ABSTRACT
Although HER2-targeted therapy has been shown to prolong the survival of patients with HER2-positive breast cancer, most patients eventually progress due to drug resistance. Novel treatment options are urgently needed to overcome resistance to HER2-targeted therapy. The VEGF/VEGFR (Vascular endothelial growth factor and its receptors) pathway is essential in tumor angiogenesis, which may be a promising target in HER2-positive breast cancer providing a rationale for the use of tyrosine kinase inhibitors (TKIs) targeting VEGFR. Here, we present a case of a heavily pretreated advanced breast cancer patient who did not respond to HER2-targeted therapy and developed resistance to multiple lines of HER2-targeted treatment. The patient was treated with apatinib at a dose of 500 mg daily, and obtained partial remission (PR) with a progression-free-stage (PFS) of 6 months. Our case indicates that apatinib might have anti-tumor activity in patients with HER2-positive breast cancer with HER2-targeted resistance. This case is of value which may provide new insights into strategies for HER2-targeted therapy resistance options in the clinic.
Introduction
Breast cancer is the most common malignancy worldwide, and the leading cause of cancer-related death amongst females.Citation1 HER2-positive breast cancer accounts for about 15–20% of all cases, with a more aggressive phenotype and a worse prognosis.Citation2 Despite effective therapy targeting the HER2 pathway, the majority of the patients ultimately progress due to drug resistance and therapeutic options remain limited.Citation3,Citation4 There is an urgent need to explore novel treatments to overcome resistance to HER2-targeted therapies which have potential to translate to the clinic.
Angiogenesis is a crucial hallmark of cancer.Citation5 It is well known that VEGF and VEGFRs serve as central mediators in neoangiogenesis of numerous cancer types including breast cancer.Citation6 Tissue VEGF level is negatively correlated with survival of breast cancer patients.Citation7 Therefore, blocking the VEGF/VEGFR axis may be a promising therapeutic option in breast cancer.Citation8 Several antiangiogenic monoclonal antibodies, including bevacizumab and ramucirumab, have shown anti-tumor activity in metastatic breast cancer (MBC) when combined with chemotherapy.Citation9-Citation16 In addition, TKIs targeting VEGFR including sorafenib, sunitinib and pazopanib in the treatment of MBC have been investigated.Citation17,Citation18 However, most of these agents only show moderate efficacy.Citation19,Citation20
Apatinib is a highly selective VEGFR2 TKI which could inhibit VEGF-stimulated endothelial cell migration and proliferation.Citation21 Apatinib has been approved as a third-line treatment for patients with advanced gastric cancer in China since 2014.Citation22 In breast cancer, two phase II trials have exhibited encouraging efficacy and manageable toxicity of apatinib monotherapy in heavily pretreated, metastatic breast cancer.Citation23,Citation24 However, to the best of our knowledge, few studies have reported the efficacy of apatinib monotherapy for heavily pretreated HER2-positive advanced breast cancer (ABC) who experienced HER2-targeted therapy resistance.
In the current report, we present a case of a patient with heavily pretreated HER2-positive ABC, who had significant response to apatinib monotherapy after failure of multiple lines of HER2-targeted therapy. This case report will provide new insights into the strategy of rescuing HER2-targeted therapy resistance in clinical practice.
Case presentation
The patient management is described in . A 28-year-old female patient complaining of a painless mass in right breast was referred to our hospital in July 2014. The patient received modified radical mastectomy for right breast cancer after comprehensive preoperative examinations. The postoperative stage was T2N0M0, stage IIA. Pathological inspection revealed invasive ductal breast carcinoma (Grade II) with immunohistochemistry (IHC) results of ER (-), PR (-), HER2 (3+) and Ki67 (50%+-75%+), VEGF (++), VEGFR2 (++), p-VEGFR2 (++) ( and ). All the nine dissected lymph nodes were shown to be negative. Fluorescence in situ hybridization (FISH) identified HER2 amplification with a HER2/CEP17 ratio of 8.56. The patient then underwent adjuvant chemotherapy with cyclophosphamide plus epirubicin for four cycles and sequential four cycles of docetaxel. Adjuvant HER2-targeted therapy was refused by the patient due to economic concerns.
