ABSTRACT
Patients’ responses to breast cancer neoadjuvant chemotherapy (NACT) differ because of heterogeneous tumor characteristics. Reports about NACT progression are sporadic. Here we enrolled 1187 patients who received NACT in our cancer center between January 1, 2007, and December 31, 2016. We analyzed the characteristics and treatments of patients with progressive disease (PD) or non-PD or pathological complete response (pCR). In total, 45 (3.8%) patients had PD. PD patients were associated with a significantly worse disease–free survival (DFS) (hazard ratio (HR) = 3.77; 95% CI, 1.77 to 8.00; P =.001) and overall survival (OS) (HR = 3.85; 95% CI, 1.77 to 8.35; P =.001). For the PD patients, 28 (62.2%) patients received mastectomy immediately after PD, and 17 (37.8%) changed to chemotherapy. DFS and OS exhibited no significant differences between these two salvage therapies. After a change to second chemotherapy, 58.8% (10/17) patients had PD or SD. With the exception of tumor size, pretreatment T stage, and histology type, no other significant differences were noted between PD and pCR patients. Our results demonstrated that PD patients were associated with a significantly worse prognosis. Based on these results, we suggest to give the addition of trastuzumab to HER-2 positive patients instead of changing the chemotherapy regimen and proceeding to surgery instead of further chemotherapy once patients have PD during NACT. Given that some similar characteristics exist between PD and pCR patients, more studies to identify novel molecular markers to predict disease response to NACT should be performed.
Introduction
For larger operable breast cancers, an increasing number of patients undergo neoadjuvant chemotherapy (NACT).Citation1 In addition to improving breast-conservation surgery rates, NACT can make inoperable locally advanced breast cancer operable. Furthermore, NACT can be used as an in vivo test for chemosensitivity by assessing pathologic complete response (pCR), which correlates with longer overall survival (OS) and disease-free survival (DFS).Citation2,Citation3 However, a small number of patients exhibit minimal sensitivity to or even progress on NACT and thus will not benefit from months of treatment, which may make breast-conservation surgery or even mastectomy impossible.Citation4
Numerous studies worldwide, including both in Western countries and Asia, have focused on NACT. However, even in Western countries, the reports about progressive disease (PD) are sporadic. To date, only 2 studies focused on PD.Citation5-Citation7 Much less is known about NACT in Asian patients, and reports on PD in Asian people are not available. The epidemiologic and clinical outcome data of breast cancer women in Asia differ from that in Western populations. Invasive breast cancer in Asia is 30% of that in Northern America and Europe.Citation8 The peak age for breast cancer in Asia is between 40 and 50 years, whereas the peak age is between 60 and 70 years in most Western countries.Citation9 Other differences include race, environmental factors, genetic variation, socioeconomic status, and stage of disease at diagnosis.Citation10 Given that most NACT strategies for breast cancer were established in the United States and Europe, the validity of NACT has been rarely evaluated in Asian patients.Citation11
Although the PD rate is low, the absolute number of PD cases tends to be high given the large population in China and other Asian countries. Consequently, even a small increase in PD may lead to a poor outcome in a large number of patients. Once a patient’s tumor progresses during NACT, different salvage treatment approaches are used, such as surgery, salvage chemotherapy and radiation therapy.Citation7 However, given the low incidence rate of PD, no standard therapy protocol is achieved. Therefore, it is desirable to gain more knowledge regarding the clinical and biological features of PD patients. Once a patient experiences PD, individualized treatment can be used based on tumor characteristics. In the present study, we evaluated Chinese PD patients’ characteristics and treatments and discerned whether PD characteristics differed between Asian and Western countries. Thus, when PD occurs, we will have protocols available to maximize salvage treatments.
Methods
Study population
All of the patients included in this retrospective study were selected from patients with invasive breast cancer treated with NACT at the Zhejiang Cancer Hospital between January 1, 2007, and December 31, 2016. Patients were excluded who were male, had distant metastasis at the time of initial diagnosis, had inflammatory breast cancer, were treated synchronously for another primary cancer, underwent excisional biopsies of the primary breast tumor before the start of chemotherapy, or had bilateral primary breast cancer.
Response criteria
The assessment of tumor burden was based on the Revised Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1)Citation12 based on the following definitions. Tumors and lymph nodes were scanned with ultrasound system prior to chemotherapy treatment, every two cycles of treatment or any point of time if necessary, and prior to surgery. PD is defined as at a minimum of a 20% increase in the sum of diameters of target lesions. Partial response (PR) is defined as a minimum of a 30% decrease in the sum of diameters of target lesions. Stable disease (SD) is defined as neither adequate decrease to qualify for PR nor sufficient increase to qualify for PD. Clinical complete response (cCR) is defined as disappearance of all target tumors by clinical evaluation. Pathologic complete response (pCR) is defined as the absence of an invasive tumor and in-situ cancer in the breast and axillary nodes by histopathologic evaluation.
