ABSTRACT
Background
Studies showed that microRNAs (miRNAs) are important regulators in drug resistance. The current study investigated the role of miR-185-3p and its predicted target gene AQP5 in 5-FU-insensitive colorectal cancer (CRC) cells.
Methods
Quantitative real-time polymerase chain reaction (qRT-PCR) and Spearman’s correlation analysis were conducted to determine the correlation of expression levels of miR-185-3p and AQP5 from CRC tissues. HCT-116 and HCT-8 cells were treated by gradient concentration of 5-FU to construct 5-FU-resistant CRC model. The inhibition and viability of 5-FU-resistant cells were detected by MTT assay, and cell migration and invasion ability were determined by wound healing and transwell assay. The expressions of miR-185-3p and AQP5 were measured by qRT-PCR. StarBase and dual-luciferase reporter assay were used to predict and confirm the interaction between miR-185-3p and AQP5. Further experiments were performed to explore the function of miR-185-3p in 5-FU-resistant cells through regulating aquaporin-5 (AQP5). The levels of EMT-associated markers and AQP5 were determined by conducting Western Blot and qRT-PCR.
Results
We found that 5-FU-resistant CRC cells showed a lower inhibition rate, and higher migration and invasion abilities. MiR-185-3p was low-expressed in CRC tissues and 5-FU-resistance cells, and it targeted and regulated the expression of AQP5, which was found up-regulated in CRC and 5-FU-resistance CRC cells (r = −0.29, P < .05). Furthermore, miR-185-3p mimic enhanced the chemo-sensitivity of 5-FU-resistant cells, while overexpressed AQP5 reversed such an effect produced by miR-185-3p mimic.
Conclusion
MiR-185-3p mimic enhances the chemosensitivity of CRC cells via AQP5. Our research provides a potential therapeutic target for 5-FU-resistant CRC cells.
Introduction
Drug resistance remains an obstacle in cancer treatment. Though multiple mechanisms underlying drug resistance in cancers, such as improved DNA repair capacity,Citation1 defects in the DNA damage response,Citation2,Citation3 anti-apoptosis,Citation4 pro-migration,Citation5 pro-proliferation effects,Citation6 autophagy,Citation7 tumor-associated compensatory pathways,Citation8 and changes in drug transport,Citation9 have been proposed, the exact mechanism of drug resistance in cancer is highly complex and has not been fully understood yet. Therefore, discoveries of new drug sensitivity-associated targets and effective drug combinations are considered as effective and promising strategies for improving the survival rate of cancer patients.Citation10
Colorectal cancer (CRC), which has a high incidence and mortality rate,Citation11 also has drug resistance. 5-FU suppresses DNA replication and transcription by blocking the activity of thymidylate synthase (TS), thereby causing cell cycle arrest and apoptosis. Based on such characteristics, 5-fluorouracil (FU) treatment is a commonly used therapeutic strategy for treating CRC or other cancers; however, the efficacy of 5-FU in the treatment of CRC is greatly limited by drug resistance.Citation12 A study showed that up-regulation of MASTL induced by 5-FU in colon cancer may contribute to chemo-resistance of the cancer cells.Citation13 A recent research indicated that reducing caspase cascade activation, ER stress responses, and immune response by lipid droplet accumulation is a novel mechanism underlying cell-death resistance to 5-FU.Citation14 Thus, to overcome and understand the complex mechanism of drug resistance in CRC, more basic researches are required to be conducted on the pathological level.
Accumulating evidence showed that microRNAs (miRNAs) are important regulators during the development of drug resistance.Citation15 Recent research indicated that miRNA-552 deficiency contributes to 5-FU resistance in CRC.Citation16 Regorafenib inhibits the development of drug-resistant cancer stem-like cells and tumorigenesis via targeting miR-34a.Citation17 MiR-195-5p-mediated inhibition of notch signaling is involved in stemness and chemoresistance in CRC.Citation18 The loss of expressions of miR-181a, miR-135a, and miR-302 c promote CRC resistance to 5-FU via targeting PLAG1.Citation19 Study also demonstrates that miR-206 is involved in 5-FU resistance of CRC cells through targeting Bcl-2.Citation20 MiR-15b-5p enhances the chemo-sensitivity of CRC cells to 5-FU by promoting cell apoptosis.Citation21 The activation of CCAT1/miR-185-3p/MLCK signaling pathway contributes to the pathogenesis of inflammatory bowel disease.Citation22 MiR-185-3p, which is a mature miRNAs from 3ʹ end of pre-miR-185, is proved to be associated with Panobinostat sensitivity in breast cancer cells.Citation23 However, the effect of miR-185-3p on 5-FU resistance in CRC has not been reported yet.
