elevated in circulating serum of mice chronically treated with conditioned media from Py230 cells.Citation53 In clinical studies, LCN2 showed regulation of epithelial–mesenchymal transition (EMT) and loss of nuclear expression of LCN2 was associated with aggressive features and poor outcome.Citation54
CST3/Cystatin C/CysC is known as an inhibitor of cysteine protease and is involved in caspase-mediated cell death.Citation55-Citation58 In cancer, CST3 is an attractive biomarker in colorectal cancer, prostate cancer, glioma, and melanoma metastases.Citation59-Citation62 However, the mechanism of CST3 in cancer progression has not been determined.Citation63
As we discovered, CCL5, IL-6, and IL-8 are critical factors in TNBC tumor progression and inhibitors of these factors, either alone or in combination with repurposed drugs maraviroc, tocilizumab, and reparixin, are effective in blocking TNBC growth and metastasis.Citation27,Citation28,Citation30 In future studies, functions of LCN2 and CST3 in the crosstalk between TNBC and stroma should be investigated, and specific inhibitors of these factors will enable identification of drug regimens with activity against TNBC that could be used to design and conduct clinical trials. The outcome of this research will be the establishment of a powerful new paradigm for discovering and evaluating new drug combinations that can be used to treat patients with TNBC, a disease for which effective therapies are currently not available.
Materials and methods
Cell lines
MDA-MB-231-luc-D3H2LN breast cancer cells were purchased from Caliper and propagated in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin (Sigma). SUM149 and SUM159 breast cancer cells were gifts from Dr. Zaver Bhujwalla (Radiology and Oncology, Johns Hopkins Medical Institutes). Cells were cultured in F-12 media supplemented with 5% FBS, 1 ng/ml hydrocortisone, 5 μg/ml insulin (Sigma), and 0.1 mM HEPES (Gibco). MDA-MB-468 breast cancer cells were purchased from ATCC and cultured in DMEM media supplemented with 10% FBS and 1% penicillin/streptomycin. LECs and MECs were purchased from Lonza and grown in EGM-2 MV culture medium. Normal human lung fibroblasts (NHLF) were purchased from Lonza and grown in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin (Sigma). Peripheral Blood Mononuclear Cells (PBMCs) were purchased from Zenbio and monocytes were differentiated into M2-type macrophages by culturing for 6 d in RPMI medium supplemented with 10% FBS, 100 ng/ml of M-CSF. Cells were maintained under standard conditions of 37°C and 5% CO2. Cells were cultured for a maximum of 4 weeks before thawing fresh, early passage cells and confirmed to be Mycoplasma negative (Hoechst stain).
Conditioned media
When MDA-MB-231, SUM149, SUM159, and MDA-MB-468 cells were confluent in T175 tissue culture flasks, the normal cancer cell growth media were replaced with 8 ml serum-free media (SFM). After 24 h incubation in a tissue culture incubator, the supernatant was centrifuged and filtered through 0.2 µm syringe filters (Corning). The resulting tumor-conditioned media (TCM) were stored in aliquots at −80°C. When LEC, MEC, fibroblasts, and macrophages reached 30-40% confluence in T75 tissue culture flasks, the growth medium (GM) was replaced with 30% TCM in GM (TCM:GM = 3:7) to allow the TCM to educate the stromal cells. For the education, the cells were allowed to grow in the media for 3–4 d then the media were replaced with 3 ml SFM with 2% FBS. After 48 h, the supernatant was centrifuged and filtered. The resulting tumor-educated stromal cells (TNBC-stroma) conditioned media were stored in aliquots at −80°C to avoid multiple freeze thaws. A diagram of the experimental procedure to collect the conditioned media from stromal cells in the crosstalk of TNBC cells is shown in .
Real-time RT-qPCR
Total RNA was extracted with Trizol reagent (Invitrogen), and cDNA was synthesized from total RNA (2 µg) using an iScript™ cDNA Synthesis Kits (Bio-rad). Aliquots of cDNA were used as templates for real-time RT-qPCR procedure. Relative quantitation of mRNA expression was achieved using real-time PCR (CFX96 Touch™ Real-Time PCR Detection System, Bio-rad laboratory, Hercules, California). The SYBR® Green PCR Supermix was used according to the manufacturer’s instruction.