Table 1. The course of disease in the female patient with HER2-positive breast cancer.
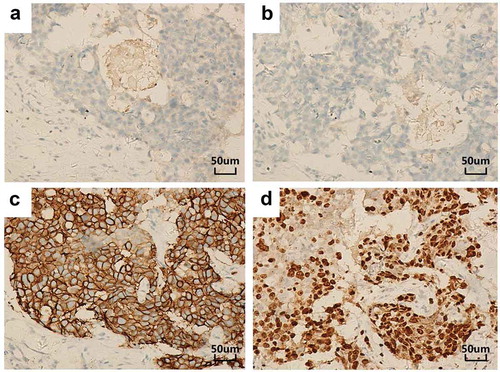
Figure 1. IHC results of the primary right breast carcinoma (original magnification, 200×). The tumor stained negative for both ER (a) and PR (b) but strongly positive for HER-2 (c) and showed a Ki-67 proliferation index of 50–75% (d).
In May 2015, follow-up chest computed tomography (CT) scan demonstrated multiple nodules in bilateral lungs and a right chest wall mass. The right chest wall lesion was further categorized as BI-RADS 4C on ultrasound. As the patient refused to undergo biopsy, recurrence and metastases were clinically diagnosed. Since May 13, 2015, this patient received second-line chemotherapy consisting of vinorelbine, capecitabine (NX) and trastuzumab, yielding a best response of stable disease (SD). However, progressive disease (PD) occurred after completion of five cycles of chemotherapy on September 21, 2015, with a PFS of 5 months. The patient underwent ultrasound-guided biopsy in the right chest wall mass and left lower lobe lesion. Results from pathological inspection and IHC confirmed the origin from breast cancer for the biopsied tissues and staining of VEGF (++), VEGFR2 (++), p-VEGFR2 (++) ().
The patient then received third-line treatment of gemcitabine, cisplatin (GP) and trastuzumab for six cycles, having the best response of SD. On February 13, 2016, CT scan showed PD in all the lesions, producing a PFS of 5 months. The patient was advised to receive lapatinib treatment but she refused the treatment for economic reasons. Systemic therapy was then switched to paclitaxel, carboplatin (TC), and trastuzumab. The patient experienced grade 2 thrombocytopenia and grade 3 neutropenia, leading to discontinuation of therapy. As a result, raltitrexed, trastuzumab and lapatinib were prescribed to the patient as fourth-line therapy. However, bilateral lung metastases and right chest wall mass progressed after four cycles of treatment ().
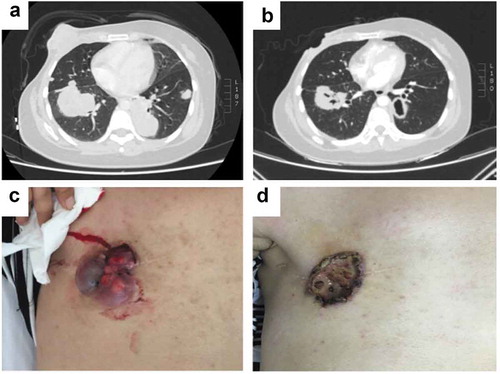
To clarify the mechanism of multi-line resistance to HER2-targeted therapy, the biopsy tissues of the postoperative lesion, left lung metastasis and chest wall recurrence lesions after failure of NX plus trastuzumab, and the blood samples after failure of raltitrexed, trastuzumab and lapatinib, were tested with next-generation sequencing (NGS) of 425-gene panel. The results showed persistent existence of HER2 amplification, HER2 V777L mutation and TP53 mutation in all samples (, ). Apatinib, at the dose of 500 mg daily, was then initiated from July 2016. Surprisingly, the lesions, especially in the right chest wall and the left lung metastasis, exhibited remarkable shrinkage after 3 months of use (). The best response was partial remission (PR) and no apatinib-related side effects were observed. Specifically, we observed clear cavitation in the above-mentioned lesions, which reflected the mechanism of action of apatinib ()).