In this study, we aimed to investigate the role of miR-185-3p in 5-FU-resistant CRC cells, hoping to provide a potential target for the treatment of 5-FU-resistant CRC.
Results
The level of miR-185-3p is down-regulated in CRC and negatively correlated with the expression of AQP5
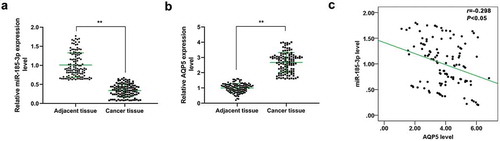
To investigate the role of miR-185-3p and its correlation with AQP5 in CRC, qRT-PCR was performed to detect the levels of miR-185-3p and AQP5 in CRC and adjacent tissues derived from 120 subjects. The results indicated that miR-185-3p was low-expressed but AQP5 was high-expressed in CRC tissues as compared with the adjacent tissues (P < .001, ). Spearman’s correlation analysis also indicated that miR-185-3p expression was negatively correlated with the level of AQP5 in CRC tissues (P < .05, r = −0.298, ).
5-FU-insensitive CRC cells show reduced inhibition rates and miR-185-3p level, and increased metastasis ability and AQP5 level
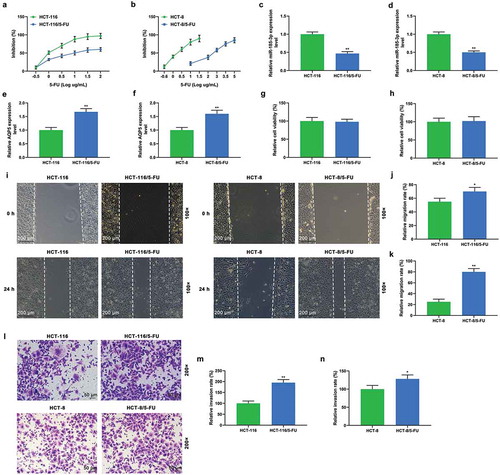
5-FU resistance was induced in HCT-116 and HCT-8 cells by treating the cells with increased gradients of 5-FU for 6 months, so as to further explore the roles of miR-185-3p and AQP5 in drug resistance of CRC cells, and the inhibition rates of HCT-116, HCT-8 cells, and the corresponding cells with 5-FU-insensitivity were determined. The result indicated that HCT-116/5-FU and HCT-8/5-FU cells showed lower inhibition rates than HCT-116 and HCT-8 cells (). When treated by the same concentrations of 5-FU, the inhibition rates of HCT-8 cells and HCT-8/5-FU cells were greatly different; similarly, for 100% inhibition of HCT-8 cells and HCT-8/5-FU cells, the concentrations of 5-FU were of great difference, suggesting that different concentrations of 5-FU resulted in varying inhibition rates (). In HCT-116/5-FU and HCT-8/5-FU cells, miR-185-3p was significantly down-regulated (P < .001, ), while the expression of AQP5 was obviously up-regulated (P < .001, ). Next, MTT assay was performed to measure the viabilities of CRC cells and corresponding 5-FU-insensitive cells (HCT-116/5-FU and HCT-8/5-FU cells); however, no change in the viability of 5-FU-insensitive CRC cells was identified compared with corresponding HCT-116 or HCT-8 CRC cells (). Furthermore, wound-healing assay and transwell assay demonstrated that the migration and invasion abilities of HCT-116/5-FU and HCT-8/5-FU cells were obviously promoted compared with HCT-116 and HCT-8 cells without treatment ().
AQP5 is directly targeted by miR-185-3p in 5-FU-insensitive CRC cells
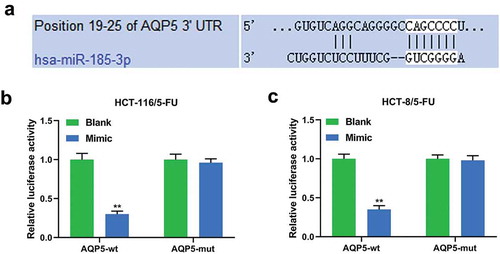
As miR-185-3p was down-regulated and AQP5 was up-regulated in 5-FU-insensitive CRC cells, we explored the relationship between miR-185-3p and AQP5 in 5-FU-insensitive CRC cells. Targetscan 7.2 predicted a binding site at position 19–25 of AQP5 3ʹUTR for has-miR-185-3p (), and dual-luciferase reporter assay performed on HCT-116/5-FU and HCT-8/5-FU cells showed that miR-185-3p mimic reduced the luciferase activity of cells transfected with AQP5-wt luciferase reporter plasmid (P < .001), while no obvious change in the luciferase activity of the cells transfected with AQP5-mut luciferase reporter plasmid was found ().