Statistical analysis
The medical record was reviewed to obtain data on patient disease, treatment characteristics, and clinical outcomes using descriptive statistics. Continuous variables were reported as the means and medians with standard deviation, and minimum and maximum ranges were also reported. Categorical variables were reported as frequency counts and proportions. T test for continuous variables and Fisher’s exact test or χ 2 test for categorical variables were used for comparisons between groups. OS was defined as the time from diagnosis to death. DFS was defined as the time from diagnosis to recurrence (local, regional or distant) or death, whichever occurred first. Patients without an event were censored at the date of last follow-up. OS and DFS for all the patients were estimated using the Kaplan–Meier method. Survival prognostic factors were examined using the log-rank test and Cox proportional hazards regression analysis. The probabilities of progression were examined by logistic regression. Both factors above were analyzed in univariate and multivariate fashion. A forward stepwise method was used to obtain the final model. A hazard ratio (HR) or odds ratio (OR) for each prognostic factor was determined with a 95% CI. P < .05 was considered to be statistically significant. Analyzes were conducted using SPSS® version 23.
Results
Patient characteristics
A total of 1187 women patients who received NACT were enrolled in the study. Baseline patient characteristics in each group are listed in . Among these patients, 45 (3.8%) patients had PD, 142 (12.0%) patients had SD, 873 (73.5%) patients had PR and 127 (10.7%) patients had cCR. Patients with PD were compared with patients without PD (including SD, PR and cCR).
Table 1. Pretreatment and post-treatment patient characteristics.
Median ages were 48 years (range, 21 to 65 years) for patients with PD and 49 years (range, 23 to 76 years) for patients without PD. Of the PD group 30 (67%) were premenopausal compared with 646 (57%) of the non-PD cases. There was no difference in body mass index (BMI) and family history of cancer between the two groups. In the PD group, 4 (8. 9%) tumors were T1, 19 (42.2%) were T2, 13 (28.9%) were T3, and 9 (20.0%) were T4. In the non-PD group, there were 77 (6.7%), 760 (66.5%), 159 (13.9%) and 146 (12.8%) patients with T1, T2, T3 and T4 disease, respectively (P = .006). Although there was no difference in tumor size between groups (39.0 mm vs. 34.5 mm, P = .133), the PD groups had larger tumors compared with the non-PD group. Additionally, no significant difference was observed between the two groups regarding lymph node status and American Joint Committee on Cancer (AJCC) stage. However, patients in the PD group were more likely to have stage IIIA-IIIC disease compared with patients in the non-PD group (55.6% vs. 45.9%, respectively). Regarding pretreatment histopathologic characteristics, the median ki-67 score was 50 in the PD group, which was increased compared with the non-PD group (median score was 30, P = .01).
In terms of tumor subtypes, the PD group was more likely to have estrogen receptor (ER)-negative (n = 31, 68.9%) and progesterone receptor (PR)-negative tumors (n = 32, 71.1%) compared with the non-PD group (with n = 465, 41.5% in ER-, P = .001 and n = 656, 58.5% in PR-, P = .016). Human epidermal growth factor receptor 2 (HER-2) status did not differ between the two groups. Histology significantly differed between the two groups. In addition to ductal and lobular histology, PD tumors exhibited greater proportions of mixed (5, 11.1%) and other (4, 8.9%) types of histology, such as metaplastic carcinomas (MPCs), invasive micropapillary carcinoma, signet ring cell carcinoma, neuroendocrine carcinoma, and sarcoma (such as fibrous histiocytoma and liposarcoma). In the non-PD group, only 21(1.8%) mixed types and 23 (2.0%) other types were observed. Although the nuclear grade was only determined in 372 (31.4%) patients, the PD group was more likely to present with grade 3 tumors (n = 14 (31.1%)) compared with the non-PD group (n = 117 (10.2%), P < .001).
Post-treatment pathologic results
The characteristics of tumors after chemotherapy also are presented in . Pathologic tumor size (median tumor size, 55.0 mm for PD and 20.0 mm for non-PD, P < .001), T stage (P < .001), N stage (P = .003) and AJCC stage (P < .001) all exhibited significant differences between the two groups. Compared with non-PD patients, PD patients were more likely to have T3-4, N2-3, and IIIA-IV stage tumors (57.8% vs. 8.8%, 51.1% vs. 39.5%, 64.5% vs. 41.3%, respectively). Although lymphovascular invasion did not differ between the two groups (P = .178), the condition was more prevalent in the PD group compared with the non-PD group with 20 (44.4%) cases in the PD group and 396 (34.7%) cases in the non-PD group. Chemotherapy regimens based on anthracycline and trastuzumab did not differ between the two groups (P = .249, and 1.000 respectively). Chemotherapy regimens based on taxane (P < .001) exhibited differences. In total, 1040 (91.1%) of non-PD patients received taxane-based chemotherapy, whereas only 31 (68.9%) of PD patients received taxane-based chemotherapy. Both PD patients and non-PD patients had similar breast-conserving therapy (BCT) and mastectomy rates. In total, 1 (2.2%) patient in the PD group and 33 (2.9%) patients in the non-PD group had BCT. In total, 44 (97.8%) patients in the PD group and 1109 (97.1%) patients in the non-PD group had a mastectomy.