Cell migration and proliferation assays
MDA-MB-231 cell migration was assessed by using the OrisTM Cell migration kit (Platypus Technology, Madison, WI), as previously described.Citation28 The conditioned media (100 μl) with or without anti-LCN2 antibodies (R&D Systems, 50 μg/ml) were added once MDA-MB-231 cells had attached. The proliferation assay was measured by MTT assay as previously described.Citation64 Cells were plated at 2.5 × 103 cells per well in 96-well plates, in triplicate, with 200 µL media, with anti-LCN2 antibody (50 μg/ml) or vehicle for 6 d. Thirty microliters of MTT solution (5 mg/mL in PBS) was added to each well and cells incubated for 2 h. Media were then aspirated and cells resuspended in 200 µL DMSO. Absorbance at 560 nm was measured, with a background at 670 nm subtracted. Triplicates were averaged for a mean absorbance, and then the fold change was calculated for the survival of antibody-treated cells versus time-matched IgG-treated cells. Experiments were performed in triplicate.
Antibody array
For reverse western blot, human cytokine antibody array kits (R&D Systems) were used, according to the manufacturer’s instructions.Citation27 The pixel densities were analyzed using ImageJ for the experimental analysis and the Array Quick Spots Tool from HLImage++ (Western Vision Software) for the bioinformatic analysis.
Bioinformatic analysis
Protein arrays with intensity values in the top 75th percentile greater than 700 were retained for bioinformatic analysis and negative values were set to zero. Replicate samples for each experimental condition were averaged. Empirical Bayes moderated paired t-tests with LIMMACitation65 were performed to compare log-transformed average protein expression values for tumor-conditioned media to stromal-conditioned media for each cell type. P-values were false discovery rate (FDR) adjusted. Although conditions were measured on different arrays, no batch correction could be performed due to the confounding of cell types with batch in the design limiting analysis between cell types. Cytokines were sorted by FDR adjusted p-values and the top ten specific to each cell type were retained for follow-up analyses using TNBC samples from TCGA.
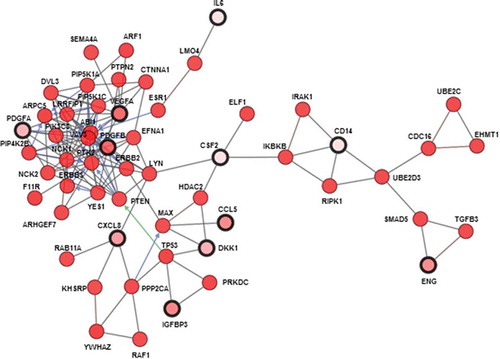
RNA-sequencing Level 3 RSEM normalized data for 76 TNBC samples from the TCGA breast cancer marker paperCitation66 were accessed from the Broad Institute TCGA GDAC Firehose (doi:10.7908/C11G0KM9) and log2-transformed. We used CIBERSORT proportions of cell types of TCGA sample from Thorsson et al.Citation67Spearman correlation between cell types and each cytokine from the array screen was computed. In addition, Wilcoxon gene set tests for canonical pathways (C2) and Hallmark pathways (H) from MSigDB Version 6.1Citation68,Citation69 were performed on the Pearson correlation coefficients between RNA expression of each of these cytokines and other genes measured with RNA-seq. Pathways with FDR adjusted p-values below 0.05 were called statistically significant and the top ten were retained for analysis. All these analyses were performed in R using build-in functions and custom scripts. Additional network analysis was performed on TNBC samples in TCGA with cBioPortalCitation70,Citation71 using the 21 proteins prioritized for qRT-PCR to initialize the network.
Supplemental Material
Download Zip (942 KB)Acknowledgments
This work was supported by startup funding from Albany College of Pharmacy and Health Sciences, the National Institutes of Health grant R01 CA138264 and P30 CA006973.
Disclosure statement
No potential conflicts of interest were disclosed.
Supplementary material
Supplemental data for this article can be accessed publisher’s website.
Additional information
Funding
References
- Malorni L, Shetty PB, De Angelis C, Hilsenbeck S, Rimawi MF, Elledge R, Osborne CK, De Placido S, Arpino G. Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat. 2012;136:795–804. doi:10.1007/s10549-012-2315-y.
- Balko JM, Giltnane JM, Wang K, Schwarz LJ, Young CD, Cook RS, Owens P, Sanders ME, Kuba MG, Sanchez V, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–245. doi:10.1158/2159-8290.CD-13-0286.