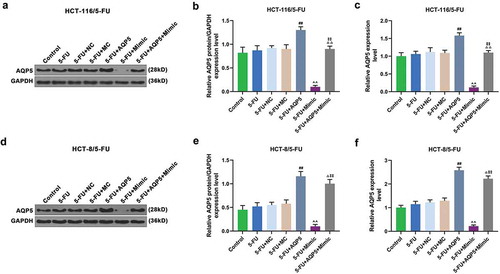
MiR-185-3p mimic enhances the sensibility of 5-FU-insensitive CRC cells to 5-FU via AQP5
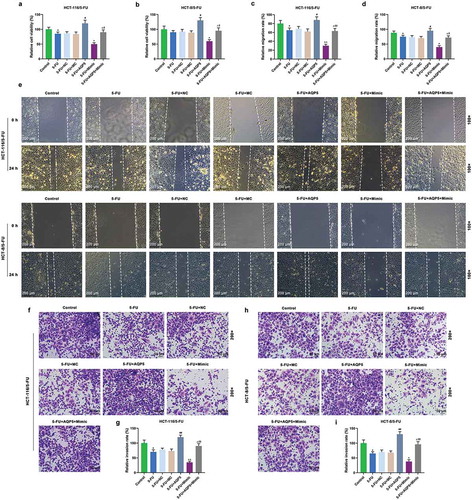
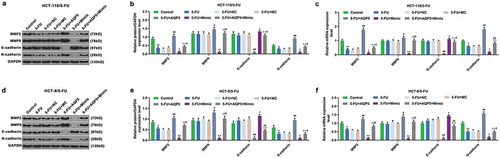
Furthermore, the cell viability, migration, and invasion, and the level of AQP5 were detected to confirm whether miR-185-3p in 5-FU-insensitive CRC cells was involved in regulating AQP5. We found that 5-FU slightly suppressed viability, migration, and invasion of 5-FU-insensitive CRC cells, and that overexpressed AQP5 enhanced the viability, migration, and invasion of 5-FU-insensitive CRC cells exposed to 5-FU; however, miR-185-3p mimic had the suppressive effects on 5-FU-insensitive CRC cells, and reduced the effect of overexpressed AQP5 on the cells (). In addition, we also detected the expression of AQP5 in 5-FU-insensitive CRC cells by performing Western Blot and qRT-PCR analysis, and the results of which indicated that 5-FU had no obvious effect on AQP5 in HCT-116/5-FU and HCT-8/5-FU cells compared with the control group. Moreover, the data also revealed that pcDNA 3.1-AQP5 up-regulated the level of AQP5; however, miR-185-3p mimic down-regulated the level of AQP5 in 5-FU+Mimic cells, compared with 5-FU cells. Subsequently, miR-185-3p mimic down-regulated the level of AQP5 in 5-FU+AQP5+ Mimic cells but was increased by AQP5 overexpression (). Furthermore, EMT-associated markers MMP2, MMP9, E-cadherin, and N-cadherin were also detected by Western blot and qRT-PCR, and we found that the expressions of MMP2 and N-cadherin were down-regulated by 5-FU treatment, while there was no obvious change observed in levels of MMP9 and E-cadherin (). More importantly, overexpression of AQP5 up-regulated the levels of MMP2, MMP9, and N-cadherin levels, but down-regulated the levels of E-cadherin, as compared with 5-FU treatment group (). MiR-185-3p mimic up-regulated the level of E-cadherin, but down-regulated the levels of MMP2, MMP9, and N-cadherin, as compared with 5-FU+ mimic control group ().
Discussion
In this study, we found that miR-185-3p was low-expressed in CRC tissues, while AQP5 was reversely up-regulated in CRC. Correlation analysis showed that miR-185-3p expression was negatively associated with AQP5 expression in CRC. Further analysis of 5-FU-resistant CRC cells confirmed that overexpressed miR-185-3p enhanced the chemo-sensitivity of CRC cells via regulating AQP5.