PD patients’ therapy
With regard to the treatments administered, 37(82.2%) patients had PD while receiving anthracycline-based therapy, and 31(68.9%) patients had PD while receiving taxane (T)-based therapy. Fourteen (31.1%) patients used a NACT regimen based on anthracycline during PD, and 23 (51.1%) patients used a NACT regimen based on anthracycline and cyclophosphamide(EC) during PD. Two (4.4%) patients used a NACT regimen based on taxane during PD. Five (11.1%) patients had SD during anthracycline and experienced PD after switching to taxane. One (2.2%) patient had SD during the EC regimen but experienced PD after changing to a vinorelbine and capecitabine regimen. Of the PD patients, 9 (20%) received EC-T regimen and of these 5 had SD during anthracycline and experienced PD after switching to taxane. Four patients experienced PD while taking an anthracycline regimen, and 3 patients experienced a second PD after switching to taxane. One patient experienced PR after switching to taxane. Sixteen (35.6%) PD patients exhibited HER-2 overexpression (HER-2+), and only 2 (12.5%) HER-2+ patients received trastuzumab therapy during progression. Three additional HER-2+ patients added trastuzumab after PD. Of these patients, 2 patients exhibited PR, and 1 patient exhibited cCR, which was confirmed as pCR after surgery.
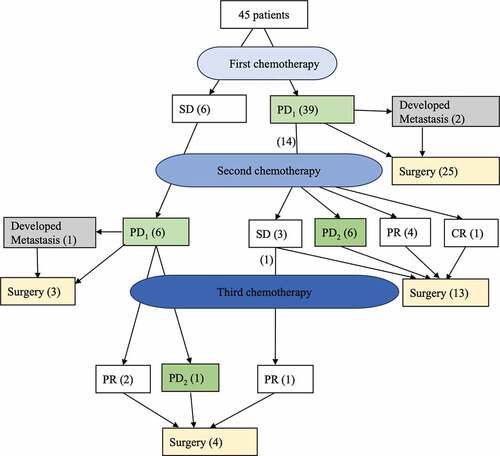
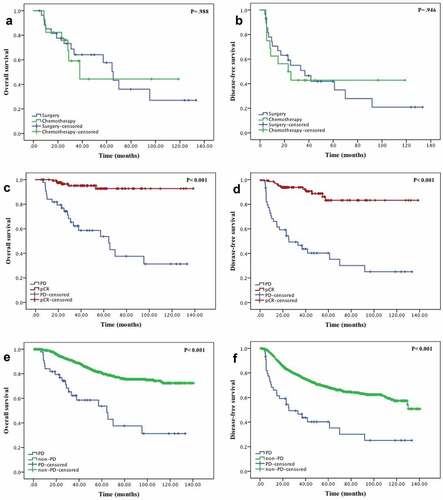
Progression was diagnosed after one cycle (n = 1), two cycles (n = 17), three cycles (n = 8), four cycles (n = 11), five cycles (n = 2), six cycles (n = 3) and eight cycles (n = 3). The median number of cycles was 3. Tumor progression was observed in 41 (91.1%) patients. Nodal progression was observed in 5 (11.1%) patients, and distant metastasis was observed in 3 (6.7%) patients. As shown in , 28 (62.2%) patients received a mastectomy immediately after PD, and 17 (37.8%) changed chemotherapy. After changing to a second chemotherapy, 7 patients still exhibited PD, 3 patients had SD, 7 patients had PR, and 1 patient exhibited a pCR after surgery. Compared with the 28 patients who switched to surgery immediately after PD and 17 patients who switched to a different chemotherapy regimen, no statistically significant differences in OS and DFS were between these two salvage therapies (,).
Figure 2. (a) Overall survival of the two salvage therapies (surgery and chemotherapy). (b) Disease-free survival of the two salvage therapies (surgery and chemotherapy. (c) Overall survival and (d) disease-free survival of patients with progression disease(PD) and pathological complete response (pCR). (e) Overall survival and (f) disease-free survival of patients with PD and non-PD.