Table 2. Tumor tissue and plasma NGS show gene amplification and mutation.
Figure 2. Resistance to trastuzumab and lapatinib induced by the HER2 V777L mutation. Representative image of read alignments visualized with IGV. HER2 V777L mutation and TP53 Q52fs shifting mutation were detected in the different tissues. The tissues 1/2/3 refer to the paraffined tissues of postoperative right breast lesion (July 2014), left lung metastasis and right chest wall recurrence lesions (September 2015). The arrow shows the position of the variant.
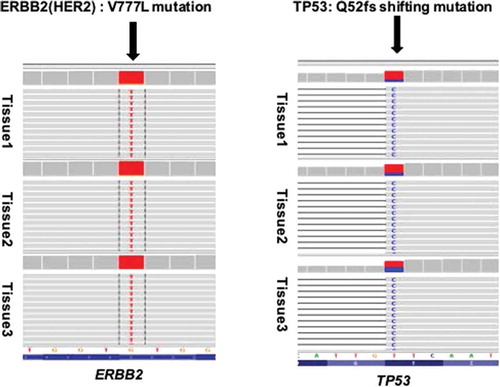
Figure 3. PR response to apatinib monotherapy treatment. (a) Chest CT scans before apatinib treatment (July 2016). (b) Chest CT scans after apatinib treatment (October 2016), the lesions especially in the right chest wall and the left lung metastasis demonstrated clear cavitation. (c) The expression of chest wall focus before apatinib treatment (July 2016). (d) The expression of chest wall focus after apatinib treatment (October 2016).
In January 2017, the patient developed persistent cough, fever and fatigue. The CT examination indicated that the lung lesions had progressed and apatinib treatment was ended at this stage, with a PFS of 6 months. The patient received no further treatment and died in March 2017. The overall survival (OS) for the patient was 32 months from the diagnosis of breast cancer and 22 months from post-operative recurrence and metastases.
Discussion
To the best of our knowledge, this is the first case of successful salvage treatment in a heavily and refractory HER2-positive advanced breast cancer patient who was resistant to multiple lines of anti-HER2 therapy with anti-VEGFR2 TKI.
Previous studies suggested that patients with HER2-positive breast cancer and concurrent HER2 activating mutations, might be resistant to trastuzumab treatment.Citation25-Citation27 Similarly, in this case, the patient with persistent HER2 amplification and HER2 V777 L mutation experienced resistance to multiple lines of HER2-targeted therapy, including trastuzumab and lapatinib. In HER2 mutant breast cancer, irreversible inhibitors such as neratinib are indicated to inhibit kinase activity and survival of HER2-mutation-driven cancer cells. However, due to the inaccessibility of neratinib at that time, and no standard-of-care at later-stage breast cancer, the patient did not receive neratinib.
It was assumed that the patient might benefit from angiogenesis inhibitors based on the assumption that HER2 amplification may induce bypass activation of the pro-angiogenic factor VEGF. As has been demonstrated in previous studies, HER2 overexpression is associated with elevated VEGF mRNA and protein levels in breast cancer cells.Citation28 Additionally, a related study indicated that VEGFR2-positive stromal vessel counts were significantly higher in HER2-positive primary breast cancer. These data imply that the effects of HER2 on tumorigenesis are at least partially mediated by stimulation of angiogenesis, which provides a strong rationale for targeting the VEGF/VEGFR2 axis in HER2-positive breast cancer.Citation29
Based on this hypothesis, several clinical trials have been conducted to explore the potential benefit of anti-HER2 and anti-angiogenesis combination therapy for HER2-positive breast cancer. Results from early phase trials have been promising.Citation30-Citation33 However, the phase III AVERAL trial did not meet the primary endpoint of prolonging investigator-assessed PFS in patients treated with bevacizumab, docetaxel and trastuzumab.Citation34 In addition, a higher incidence of cardiovascular toxicity from the combination therapy was a significant concern. Novel angiogenesis inhibitors with improved efficacy and manageable toxicities are urgently needed.