Previous study showed that miR-185 (miR-185-3p) is a tumor suppressor in CRC.Citation24 MiR-185-3p is a mature miRNA from 3ʹ end of pre-miR-185, and the study demonstrated that miR-185, which is involved in DC-SIGN-LEF1/TCF1-miR-185 feedback loop, regulates the invasion and metastasis of CRCCitation25 and can also enhance the radiosensitivity of CRC cells.Citation26 The function of miR-185 in CRC has been extensively explored; however, the role of miR-185-3p in CRC, especially in 5-FU resistance, has been relatively less reported. Early study found that miR-185-3p inhibits cell growth through modulating the cellular growth signaling pathway.Citation27 In nasopharyngeal carcinoma, silencing miR-185-3p promotes the metastasis of 5–8 F NPC cells.Citation28 However, the role of miR-185-3p in CRC has been less reported. In the current research, being similar to the expression pattern of miR-185 in CRC, miR-185-3p was found down-regulated in CRC tissues. To explore the function of miR-185-3p in CRC, 5-FU-resistant CRC cells were successfully constructed, and this was supported by the fact that the cells showed a lower inhibition rate of growth in the presence of 5-FU, but higher cell migration and invasion rates. The results indicated that miR-185-3p expression was reduced in 5-FU-resistant CRC cells, suggesting a potential function of miR-185-3p in 5-FU-resistant CRC cells.
We also found that AQP5 was up-regulated in CRC tissues and 5-FU-resistant HCT-116 and HCT-8 cells, and had a significantly negative correlation with miR-185-3p. Further experiments verified that AQP5 was a target gene for miR-185-3p. AQP5 belongs to water channel proteins and is responsible for the regulation of permeability in mammalian cells, digestive and nervous systems.Citation29–Citation31 Dysregulation of AQP5 is involved in Sjögren’s syndrome,Citation32 autosomal-dominant diffuse nonepidermolytic palmoplantar keratoderma,Citation33 and 30-day survival of patients with severe sepsis.Citation34 Recently, research on AQP5 has been extended to cancer progress,Citation35 for example, Zhu L et al. suggest that Aquaporin mRNA levels, including AQP5 level, are down-regulated in breast cancer, and some of them can act as potential prognostic markers.Citation36,Citation37 Recent studies showed that AQP5 is high-expressed in cancer, and silencing AQP5 suppresses the growth of cancer cells.Citation38,Citation39 Taken together, AQP5 can be considered as an oncogene in cancer. Apart from its role in growth, metastasis of cancer, AQP5 is also involved in chemotherapy sensibility of cancer,Citation40 specifically, according to a previous study, AQP5 has been found to enhance the drug resistance of CRC cells to 5-FU via modulating the Wnt-β-catenin signaling pathway.Citation41
In the current research, we also found that the epithelial–mesenchymal transition (EMT) program is also involved in CRC cells developing 5-FU resistance. EMT participates in developmental and morphogenetic processes during which adherent cells acquire migration ability.Citation42 Moreover, EMT is considered to be related to metastasis, stemness, and drug resistance in cancer treatment.Citation43 In CRC, M2 macrophage regulates 5-FU-induced chemo-resistance through EMT, PI3 K/AKT pathway, and cell apoptosis.Citation44 Inhibition of PAR2 suppresses EMT and promotes the chemo-resistance of CRC to 5-FU.Citation45 Previous study showed that AQP5 silencing has an anti-cancer effect via regulating Wnt/β-catenin pathway and EMT progress,Citation46,Citation47 which is consistent with our research. Additionally, AQP5 promotes hepatocellular carcinoma metastasis via modulating NF-κB-regulated EMT progress.Citation48 Taken together, we consider that AQP5 promotes metastasis of cancer cells through indirectly regulating EMT progress. A recent study indicated that AQP5 promotes EMT and metastasis via regulating cairicoside E (CE), which is a natural resin glycoside compound isolated from Ipomoea cairica and has been observed to suppress EMT and Smad2/3 pathway.Citation48
Consistent with previous studies, we confirmed that overexpressed AQP5 enhanced the resistance of CRC cells to 5-FU, which was supported by increased viability, migration, invasion rates, and EMT-associated gene expressions of CRC cells. We speculated that NF-κB pathway, Wnt/β-catenin pathway, or other signaling molecules such as cairicoside E might be involved in EMT regulated by AQP5. However, the mechanism of AQP5 regulating EMT progress in CRC remains unclear and should be further explored.
In conclusion, miR-185-3p is down-regulated in CRC tissues and 5-FU- insensitive CRC cells. AQP5 is predicted to be a downstream target gene for miR-185-3p and is inversely up-regulated in CRC and 5-FU-resistant CRC cells. Furthermore, miR-185-3p mimic enhances the chemo-sensitivity of 5-FU-resistant CRC cells, as it reduces viability, invasion, and migration rates and the expressions of EMT-associated markers of the cells, while overexpressed AQP5 can reverse the effect of miR-185-3p mimic on 5-FU-insensitive CRC cells. Taken together, we consider that miR-185-3p is a potential target for the treatment of 5-FU-insensitive CRC.