Comparison of tumor characteristics between PD and pCR patients
Patients with PD were also compared with patients with pCR after surgery (). A pathologic complete response in both the breast and axilla occurred in 133 (11.2%) of all NACT patients (including one PD patient who changed chemotherapy after PD was noted and exhibited pCR after surgery). No significant differences were noted in age, menopausal status, family history of cancer, body mass index (BMI), prechemotherapy T stage, prechemotherapy AJCC stage, nuclear grade, Topoisomerase (TOPO) II, Ki-67 score, ER status, PR status, Her-2 status, and surgery type. The following pretreatment tumor features were different: pretreatment tumor size (with median size, 38 mm vs. 31 mm, p = .015) and pretreatment T stage (p < .001). In total, 48.9% of PD patients had pretreatment T3-4 tumors, whereas 72.2% of pCR patients had pretreatment T2 tumors. Histological type also differed (p < .001). Only 34 (75.6%) tumors in PD patients were classified as ductal type, whereas 127 (95.5%) pCR patients exhibited this type of disease. Pretreatment chemotherapy features that exhibited significant differences included taxane-based regimen (p < .001) and trastuzumab-based regimen (p < .001). Of the 116 HER2+ patients receiving trastuzumab-containing regimens, 2 (1.72%) had PD, and 40 (34.48%) had pCR. Of the 317 HER2+ patients receiving NACT without trastuzumab, 14 (4.42%) had PD and 24 (7.57%) had pCR (). Additionally, tumor subtypes were evaluated (). Of the 234 patients with hormone receptor (HR)+ and HER-2+ tumors, 4 (1.71%) patients had PD and 32 (13.68%) patients had pCR. Of the 383 patients with HR+ and HER-2- disease, 9 (2.35%) patients had PD and 23 (6.01%) patients had pCR. Of the 198 patients with HR- and HER-2+ tumors, 12 (6.06%) patients had PD and 32 (16.16%) patients had pCR. Of the 207 patients with HR- and HER-2- tumors, 12 (5.80%) patients had PD and 30 (14.49%) patients had pCR. Patients with pCR exhibited significantly longer OS and DFS compared with patients with PD (,)
Table 2. Patients’ and tumor characteristics of PD and pCR.
Predictors of PD
Predictors of tumor progression are listed in . In univariate analysis, predictors of PD included prechemotherapy tumor size (P = .044), prechemotherapy T stage (P = .009), ER negative (P = .001), PR negative (P = .009), high nuclear grade (P = .001), high Ki-67 score (P = .001), mixed or other histology types (P < .001), and non-taxane based regimen (P < .001). In multivariate analysis, significant predictors of progression including nuclear grade 3 (OR = 5.05; 95% CI, 1.18 to 21.61; P = .029) and high Ki-67 score (OR = 1.03; 95% CI, 1.00 to 1.06; P = .047).
Table 3. Predictors of PD.
Survival analysis
The median follow-up time of OS and DFS was 45.47 months (range, 0.80 to 140.53 months). The OS rates determined by Kaplan–Meier analysis for the PD and non-PD groups are presented in . The median OS time was 64.40 months in patients with PD and was not achieved in the non-PD group. Factors significantly affecting OS (listed in ) in univariate analysis were PD (HR 3.85, P < .001), BMI (HR 0.95, P = .015), prechemotherapy size (HR = 1.02, P < .001), prechemotherapy T3 and T4 stage (with T3 HR = 3.12, P = .002 and T4 HR = 2.94, P = .003), prechemotherapy N3 stage (HR 4.17, P < .001), prechemotherapy IIIB/IIIC stage (HR = 4.23, P < .001), ER positive (HR = .59, P < .001), PR positive (HR = .61, P = .001), lymphovascular invasion (HR = 2.09, P < .001), nuclear grade 3 (HR = 10.88, P = .019) and trastuzumab therapy (HR = .39, P = .009). In multivariate analysis, PD (HR = 3.85, P = .001), prechemotherapy size (HR = 1.01, P = .035), prechemotherapy N3 stage (HR = 3.07, P = .032), ER positive (HR = .42, P = .001), and trastuzumab therapy (HR = 0.10, P = .023) were predictive of OS. The DFS rates based on Kaplan-Meier analysis for the PD and non-PD groups are presented in . The median DFS time was 25.30 months in the patients with PD and was not achieved in the non-PD group. Factors affecting DFS (listed in ) in univariate analysis were PD (HR 3.00, P < .001); BMI (HR = .96, P = .036); prechemotherapy size (HR = 1.02, P < .001); prechemotherapy T3 and T4 stage (with T3 HR = 2.89, P < .001 and T4 HR = 2.55, P = .001); prechemotherapy N1, N2 and N3 stage (with N1 HR = 1.52, P = .041, N2 HR = 1.74, P = .016 and N3 HR = 4.70, P < .001); prechemotherapy IIB/IIIA and IIIB/IIIC stage (with IIB/IIIA HR = 2.01, P = .002 and IIIB/IIIC HR = 4.57, P < .001); ER positive (HR = .68, P < .001); PR positive (HR .66, P < .001); lymphovascular invasion (HR 1.99, P < .001); nuclear grade 3 (HR 4.13, P = .018); Ki-67 index score (HR = 1.01, P = .028); and trastuzumab therapy (HR = .55, P = .006). In multivariate analysis, factors that were predictive for DFS included PD (HR = 3.77, P = .001), prechemotherapy size (HR 1.02, P = .010), prechemotherapy N3 stage (HR 3.14, P = .040), ER positive (HR = .47, P = .002) and lymphovascular invasion (HR = 1.90, P = .008).
Table 4. Univariate and multivariate effects of potentially prognostic factors on OS and DFS.
Discussion
In this study, we described predictors of progression during NACT in China. To our knowledge, this is the largest study to date on PD during NACT in Asian patients. In China, statistical results about NACT are rarely studied or recorded by researchers, and considerably less is known about NACT in China compared with Western countries. Therefore, we collected detailed information on factors that might influence NACT response.