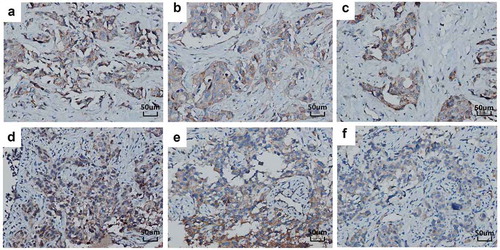
Apatinib is a highly selective and potent VEGFR2 TKI. Compared to other VEGFR TKI such as sorafenib, sunitinib and pazopanib, apatinib is more potent in inhibiting the activity of VEGFR2 with IC50 value of 1 nM.Citation35-Citation37 In two phase II trials, apatinib monotherapy demonstrated a promising median PFS of 3.8 and 4.0 months, respectively, with manageable toxicity profiles for patients having metastatic breast cancer.Citation23,Citation24 Exploratory analysis combined data from the two trials suggested that tumor p-VEGFR2 expression (adjusted HR, 0.40; P = .013) is an independent predictor of both PFS and clinical benefit rate.Citation38 Similarly, IHC of postoperative and biopsy tissues in the current patient showed moderate expressions of VEGFR2, VEGF and p-VEGFR2 (). Based on these lines of evidence and clinical experience, the patient was treated with apatinib monotherapy. Surprisingly, the patient obtained PR after 3 months of treatment, and a PFS of 6 months despite resistance to multiple lines of anti-HER2 treatment.Citation23
Figure 4. IHC of tumor tissue using anti-VEGF, VEGFR2 and p-VEGFR2 antibodies (original magnification, 200×). The tissues with medium positive staining for VEGF, VEGFR2 and p-VEGFR2 of postoperative breast tissue (a–c). The tissues with medium positive staining for VEGF, VEGFR2 and p-VEGFR2 of left lung biopsy tissue (d–f).
VEGFR2 is the canonical member of the VEGFR family in angiogenic signaling.Citation39,Citation40 Theoretically, cells with high VEGFR2 expression could be stimulated using VEGF, and the proliferation of these stimulated cells could be inhibited by anti-VEGF antibody.Citation41 Therefore, both the high expressions of VEGF and VEGFR2 are necessary for activation of the VEGF/VEGFR2 pathway. VEGFR2 is phosphorylated upon VEGF/VEGFR2 engagement which is essential to tumor growth and survival.Citation42 Hence, p-VEGFR2 levels may predict the efficacy of anti-angiogenic therapy in patients with advanced breast cancer.Citation38
We speculated that the high VEGF expression in this case stemmed from HER2 amplification and mutation. The high expression of VEGFR2 may be a result of the TP53(Q52fs) truncation mutation, which probably binds near the VEGFR2 promoter initiation site which has been reported previously.Citation43 Considering the consistent high mutation abundance in the breast, lung, chest wall and serum of this patient, we postulate that the TP53 truncation frameshift mutation (Q52fs) may synergistically promote expression of VEGFR2 which is highly pre-stimulated by VEGF. The highly activating p-VEGFR2 may then sensitize the inhibitory effect of apatinib and so apatinib rescues the refractory ABC patient.
Conclusions
In conclusion, we present a case of apatinib monotherapy for a patient with HER2-positive breast cancer who developed resistance to multiple lines of anti-HER2 therapy. Apatinib obtained PR with a PFS of 6 months. We hypothesized that HER-2 amplification and concurrent HER2 activating mutation might promote tumor angiogenesis and subsequent resistance of HER2-targeted therapies. In the future, further large-scale clinical trials are required to clarify the role of apatinib in refractory HER2-targeted resistant breast cancer.
Disclosure of Potential Conflicts of Interest
The authors report no conflict of interest.
Acknowledgments
We would like to thank MD. Shaodong Hong from Sun Yat-sen University Cancer Hospital for a critical revision of the article.
Additional information
Funding
References
- Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol. 2005;34:405–412. doi:10.1093/ije/dyh414.
- Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017;389:2415–2429. doi:10.1016/S0140-6736(16)32417-5.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, Castro G Jr., Untch M, Smith I, Gianni L, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. doi:10.1016/S0140-6736(16)32616-2.
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi:10.1056/NEJM200103153441101.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi:10.1016/j.cell.2011.02.013.
- Banerjee S, Dowsett M, Ashworth A, Martin LA. Mechanisms of disease: angiogenesis and the management of breast cancer. Nat Clin Pract Oncol. 2007;4:536–550. doi:10.1038/ncponc0905.
- Schirosi L, De Summa S, Tommasi S, Paradiso A, Gasparini G, Popescu O, Simone G, Mangia A. VEGF and TWIST1 in a 16-biomarker immunoprofile useful for prognosis of breast cancer patients. Int J Cancer. 2017;141:1901–1911. doi:10.1002/ijc.30868.
- Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–1914. doi:10.1158/0008-5472.CAN-11-3406.
- Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi:10.1200/JCO.2008.21.6457.
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi:10.1056/NEJMoa072113.
- Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi:10.1200/JCO.2010.28.0982.
- Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, Rugo HS. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–4293. doi:10.1200/JCO.2010.34.1255.
- Vahdat LT, Layman R, Yardley DA, Gradishar W, Salkeni MA, Joy AA, Garcia AA, Ward P, Khatcheressian J, Sparano J, et al. Randomized Phase II study of ramucirumab or icrucumab in combination with capecitabine in patients with previously treated locally advanced or metastatic breast cancer. Oncologist. 2017;22:245–254. doi:10.1634/theoncologist.2016-0265.
- Yardley DA, Reeves J, Dees EC, Osborne C, Paul D, Ademuyiwa F, Soliman H, Guthrie T, Andersen J, Krekow L, et al. Ramucirumab with eribulin versus eribulin in locally recurrent or metastatic breast cancer previously treated with anthracycline and taxane therapy: a multicenter, randomized, Phase II study. Clin Breast Cancer. 2016;16:471–9 e1. doi:10.1016/j.clbc.2016.07.005.
- Mackey JR, Ramos-Vazquez M, Lipatov O, McCarthy N, Krasnozhon D, Semiglazov V, Manikhas A, Gelmon KA, Konecny GE, Webster M, et al. Primary results of ROSE/TRIO-12, a randomized placebo-controlled phase III trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. J Clin Oncol. 2015;33:141–148. doi:10.1200/JCO.2014.57.1513.
- Spera G, Fresco R, Fung H, Dyck JRB, Pituskin E, Paterson I, Mackey JR. Beta blockers and improved progression-free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol. 2017;28:1836–1841. doi:10.1093/annonc/mdx264.
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337.
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi:10.1038/nrd2130.
- Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, Laferriere N, Pena C, Lathia C, Bergamini L, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009;20:616–624. doi:10.1097/CAD.0b013e32832b2ea0.
- Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM Jr., Wiesenfeld M, Flynn PJ, Fitch TR, Perez EA. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi:10.1200/JCO.2007.15.5242.
- Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer. 2010;10:529. doi:10.1186/1471-2407-10-529.
- Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, et al. Randomized, double-blind, placebo-controlled Phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–1454. doi:10.1200/JCO.2015.63.5995.
- Hu X, Cao J, Hu W, Wu C, Pan Y, Cai L, Tong Z, Wang S, Li J, Wang Z, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer. 2014;14:820. doi:10.1186/1471-2407-14-820.
- Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961–1969. doi:10.1002/ijc.28829.
- Hirotsu Y, Nakagomi H, Amemiya K, Oyama T, Inoue M, Mochizuki H, Omata M. Intrinsic HER2 V777L mutation mediates resistance to trastuzumab in a breast cancer patient. Med Oncol. 2017;34:3. doi:10.1007/s12032-016-0857-2.
- Tortora G. Mechanisms of resistance to HER2 target therapy. J Natl Cancer Inst Monogr. 2011;2011:95–98. doi:10.1093/jncimonographs/lgr026.