Materials and methods
Sample collection
One hundred and twenty CRC tissues and adjacent tissues were collected from July 2017 to March 2018 at the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. The samples collected from the surgical resection were stored at −80°C. The research had been approved by the Ethical Committee of the Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine. All patients had signed the informed consent form.
Cell culture and treatment
CRC cell lines, namely, HCT-116 (CCL-247) and HCT-8 (CCL-244) were purchased from American Type Culture Collection. The cells were cultured in RPMI-1640 medium (61870044, Gibco, ThermoFisher, USA) supplemented with L-Glut (4 mM, 25030081, Gibco, ThermoFisher, USA), 10% fetal bovine serum (16140071, Gibco, ThermoFisher, USA), and 1% penicillin/streptomycin (100 U/ml – 100 µg/ml, 15070063, Gibco, ThermoFisher, USA). The two cell lines were incubated in a humidified cell incubator with 5% CO2 at 37°C.
For the construction of 5-FU-resistant CRC cells, the dose of 5-FU was gradually increased until the cells were able to tolerate high doses of 5-FU. Briefly, 5-FU (04541, Sigma, USA) diluted into DMSO was added into cell culture medium at final concentrations of log −0.5 ug/mL, log 0 µg/mL, log 0.5 µg/mL, log 1 µg/mL, log 1.5 µg/mL, log 2 µg/mL. HCT-116 and HCT-8 cells were exposed to a low dose of 5-FU and cultured for 3 weeks; then, one part of the cells was stored for further experiments, while the rest was exposed to a higher dose of 5-FU. When the inhibition rate reached 100%, 5-FU-resistant cells were selected and named as HCT-116/5-FU and HCT-8/5-FU cells.
Qualitative real-time PCR analysis
Total RNAs from tissues and cells were collected by chloroform and isopropanol methods at 4ºC. Parts of the tissues were cut into sections and homogenized by TRIzol® reagent (15596018, Invitrogen, Thermofisher, USA) for 5 min on ice. Then, the total RNAs in homogenates were collected by chloroform and isopropanol methods and centrifuged at 500 x g, 4ºC for 15 min to 20 min. The RNAs were treated by TRIzol® reagent, isolated by chloroform and isopropanol methods, and centrifuged. After RNA collection, PrimeScript™ II 1st Strand cDNA Synthesis Kit (6210B, Takara, Japan) was used for reverse-transcription, and qRT-PCR was conducted using SYBR® Green PCR Master Mix (4312704, ABI, USA). The PCR reaction was performed on Bio-Rad CFX 96 Touch Real-Time PCR Detection System (1855196, Bio-Rad, China). GAPDH served as endogenous reference gene, and the 2 −ΔΔCt method was used to calculate gene expression levels. TaqMan™ MicroRNA Reverse Transcription Kit (4366597, Applied Biosystems, Thermofisher, USA) was used for the detection of hsa-miR-185-3p. The specific RT primer for hsa-miR-185-3p was 5ʹ-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACGACCAGAG-3ʹ. Then, SYBR® Green PCR Master Mix (4312704, ABI, USA) was used for qRT-PCR. U6 snRNA served as a reference gene. The reaction condition for PCR reactions was as follows: at 95°C for 5 min, 40 cycles at 95°C for 15 s, at 60°C for 30 s, and at 70°C for 10 s. Primers used in this research are listed in .
Table 1. qRT-PCR primers.
MTT assay
The inhibition rate of CRC cells was determined by MTT assay. The cells were exposed to different doses of 5-FU for 3 weeks, collected and seeded into 96-well plate. Prior to the experiment, the cells were cultured under normal conditions without 5-FU for 1 week and then exposed to 5-FU at log 2 µg/mL for 24 h. The cells were then washed by PBS, stained by MTT (V13154, Invitrogen, Thermofisher, USA) at 37°C for 2 h. Then, the purple precipitates were dissolved by DMSO at room temperature. Finally, the absorbance at 570 nm was measured using a microplate reader (PLUS 384, Molecular Devices, USA). Ten parallel wells in each group were set up. The cells without any treatment were considered as reference group. The inhibition rate was calculated by (the OD 570 nm reference group -OD 570 nm treatment group)/(OD 570 nm reference group- OD 570 nm blank)*100%.