PD occurs in 3 – 6% in NACT cases.Citation5,Citation7 This finding is in accordance with our study. The pretreatment predictors of disease progression include large tumor size, T stage, ER and PR negativity, high Ki-67 scores, and high nuclear grade. All these results are similar to the study performed at the MD Anderson Cancer Center by Caudle et al.Citation5 In contrast to other reports, our study discovered that in addition to ductal and lobular histology, PD tumors exhibited increased proportions of mixed and other special types of histology compared with the non-PD group as mentioned above. Compared with invasive ductal carcinoma and invasive lobular carcinoma, these special types of breast carcinoma exhibit a more aggressive behavior. These carcinomas exhibit an increased propensity of local and distant recurrence and poorer overall survival and disease-free survival regardless of similar age and disease stages at presentation and the relative proportion of these special carcinoma components.Citation13-Citation16 For instance, invasive micropapillary carcinoma of the breast exhibited increased frequencies of lymphovascular invasion and lymph nodal metastasis.Citation17 Merino et al. reported that primary signet ring cell carcinoma-related death occurred in 60% of 24 patients within 7 years.Citation16 Standard chemotherapy is typically unsuccessful in the treatment of these patients.Citation13,Citation18,Citation19 The study by Tanabe et al.Citation20 revealed that 82% of MPCs experienced PD during NACT. Hennessy et al.Citation21 reported that metaplastic sarcomatoid carcinomas were less sensitive to primary anthracycline/taxane-based chemotherapy compared with that reported in conventional breast adenocarcinoma. Chen et al.Citation22 demonstrated that none of the MPCs patients who received anthracycline, vinorelbine, or cyclophosphamide-based chemotherapy responded, and only 17.6% of patients who received taxane-based chemotherapy exhibited a partial response. The above information potentially suggests that therapies other than regular NACT may be suggested when we encounter these special types of histology.
Currently, there is no standard treatment protocol for PD given the low rate and the rarity of large prospective clinical studies. In our study, 28 patients switched to surgery immediately after PD, and 17 patients switched to a different chemotherapy. For the 17 patients who changed chemotherapy regimens, 10 (58.8%) patients continued to exhibit PD or SD (). Furthermore, the addition of taxane to an anthracycline-based regimen, particularly when added sequentially, was confirmed to result in increased response rates, DFS and OS in breast cancer patients.Citation23,Citation24 In this study, after experiencing no response to the EC regimen (either SD or PD), 9 patients added taxane sequentially. Only 1 patient had PR, and the remaining 8 patients continued experienced PD. All these results suggest that patients whose tumors do not exhibit a response to first-line chemotherapy may not exhibit further tumor size shrinkage as a result of any further changes or additions to the chemotherapy regimen. Other neoadjuvant trials also confirmed this phenomenon. Both the GeparTrio phase III trialCitation25 and the Aberdeen trialCitation26,Citation27 demonstrated that compared with the early responders to chemotherapy, switching the early chemotherapy of nonresponders to a different chemotherapy regimen before surgery did not result in the achievement of pCR. Furthermore, we did not observe statistically significant differences in either DFS or OS between patients undergoing surgery or changing chemotherapy after PD (,). Based on all these results, we conclude that once a patient experiences PD, surgery should be suggested instead of further chemotherapy.
Breast cancer is a clinically heterogeneous disease; thus, not all breast cancer patients respond equally to chemotherapy and exhibit the same prognoses. Most patients experience clinical responses, with the rate ranging from 50 to 90%. A small number of patients experience pCR after surgery, and rates ranged from 2 to 27%. An even smaller number of patients (approximately 2-5%) experience PD during treatment.Citation28,Citation29 This study implied that tumor characteristics that correlated with progression were also associated with the likelihood of a complete response to NACT.Citation5 However, PD and pCR had two polarizing prognoses. The long-term data from National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 demonstrated that the achievement of pCR in both breast and axillary nodes after NACT clearly predict favorable long-term outcomes.Citation4 In contrast, once patients experience PD, they may incorrectly perceive a risk in delaying surgery or even losing the opportunity for operation. The characteristics and clinical outcomes of PD compared with pCR have not been reported to date. In this study, we compared the characteristics of these two groups of patients. As presented in , our study revealed no differences in age, menopausal status, family history of cancer, BMI, pretreatment N stage, AJCC stage, nuclear grade, TOPO II, ER expression, PR expression, HER-2 expression, anthracycline-based chemotherapy and types of surgery between PD and pCR patients. Chemotherapy regimens based on taxane exhibited significance (P < .001) (also see the difference in the comparison between PD and non-PD group) because PD occurred within 4 cycles of NACT in 37 (82.2%). These patients may transfer to surgery or other salvage chemotherapies and thus do not use taxane-based sequential therapy. A neoadjuvant combination of trastuzumab and chemotherapy resulted in an increased pCR rate in HER-2+ primary breast cancer.Citation30 In this study, a chemotherapy regimen based on Trastuzumab also exhibited significance (P < .001). As shown in , of the HER-2+ patients not receiving trastuzumab, 4.42% had PD and 7.57% had pCR. However, of the HER-2+ patients receiving trastuzumab, only 1.72% had PD and 34.48% had pCR. Moreover, after administering trastuzumab to HER-2+ PD patients, the patients experienced further PR, and some patients even achieved pCR after surgery. This finding suggests that the addition of trastuzumab to NACT may benefit HER-2+ patients. Once a HER-2+ patient experiences PD during NACT, the addition of trastuzumab instead of changing the chemotherapy regimen might result in an increased tumor response. It is noteworthy that although PD patients presented with a significantly higher Ki-67 index compared with the non-PD group (P = .01, as shown in ), no significant difference in Ki-67 index values are noted between PD and pCR patients. Both groups exhibit a median Ki-67 index > 40%. Ki-67 is present in all proliferating cells and serves as a proliferation marker.Citation31 Several retrospective studies confirmed that an increased Ki67 index is associated with an increased pCR rate.Citation32,Citation33 Simultaneously, some studies reported that high Ki67 correlated with progressive disease.Citation5 Our results are consistent with previous studies, suggesting that patients with a risk of progression are also more likely to exhibit a complete response to NACT. This finding suggests that high cell proliferation might exhibit a bilateral relation to chemosensitivity. However, this result is not as obvious in reports that suggest it directly reflects achieving pCR.Citation34
Different subsets of breast cancers have different degrees of chemotherapy sensitivity.Citation35 In this report, we studied the response of different tumor subtypes to therapy. As shown in , the HR-/HER-2+ subgroups exhibited the highest rates of both PD and pCR. The PD rate was lowest in the HR+/HER-2+ subgroup, and the pCR rate was lowest in the HR+/HER-2- subgroup. This effect has also been observed in the study by Caudle et al.Citation5 Although no additional studies about PD in subset groups were reported, the pCR rate was the highest in the HR-/HER2+ subset and lowest in HR+/HER-2- subset as confirmed in the I-SPY 1 TRIAL,Citation33 Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization (Neo ALTTO),Citation36 and Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation (NeoSphere).Citation37 When combining previous studies with our study, we conclude that patients who are at greatest risk of PD are more likely to exhibit pCR. However, compared with pCR patients, PD patients are more likely to present with a large tumor size and more advanced stages of disease at diagnosis. This finding suggests that we should be aware that HR-/HER-2+ subgroup tumors with larger tumor size or advanced T stages exhibit PD during NACT. Given that most PD occurred during the first few chemotherapy cycles, frequent and comprehensive inspection is required to prevent PD. Given that DFS and OS curves differed significantly between PD and pCR patients (P < .001), there is an urgent need to identify novel molecular markers to predict disease response to NACT, thus allowing more individuals to obtain a pCR and facilitating the identification of individuals with low or no benefit to NACT.
Compared with most Western countries, one striking feature about Asian patients is the age at diagnosis. The median age in our study was 49, and 57.3% of patients were premenopausal. This finding is in accordance with other studies in Asian countries, with the rage between 40–50 years. In contrast, the peak age in Western countries is 10 years older, and greater than half of patients were perimenopausal/postmenopausal.Citation5,Citation9,Citation38 Among patients who received NACT in our cancer center, 65.6% were T2 stage and 57.7% were N1 stage. Clinical stage at diagnosis was IIB-IIIA in 65.2% and IIIB-IIIC in 22.3% patients. The diagnosis at later stages compared with Western countries may be attributed to poor health awareness and education, lack of good screening instruments and medical examination per-year for early diagnosis and treatment. Moreover, patients in our country exhibit fear and misunderstanding of cancer treatment and outcomes. Patients become pale at the mention of cancers and therefore miss the best time for treatment. In our study, the PD, SD and pCR rates are similar to the randomized trial of NSABP B-18,Citation39 which demonstrated PD, SD and pCR rates of 3%, 17% and 13%, respectively. However, the cCR rate in NSABP B-18 (36%) was increased compared with our study. This finding may be attributed to the fact that the B-18 trial only enrolled patients with operable and palpable breast cancer (T1-3 N0-1 M0). In contrast, in our study, greater than 22.3% of patients were inoperable. In our study, the 5-year OS and DFS was similar to that of NSABP B-18. The results presented above demonstrated that although differences in age, menopausal status, race, genetic variation, lifestyle, environmental factors, and stage of disease at diagnosis between Asian and Western countries as reported previously [10], the validity of NACT in our country was similar to that of Western countries. Of note, our NACT regimens and doses were based on Western standards. However, compared with Western countries, our patients presented with a smaller BMI index and later tumor stages. This finding suggests that individual treatment is necessary to obtain a better prognosis. The other striking data from our center is that the breast conservation rate after NACT was only 2.9%, and most patients experienced mastectomy (97.1%). The BCT rate was considerably reduced compared with other Western cancer centers. Specifically, the BCT rate at the MD Anderson Cancer Center was greater than 30%.Citation40 This difference may be related to a combination of factors, including socioeconomic factors in our country, a lack of radiotherapy equipment, patient-surgeon interactions, cognition of breast-conserving surgery, and difficulty in achieving negative margins of the primary tumor after NACT.