- Herter-Sprie GS, Greulich H, Wong KK. Activating mutations in ERBB2 and their impact on diagnostics and treatment. Front Oncol. 2013;3:86. doi:10.3389/fonc.2013.00086.
- Alameddine RS, Otrock ZK, Awada A, Shamseddine A. Crosstalk between HER2 signaling and angiogenesis in breast cancer: molecular basis, clinical applications and challenges. Curr Opin Oncol. 2013;25:313–324. doi:10.1097/CCO.0b013e32835ff362.
- Nasir A, Holzer TR, Chen M, Man MZ, Schade AE. Differential expression of VEGFR2 protein in HER2 positive primary human breast cancer: potential relevance to anti-angiogenic therapies. Cancer Cell Int. 2017;17:56. doi:10.1186/s12935-017-0427-5.
- Rugo HS, Chien AJ, Franco SX, Stopeck AT, Glencer A, Lahiri S, Arbushites MC, Scott J, Park JW, Hudis C, et al. A phase II study of lapatinib and bevacizumab as treatment for HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2012;134:13–20. doi:10.1007/s10549-011-1918-z.
- Schwartzberg LS, Badarinath S, Keaton MR, Childs BH. Phase II multicenter study of docetaxel and bevacizumab with or without trastuzumab as first-line treatment for patients with metastatic breast cancer. Clin Breast Cancer. 2014;14:161–168. doi:10.1016/j.clbc.2013.12.003.
- Lin NU, Seah DS, Gelman R, Desantis S, Mayer EL, Isakoff S, Dipiro P, Krop IE, Come SE, Weckstein D, et al. A phase II study of bevacizumab in combination with vinorelbine and trastuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2013;139:403–410. doi:10.1007/s10549-013-2551-9.
- Martin M, Makhson A, Gligorov J, Lichinitser M, Lluch A, Semiglazov V, Scotto N, Mitchell L, Tjulandin S. Phase II study of bevacizumab in combination with trastuzumab and capecitabine as first-line treatment for HER-2-positive locally recurrent or metastatic breast cancer. Oncologist. 2012;17:469–475. doi:10.1634/theoncologist.2011-0344.
- Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. doi:10.1200/JCO.2012.44.7912.
- Bergh J, Bondarenko IM, Lichinitser MR, Liljegren A, Greil R, Voytko NL, Makhson AN, Cortes J, Lortholary A, Bischoff J, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–929. doi:10.1200/JCO.2011.35.7376.
- Baselga J, Segalla JG, Roche H, Del Giglio A, Pinczowski H, Ciruelos EM, Filho SC, Gomez P, Van Eyll B, Bermejo B, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30:1484–1491. doi:10.1200/JCO.2011.36.7771.
- Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, Li J, Lou L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–1380. doi:10.1111/j.1349-7006.2011.01939.x.
- Fan M, Zhang J, Wang Z, Wang B, Zhang Q, Zheng C, Li T, Ni C, Wu Z, Shao Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat. 2014;143:141–151. doi:10.1007/s10549-013-2793-6.
- Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007;19:2003–2012. doi:10.1016/j.cellsig.2007.05.013.
- Casella I, Feccia T, Chelucci C, Samoggia P, Castelli G, Guerriero R, Parolini I, Petrucci E, Pelosi E, Morsilli O, et al. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood. 2003;101:1316–1323. doi:10.1182/blood-2002-07-2184.
- Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13:905–919. doi:10.1677/erc.1.01221.
- Weigand M, Hantel P, Kreienberg R, Waltenberger J. Autocrine vascular endothelial growth factor signalling in breast cancer. Evidence from cell lines and primary breast cancer cultures in vitro. Angiogenesis. 2005;8:197–204. doi:10.1007/s10456-005-9010-0.
- Pfister NT, Fomin V, Regunath K, Zhou JY, Zhou W, Silwal-Pandit L, Freed-Pastor WA, Laptenko O, Neo SP, Bargonetti J, et al. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulate VEGFR2 in breast cancer cells. Genes Dev. 2015;29:1298–1315. doi:10.1101/gad.263202.115.