For the detection of cell viability, the cells (3,000 cells/well) after the treatment were seeded into a 96-well plate containing normal medium but without 5-FU. After culture for 48 h, the cells were washed by PBS, and MTT assay was performed. The cells without any treatment were considered a control group. The cell viability was calculated by (the OD 570 nm treatment group -OD 570 nm blank)/(OD 570 nm control group- OD 570 nm blank)*100%.
Wound healing
The cells after the corresponding treatment were grown in a 6-well plate and then a wound was created using a 10 µL pipette tip. After removing floating cells, the cells were cultured for another 48 h. The scratches were observed under a microscope (TS100, Nikon, Japan) at 0 h and 48 h.
Transwell assay
The cells (2 x 103 cells/well) were grown in the upper chamber of a transwell plate (3428, Corning, USA) pre-coated with matrigel, whereas the lower chamber was added with 600 µL normal medium containing 10% FBS. After incubation for 34 h, the cells invaded through the transwell pore under the membrane were fixed by 4% methanol at room temperature for 5 min and stained by 0.1% crystal violet (548-62-9, Aladdin, China) at temperature for 20 min. Finally, the number of cells were counted from 10 randomly selected fields at magnification 200 x.
Cell transfection
Mimic control (MC, miR1N0000001-1-1) and miR-185-3p mimic (miR10004611-1-5) were purchased from RiboBio, China. The full-length cDNA of AQP5 was amplified and inserted into pcDNA 3.1 empty vector as pcDNA 3.1- AQP5 overexpression plasmid. The empty plasmid of pcDNA 3.1served as a negative control. The cells (2x105 cells/well) were grown in a 6-well plate and cultured for 24 h at 37°C. When the cells reached 80% confluence, 2 µg plasmid or 100 pmol mimics mixed with Opti-MEM (11058021, Invitrogen, USA) containing Lipofectamine® 2000 (11668019, Invitrogen, USA) was, respectively, transfected into the cells. After incubation at 37°C for 8 h, the medium was replaced by fresh medium. After culture for another 24 h, the cells were collected for further experiments.
Dual-luciferase reporter assay
Targetscan 7.2 was used to predict the interaction between miR-185-3p and AQP5. The cDNA of AQP5- 3ʹUTR- wt and AQP5- 3ʹUTR- mut were synthesized BY Tsingke CO., Ltd. and, respectively, inserted into a luciferase reporter gene vector (pmirGLO, E1330, Promega, USA) as AQP5- 3ʹUTR- wt reporter plasmid and AQP5- 3ʹUTR- mut reporter plasmid. Next, the cells were co-transfected with reporter plasmid and mimic (miR-185-3p mimic or mimic control). The renilla luciferase gene on the reporter plasmid served as an internal reporter control. After incubation for 48 h, the luciferase activity was measured by dual-luciferase assay system (E1910, Promega, USA) and a microplate luminometer (11300010, Berthold, Germany). The firefly luciferase activity against renilla luciferase activity was calculated. Six parallel wells were analyzed in each group.
Western blot
The cells were treated by cell extraction buffer (FNN0011, Invitrogen, Thermofisher, USA) containing PMSF protease inhibitor (36978, Thermo Scientific, USA) at 4ºC for 30 min. The proteins were isolated from the cells by centrifuging the cells at 12000 x g for 30 min 4ºC. Next, the proteins (50 μg) in each group were separated on 10% SDS-PAGE and then transferred to a PVDF membrane (LC2002, Invitrogen, Thermofisher, USA). After blocking the membrane by 5% nonfat milk (PA201-01, BioMed, China) for 10 min at room temperature, the PVDF membrane was exposed to primary antibodies (AQP5 (ab92320, 1:2000, Abcam, UK), MMP-9 (ab73734, 1:2000, Abcam, UK), MMP2 (ab37150, 1:2000, Abcam, UK), E-cadherin (ab40772, 1:2000, Abcam, UK), N-cadherin (ab18203, 1:2000, Abcam, UK), and Anti-GAPDH (ab181602, 1:5000, Abcam, UK)) at 4°C for 8 h. Next, the membrane was washed by 1 X PBST and incubated with HRP-linked anti-rabbit IgG antibody (1:5000, 7074, CST, USA). Finally, the signals were developed by SignalFir ECL reagent (6883, CST, USA), and captured by ImageQuant ECL Imager (28-9605-63, GE Healthcare, USA).
Statistical analysis
Graphpad Prism 5.02 software (La Jolla, CA, USA) was used for statistical analysis. Spearman’s correlation analysis was performed to analyze the correlation between miR-185-3p and AQP5 expressions. The results are shown as the mean ± SD, and the data were analyzed using Student’s t-test or ANOVA followed by Tukey’s post hoc test. P < .05 was considered as statistically significant.