Although the data presented in our cancer center cannot represent the entire country, these data provide some useful insights into PD patients in Asian countries. However, some limitations should be noted. First, this is a retrospective study, and the number of patients enrolled was small, resulting in an even small number of PD patients. Furthermore, some patient information was incomplete, such as Ki-67 index, nuclear grade and Her-2 status. Thus, it was difficult to properly assess these patients. Thus, the results should be interpreted with caution, and further well-designed studies are urgently needed.
Conclusion
In summary, we conclude that predictors of progression during NACT include prechemotherapy size, prechemotherapy T stage, HR-negative, histology with mixed or other types, nuclear grade 3, and high Ki-67 score. In multivariate analysis, significant predictors of progression including nuclear grade 3 and high Ki-67 score. PD patients were associated with significantly worse DFS and OS. Once patients experience PD during NACT, the addition of trastuzumab to HER-2+ patients instead of changing the chemotherapy regimen and proceeding to surgery or further chemotherapy is suggested. We also demonstrate that patients who are at greater risk of PD are more likely to experience pCR. We should be aware that HR-/HER-2+ subgroup tumors with larger tumor sizes or advanced T stages are likely to exhibit PD during NACT. Frequent and comprehensive inspection is needed to prevent PD in early chemotherapy cycles. The search for biological markers for PD at the molecular level is urgent to select the best treatments for patients.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Kaufmann M, Von Minckwitz G, Bear H, Buzdar A, McGale P, Bonnefoi H, Colleoni M, Denkert C, Eiermann W, Jackesz R. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18:1927–1934. doi:10.1093/annonc/mdm201.
- Kaufmann M, Von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516. doi:10.1245/s10434-011-2108-2.
- Kaufmann M, Von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, Howell A, Costa SD, Beuzeboc P, Untch M. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21:2600–2608. doi:10.1200/JCO.2003.01.136.
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi:10.1200/JCO.2007.15.0235.
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, Liu P, Pusztai L, Symmans WF, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Predictors of tumor progression during neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:1821–1828. doi:10.1200/JCO.2009.25.3286.
- Caudle AS, Gonzalez-Angulo AM, Hunt KK, Pusztai L, Kuerer HM, Mittendorf EA, Hortobagyi GN, Meric-Bernstam F. Impact of progression during neoadjuvant chemotherapy on surgical management of breast cancer. Ann Surg Oncol. 2011;18:932–938. doi:10.1245/s10434-010-1390-8.
- Raphael J, Paramsothy T, Li N, Lee J, Gandhi S. A single-institution experience of salvage therapy for patients with early and locally advanced breast cancer who progress during neoadjuvant chemotherapy. Breast Cancer Res Treat. 2017;163:11–19. doi:10.1007/s10549-017-4167-y.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015:136(5):E359–386. doi:10.1002/ijc.29210.
- Leong SP, Shen -Z-Z, Liu T-J, Agarwal G, Tajima T, Paik N-S, Sandelin K, Derossis A, Cody H, Foulkes WD. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–2324. doi:10.1007/s00268-010-0683-1.
- Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu M-C, Martin M, Namer M. The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer. 2005;6:391–401. doi:10.3816/CBC.2005.n.043.
- Bhoo-Pathy N, Yip CH, Hartman M, Saxena N, Taib NA, Ho GF, Looi LM, Bulgiba AM, van der Graaf Y, Verkooijen HM. Adjuvant! Online is overoptimistic in predicting survival of Asian breast cancer patients. Eur J Cancer. 2012;48:982–989. doi:10.1016/j.ejca.2012.01.034.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247.
- Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, Abraham S, Nayeemuddin K, Hunt K, Wu Y. Invasive neuroendocrine carcinoma of the breast: a distinctive subtype of aggressive mammary carcinoma. Cancer. 2010;116:4463–4473. doi:10.1002/cncr.25352.
- Chen L, Fan Y, Lang R, Guo X, Sun Y, Fu L. Diagnosis and prognosis study of breast carcinoma with micropapillary component. Zhonghua Bing Li Xue Za Zhi= Chin J Pathol. 2007;36:228–232.
- Wu X, Zhang Z, Li X, Lin Q, Chen G, Lu J, Zeng Y, Hu D, Huang K, Lin Z. Poorer prognosis of primary signet-ring cell carcinoma of the breast compared with Mucinous carcinoma. PLoS One. 2016;11:e0162088. doi:10.1371/journal.pone.0162088.
- Merino MJ, Livolsi VA. Signet ring carcinoma of the female breast: a clinicopathologic analysis of 24 cases. Cancer. 1981;48:1830–1837. doi:10.1002/1097-0142(19811015)48:8<1830::AID-CNCR2820480821>3.0.CO;2-H.
- Yang Y-L, Liu -B-B, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: an update. Arch Pathol Lab Med. 2016;140:799–805. doi:10.5858/arpa.2016-0040-RA.