- Dotto GP, Weinberg RA, Ariza A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc Natl Acad Sci U S A. 1988;85:6389–6393. doi:10.1073/pnas.85.17.6389.
- Rudnick JA, Arendt LM, Klebba I, Hinds JW, Iyer V, Gupta PB, Naber SP, Kuperwasser C. Functional heterogeneity of breast fibroblasts is defined by a prostaglandin secretory phenotype that promotes expansion of cancer-stem like cells. PLoS One. 2011;6:e24605. doi:10.1371/journal.pone.0024605.
- Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2:e119. doi:10.1371/journal.pgen.0020119.
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe F, Itohara S,Werb Z, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi:10.1038/35036374.
- Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, DiPrete B, Betz KS, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi:10.1016/j.ccr.2011.01.020.
- Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi:10.1093/jnci/djm135.
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi:10.1038/nri2216.
- Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi:10.1038/nrd3626.
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JPA, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–867. doi:10.1200/JCO.2011.41.0902.
- Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–618. doi:10.1093/annonc/mdt556.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi:10.1016/j.cell.2010.03.014.
- Tu E, Chia PZ, Chen W. TGFbeta in T cell biology and tumor immunity: angel or devil? Cytokine Growth Factor Rev. 2014;25:423–435. doi:10.1016/j.cytogfr.2014.07.014.
- Kelly PM, Davison RS, Bliss E, McGee JO. Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer. 1988;57:174–177. doi:10.1038/bjc.1988.36.
- Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao Y-W, Wei Y-Q. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi:10.1371/journal.pone.0050946.
- Zhou J, Wang XH, Zhao YX, Chen C, Xu XY, Sun Q, Wu H-Y, Chen M, Sang J-F, Su L, et al. Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J Cancer. 2018;9:4635–4641. doi:10.7150/jca.28583.
- Lacalle RA, Blanco R, Carmona-Rodriguez L, Martin-Leal A, Mira E, Manes S. Chemokine receptor signaling and the hallmarks of cancer. Int Rev Cell Mol Biol. 2017;331:181–244.
- Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017;6:2115–2125. doi:10.1002/sctm.17-0138.
- Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–725. doi:10.4161/onci.20068.
- Liu Q, Zhang H, Jiang X, Qian C, Liu Z, Luo D. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer. 2017;16:176. doi:10.1186/s12943-017-0742-4.
- Velaei K, Samadi N, Barazvan B, Soleimani Rad J. Tumor microenvironment-mediated chemoresistance in breast cancer. Breast. 2016;30:92–100. doi:10.1016/j.breast.2016.09.002.
- Naik A, Al-Zeheimi N, Bakheit CS, Al Riyami M, Al Jarrah A, Al Moundhri MS, Al Habsi Z, Basheer M, Adham SA. Neuropilin-1 associated molecules in the blood distinguish poor prognosis breast cancer: a cross-sectional study. Sci Rep. 2017;7:3301. doi:10.1038/s41598-017-03280-0.
- Oh N, Park JI, Park JH, Kim KS, Lee DR, Park KS. The role of ELK3 to regulate peritumoral lymphangiogenesis and VEGF-C production in triple negative breast cancer cells. Biochem Biophys Res Commun. 2017;484:896–902. doi:10.1016/j.bbrc.2017.02.030.
- Ibrahim SA, Gadalla R, El-Ghonaimy EA, Samir O, Mohamed HT, Hassan H, Greve B, El-Shinawi M, Mohamed MM, Götte M, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. 2017;16:57. doi:10.1186/s12943-017-0621-z.
- Fertig EJ, Lee E, Pandey NB, Popel AS. Analysis of gene expression of secreted factors associated with breast cancer metastases in breast cancer subtypes. Sci Rep. 2015;5:12133. doi:10.1038/srep12133.
- Jin K, Pandey NB, Popel AS. Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget. 2017;8:60210–60222. doi:10.18632/oncotarget.19417.
- Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun. 2014;5:4715. doi:10.1038/ncomms5715.
- Dore-Savard L, Lee E, Kakkad S, Popel AS, Bhujwalla ZM. The Angiogenic Secretome in VEGF overexpressing breast cancer xenografts. Sci Rep. 2016;6:39460. doi:10.1038/srep39460.
- Jin K, Pandey NB, Popel AS. Simultaneous blockade of IL6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res. 2018;20:54. doi:10.1186/s13058-018-0981-3.