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Additional information
Funding
References
- Mathews MJ, Mead RN, Galizio M. Effects of N-Methyl-D-aspartate (NMDA) antagonists ketamine, methoxetamine, and phencyclidine on the odor span test of working memory in rats. Exp Clin Psychopharmacol. 2018;26:6–17.
- He YJ, Meghani K, Caron MC, Yang C, Ronato DA, Bian J, Sharma A, Moore J, Niraj J, Detappe A, et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature. 2018;563:522–526.
- Germano G, Amirouchene-Angelozzi N, Rospo G, Bardelli A. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov. 2018;8:1518–1528.
- Aird D, Teng T, Huang CL, Pazolli E, Banka D, Cheung-Ong K, Eifert C, Furman C, Wu ZJ, Seiler M, et al. Sensitivity to splicing modulation of BCL2 family genes defines cancer therapeutic strategies for splicing modulators. Nat Commun. 2019;10:137.
- Yin Y, Xu L, Chang Y, Zeng T, Chen X, Wang A, Groth J, Foo WC, Liang C, Hu H, et al. N-Myc promotes therapeutic resistance development of neuroendocrine prostate cancer by differentially regulating miR-421/ATM pathway. Mol Cancer. 2019;18:11.
- Borgo C, Ruzzene M. Role of protein kinase CK2 in antitumor drug resistance. J Exp Clin Cancer Res. 2019;38:287.
- Shan Z, Wei L, Yu S, Jiang S, Ma Y, Zhang C, Wang J, Gao Z, Wan F, Zhuang G, et al. Ketamine induces reactive oxygen species and enhances autophagy in SV-HUC-1 human uroepithelial cells. J Cell Physiol. 2019;234:2778–2787.
- Saleem H, Kulsoom Abdul U, Kucukosmanoglu A, Houweling M, Cornelissen FMG, Heiland DH, Hegi ME, Kouwenhoven MCM, Bailey D, Wurdinger T, et al. The TICking clock of EGFR therapy resistance in glioblastoma: target Independence or target Compensation. Drug Resist Updates. 2019;43:29–37.
- Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, Yang DH, Chen ZS. Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updates. 2016;27:14–29.
- Han K, Jeng EE, Hess GT, Morgens DW, Li A, Bassik MC. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat Biotechnol. 2017;35:463–474.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
- Khalilzadeh B, Shadjou N, Kanberoglu GS, Afsharan H, de la Guardia M, Charoudeh HN, Ostadrahimi A, Rashidi MR. Advances in nanomaterial based optical biosensing and bioimaging of apoptosis via caspase-3 activity: a review. Mikrochim Acta. 2018;185:434.
- Uppada SB, Gowrikumar S, Ahmad R, Kumar B, Szeglin B, Chen X, Smith JJ, Batra SK, Singh AB, Dhawan P. MASTL induces colon cancer progression and Chemoresistance by promoting Wnt/beta-catenin signaling. Mol Cancer. 2018;17:111.
- Cotte AK, Aires V, Fredon M, Limagne E, Derangere V, Thibaudin M, Humblin E, Scagliarini A, de Barros JP, Hillon P, et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat Commun. 2018;9:322.
- Marjaneh RM, Khazaei M, Ferns GA, Avan A, Aghaee-Bakhtiari SH. The role of microRNAs in 5-FU resistance of colorectal cancer: possible mechanisms. J Cell Physiol. 2019;234:2306–2316.
- Zhao P, Ma YG, Zhao Y, Liu D, Dai ZJ, Yan CY, Guan HT. MicroRNA-552 deficiency mediates 5-fluorouracil resistance by targeting SMAD2 signaling in DNA-mismatch-repair-deficient colorectal cancer. Cancer Chemother Pharmacol. 2019;84:427–439.
- Cai MH, Xu XG, Yan SL, Sun Z, Ying Y, Wang BK, Tu YX. Regorafenib suppresses colon tumorigenesis and the generation of drug resistant cancer stem-like cells via modulation of miR-34a associated signaling. J Exp Clin Cancer Res. 2018;37:151.
- Han X, Wu YC, Meng M, Sun QS, Gao SM, Sun H. Linarin prevents LPSinduced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NFkappaB pathways. Int J Mol Med. 2018;42:1460–1472.
- Shi L, Li X, Wu Z, Li X, Nie J, Guo M, Mei Q, Han W. DNA methylation-mediated repression of miR-181a/135a/302c expression promotes the microsatellite-unstable colorectal cancer development and 5-FU resistance via targeting PLAG1. J Genet Genomics. 2018;45:205–214.