- Kanat O, Kilickap S, Korkmaz T, Oven BBU, Canhoroz M, Cubukcu E, Tolunay S, Evrensel T, Manavoglu O. Primary small cell carcinoma of the breast: report of seven cases and review of the literature. Tumori J. 2011;97:473–478. doi:10.1177/030089161109700410.
- Lim SZ, Ong KW, Tan BKT, Selvarajan S, Tan PH. Sarcoma of the breast: an update on a rare entity. J Clin Pathol. 2016;69(5):373–381. doi:10.1136/jclinpath-2015-203545.
- Tanabe Y, Tsuda H, Yoshida M, Yunokawa M, Yonemori K, Shimizu C, Yamamoto S, Kinoshita T, Fujiwara Y, Tamura K. Pathological features of triple‐negative breast cancers that showed progressive disease during neoadjuvant chemotherapy. Cancer Sci. 2017;108:1520–1529. doi:10.1111/cas.13274.
- Hennessy B, Giordano S, Broglio K, Duan Z, Trent J, Buchholz T, Babiera G, Hortobagyi G, Valero V. Biphasic metaplastic sarcomatoid carcinoma of the breast. Ann Oncol. 2006;17:605–613. doi:10.1093/annonc/mdl006.
- Chen I, Lin C, Huang C, Lien H, Hsu C, Kuo W, Lu Y, Cheng A. Lack of efficacy to systemic chemotherapy for treatment of metaplastic carcinoma of the breast in the modern era. Breast Cancer Res Treat. 2011;130:345. doi:10.1007/s10549-011-1686-9.
- Bear HD, Anderson S, Smith RE, Jr CE G, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi:10.1200/JCO.2005.04.1665.
- Goble S, Bear HD. Emerging role of taxanes in adjuvant and neoadjuvant therapy for breast cancer: the potential and the questions. Surg Clin North Am. 2003;83:943–971. doi:10.1016/S0039-6109(03)00071-9.
- von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–3630. doi:10.1200/JCO.2012.45.0940.
- Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, Ah-See AK, Eremin O, Walker LG, Sarkar TK. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20:1456–1466. doi:10.1200/JCO.2002.20.6.1456.
- Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, Smith I, Walker LG, Eremin O. Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer. 2002;3:S69–S74. doi:10.3816/CBC.2002.s.015.
- Alba E, Calvo L, Albanell J, De la Haba J, Arcusa Lanza A, Chacon J, Sanchez-Rovira P, Plazaola A, Lopez Garcia-Asenjo J, Chemotherapy BB. (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23:3069–3074. doi:10.1093/annonc/mds132.
- Berruti A, Brizzi MP, Generali D, Ardine M, Dogliotti L, Bruzzi P, Bottini A. Presurgical systemic treatment of nonmetastatic breast cancer: facts and open questions. Oncologist. 2008;13:1137–1148. doi:10.1634/theoncologist.2008-0162.
- Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, Camara O, Müller V. du Bois A, Kühn T. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2–overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357.
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi:10.1002/ijc.2910310104.
- Denkert C, Loibl S, Müller B, Eidtmann H, Schmitt W, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J. Ki67 levels as predictive and prognostic parameters in pretherapeutic breast cancer core biopsies: a translational investigation in the neoadjuvant GeparTrio trial. Ann Oncol. 2013;24:2786–2793. doi:10.1093/annonc/mdt350.
- Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, Hudis C, Gray JW, Perou C, Yau C. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL—CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242. doi:10.1200/JCO.2011.39.2779.
- Alba E, Lluch A, Ribelles N, Anton-Torres A, Sanchez-Rovira P, Albanell J, Calvo L, JAL G-A, Palacios J, Chacon JI. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. Oncologist. 2016;21:150–155. doi:10.1634/theoncologist.2015-0312.
- Lee JK, Coutant C, Kim Y-C, Qi Y, Theodorescu D, Symmans WF, Baggerly K, Rouzier R, Pusztai L. Prospective comparison of clinical and genomic multivariate predictors of response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2010;1078–0432:CCR-09-2247.
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, De Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi:10.1016/S0140-6736(11)61847-3.
- Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, Lluch A, Staroslawska E, de la Haba-rodriguez J, Im S-A. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi:10.1016/S1470-2045(11)70336-9.
- Li J, Zhang BN, Fan JH, Pang Y, Zhang P, Wang SL, Zheng S, Zhang B, Yang HJ, Xie XM, et al. A Nation-Wide multicenter 10-year (1999–2008) retrospective clinical epidemiological study of female breast cancer in china. BMC Cancer. 2011;11:364. doi:10.1186/1471-2407-11-364.
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from national surgical adjuvant breast and bowel project B-18. JNCI Monogr. 2001;2001:96–102. doi:10.1093/oxfordjournals.jncimonographs.a003469.
- Mazouni C, Peintinger F, Wan-Kau S, Andre F, Gonzalez-Angulo AM, Symmans WF, Meric-Bernstam F, Valero V, Hortobagyi GN, Pusztai L. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25:2650–2655. doi:10.1200/JCO.2006.08.2271.