- Norton KA, Jin K, Popel AS. Modeling triple-negative breast cancer heterogeneity: effects of stromal macrophages, fibroblasts and tumor vasculature. J Theor Biol. 2018;452:56–68. doi:10.1016/j.jtbi.2018.05.003.
- Lee E, Pandey NB, Popel AS. Pre-treatment of mice with tumor-conditioned media accelerates metastasis to lymph nodes and lungs: a new spontaneous breast cancer metastasis model. Clin Exp Metastasis. 2013;31:67–79. doi:10.1007/s10585-013-9610-9.
- Lee E, Pandey NB, Popel AS. Crosstalk between cancer cells and blood endothelial and lymphatic endothelial cells in tumour and organ microenvironment. Expert Rev Mol Med. 2015;17:e3. doi:10.1017/erm.2015.2.
- Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey NB, Popel AS. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Sci Rep. 2014;4:7139. doi:10.1038/srep07139.
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi:10.1172/JCI45014.
- Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. Onco Targets Ther. 2018;11:8099–8106. doi:10.2147/OTT.
- Jung M, Oren B, Mora J, Mertens C, Dziumbla S, Popp R, Weigert A, Grossmann N, Fleming I, Brüne B, et al. Lipocalin 2 from macrophages stimulated by tumor cell-derived sphingosine 1-phosphate promotes lymphangiogenesis and tumor metastasis. Sci Signal. 2016;9(434):ra64. doi:10.1126/scisignal.aaf3241.
- Oren B, Urosevic J, Mertens C, Mora J, Guiu M, Gomis RR, Weigert A, Schmid T, Grein S, Brüne B, et al. Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J Pathol. 2016;239(3):274–285. doi:10.1002/path.4724.
- Leng X, Wu Y, Arlinghaus RB. Relationships of lipocalin 2 with breast tumorigenesis and metastasis. J Cell Physiol. 2011;226:309–314. doi:10.1002/jcp.22403.
- Leng X, Ding T, Lin H, Wang Y, Hu L, Hu J, Feig B, Zhang W, Pusztai L, Symmans WF, et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009;69:8579–8584. doi:10.1158/0008-5472.CAN-09-1934.
- Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106:3913–3918. doi:10.1073/pnas.0810617106.
- Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–789. doi:10.1038/s41577-018-0066-7.
- Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15:210. doi:10.1186/bcr3436.
- Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–360. doi:10.2217/imt-2016-0141.
- Moschen AR, Adolph TE, Gerner RR, Wieser V, Tilg H. Lipocalin-2: a master mediator of intestinal and metabolic inflammation. Trends Endocrinol Metab. 2017;28:388–397. doi:10.1016/j.tem.2017.01.003.
- Bartsch DK, Gercke N, Strauch K, Wieboldt R, Matthai E, Wagner V, Rospleszcz S, Schäfer A, Franke F, Mintziras I, et al. 2018. The combination of MiRNA-196b, LCN2, and TIMP1 is a potential set of circulating biomarkers for screening individuals at risk for familial pancreatic cancer. J Clin Med. 7. DOI:10.3390/jcm7100295.
- Dokun OY, Florl AR, Seifert HH, Wolff I, Schulz WA. Relationship of SNCG, S100A4, S100A9 and LCN2 gene expression and DNA methylation in bladder cancer. Int J Cancer. 2008;123:2798–2807. doi:10.1002/ijc.23893.
- Meka P, Jarjapu S, Nanchari SR, Vishwakarma SK, Edathara PM, Gorre M, Cingeetham A, Vuree S, Annamaneni S, Dunna NR, et al. LCN2 promoter methylation status as novel predictive marker for microvessel density and aggressive tumor phenotype in breast cancer patients. Asian Pac J Cancer Prev. 2015;16:4965–4969. doi:10.7314/APJCP.2015.16.12.4965.
- Slater EP, Fendrich V, Strauch K, Rospleszcz S, Ramaswamy A, Matthai E. LCN2 and TIMP1 as potential serum markers for the early detection of familial pancreatic cancer. Transl Oncol. 2013;6:99–103. doi:10.1593/tlo.12373.
- Cheng G, Sun X, Wang J, Xiao G, Wang X, Fan X, Zu L, Hao M, Qu A, Mao Y, et al. HIC1 silencing in triple-negative breast cancer drives progression through misregulation of LCN2. Cancer Res. 2014;74:862–872. doi:10.1158/0008-5472.CAN-13-2420.
- Guo P, Yang J, Jia D, Moses MA, Auguste DT. ICAM-1-targeted, Lcn2 siRNA-encapsulating liposomes are potent anti-angiogenic agents for triple negative breast cancer. Theranostics. 2016;6:1–13. doi:10.7150/thno.12167.
- Guo P, Yang J, Huang J, Auguste DT, Moses MA. Therapeutic genome editing of triple-negative breast tumors using a noncationic and deformable nanolipogel. Proc Natl Acad Sci U S A. 2019;116:18295–18303. doi:10.1073/pnas.1904697116.
- Meade KJ, Sanchez F, Aguayo A, Nadales N, Hamalian SG, Uhlendorf TL, Banner LR, Kelber JA. Secretomes from metastatic breast cancer cells, enriched for a prognostically unfavorable LCN2 axis, induce anti-inflammatory MSC actions and a tumor-supportive premetastatic lung. Oncotarget. 2019;10:3027–3039. doi:10.18632/oncotarget.v10i32.
- Kurozumi S, Alsaeed S, Orah N, Miligy IM, Joseph C, Aljohani A, Toss MS, Fujii T, Shirabe K, Green Ar, et al. Clinicopathological significance of lipocalin 2 nuclear expression in invasive breast cancer. Breast Cancer Res Treat. 2020;179:557–564.
- Abrahamson M. Cystatins. Methods Enzymol. 1994;244:685–700.
- Ochieng J, Chaudhuri G. Cystatin superfamily. J Health Care Poor Underserved. 2010;21:51–70. doi:10.1353/hpu.0.0257.
- Turk B, Stoka V. Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett. 2007;581:2761–2767. doi:10.1016/j.febslet.2007.05.038.
- Benchoua A, Braudeau J, Reis A, Couriaud C, Onteniente B. Activation of proinflammatory caspases by cathepsin B in focal cerebral ischemia. J Cereb Blood Flow Metab. 2004;24:1272–1279. doi:10.1097/01.WCB.0000140272.54583.FB.
- Gora J, Latajka R. Involvement of cysteine proteases in cancer. Curr Med Chem. 2015;22:944–957. doi:10.2174/0929867321666141106115624.
- Kos J, Stabuc B, Schweiger A, Krasovec M, Cimerman N, Kopitar-Jerala N, Vrhovec I. Cathepsins B, H, and L and their inhibitors stefin A and cystatin C in sera of melanoma patients. Clin Cancer Res. 1997;3:1815–1822.
- Wegiel B, Jiborn T, Abrahamson M, Helczynski L, Otterbein L, Persson JL, Bjartell A. Cystatin C is downregulated in prostate cancer and modulates invasion of prostate cancer cells via MAPK/Erk and androgen receptor pathways. PLoS One. 2009;4:e7953. doi:10.1371/journal.pone.0007953.
- Nakabayashi H, Hara M, Shimuzu K. Clinicopathologic significance of cystatin C expression in gliomas. Hum Pathol. 2005;36:1008–1015. doi:10.1016/j.humpath.2005.06.021.
- Yan Y, Fan Q, Wang L, Zhou Y, Li J, Zhou K. LncRNA Snhg1, a non-degradable sponge for miR-338, promotes expression of proto-oncogene CST3 in primary esophageal cancer cells. Oncotarget. 2017;8:35750–35760. doi:10.18632/oncotarget.16189.
- Jin K, Park S, Teo WW, Korangath P, Cho SS, Yoshida T. HOXB7 is an ERalpha cofactor in the activation of HER2 and multiple ER target genes leading to endocrine resistance. Cancer Discov. 2015;5:944–959. doi:10.1158/2159-8290.CD-15-0090.
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi:10.1093/nar/gkv007.
- Cancer Genome AN. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Silveira HCS, Vidal DO, Burnette A, Eschbacher J, et al. The immune landscape of cancer. Immunity. 2018;48:812–30 e14. doi:10.1016/j.immuni.2018.03.023.
- Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi:10.1016/j.cels.2015.12.004.
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi:10.1073/pnas.0506580102.
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Thorsson V, Gibbs DL, Brown SD, Wolf D, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi:10.1126/scisignal.2004088.
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi:10.1158/2159-8290.CD-12-0095.