- Wen Y, Liu YR, Tang TT, Pan MM, Xu SC, Ma KL, Lv LL, Liu H, Liu BC. mROS-TXNIP axis activates NLRP3 inflammasome to mediate renal injury during ischemic AKI. Int J Biochem Cell Biol. 2018;98:43–53.
- Zhao C, Zhao Q, Zhang C, Wang G, Yao Y, Huang X, Zhan F, Zhu Y, Shi J, Chen J, et al. miR-15b-5p resensitizes colon cancer cells to 5-fluorouracil by promoting apoptosis via the NF-kappaB/XIAP axis. Sci Rep. 2017;7:4194.
- Ma D, Cao Y, Wang Z, He J, Chen H, Xiong H, Ren L, Shen C, Zhang X, Yan Y, et al. CCAT1 lncRNA promotes inflammatory bowel disease malignancy by destroying intestinal barrier via downregulating miR-185-3p. Inflamm Bowel Dis. 2019;25:862–874.
- Uhr K, Prager-van der Smissen WJC, Heine AAJ, Ozturk B, van Jaarsveld MTM, Boersma AWM, Jager A, Wiemer EAC, Smid M, Foekens JA, et al. MicroRNAs as possible indicators of drug sensitivity in breast cancer cell lines. PLoS One. 2019;14:e0216400.
- Lu ZJ, Lu LG, Tao KZ, Chen DF, Xia Q, Weng JJ, Zhu F, Wang XP, Zheng P. MicroRNA-185 suppresses growth and invasion of colon cancer cells through inhibition of the hypoxia-inducible factor-2alpha pathway in vitro and in vivo. Mol Med Rep. 2014;10:2401–2408.
- Yuan M, Zhang X, Zhang J, Wang K, Zhang Y, Shang W, Zhang Y, Cui J, Shi X, Na H, et al. DC-SIGN-LEF1/TCF1-miR-185 feedback loop promotes colorectal cancer invasion and metastasis. Cell Death Differ. 2020;27:379–395.
- Afshar S, Najafi R, Sedighi Pashaki A, Sharifi M, Nikzad S, Gholami MH, Khoshghadam A, Amini R, Karimi J, Saidijam M. MiR-185 enhances radiosensitivity of colorectal cancer cells by targeting IGF1R and IGF2. Biomed Pharmacother. 2018;106:763–769.
- Liao JM, Lu H. Autoregulatory suppression of c-Myc by miR-185-3p. J Biol Chem. 2011;286:33901–33909.
- Liu C, Li G, Ren S, Su Z, Wang Y, Tian Y, Liu Y, Qiu Y. miR-185-3p regulates the invasion and metastasis of nasopharyngeal carcinoma by targeting WNT2B in vitro. Oncol Lett. 2017;13:2631–2636.
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem. 1999;68:425–458.
- Zhu S, Ran J, Yang B, Mei Z. Aquaporins in digestive system. Adv Exp Med Biol. 2017;969:123–130.
- Xu M, Xiao M, Li S, Yang B. Aquaporins in Nervous System. Adv Exp Med Biol. 2017;969:81–103.
- Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357:688–689.
- Blaydon DC, Lind LK, Plagnol V, Linton KJ, Smith FJ, Wilson NJ, McLean WH, Munro CS, South AP, Leigh IM, et al. Mutations in AQP5, encoding a water-channel protein, cause autosomal-dominant diffuse nonepidermolytic palmoplantar keratoderma. Am J Hum Genet. 2013;93:330–335.
- Adamzik M, Frey UH, Mohlenkamp S, Scherag A, Waydhas C, Marggraf G, Dammann M, Steinmann J, Siffert W, Aquaporin PJ. 5 gene promoter–1364A/C polymorphism associated with 30-day survival in severe sepsis. Anesthesiology. 2011;114:912–917.
- Direito I, Madeira A, Brito MA, Soveral G. Aquaporin-5: from structure to function and dysfunction in cancer. Cell Mol Life Sci. 2016;73:1623–1640.
- Zhu L, Ma N, Wang B, Wang L, Zhou C, Yan Y, He J, Ren Y. Significant prognostic values of aquaporin mRNA expression in breast cancer. Cancer Manag Res. 2019;11:1503–1515.
- Zhu Z, Jiao L, Li T, Wang H, Wei W, Qian H. Expression of AQP3 and AQP5 as a prognostic marker in triple-negative breast cancer. Oncol Lett. 2018;16:2661–2667.
- Zhang Z, Han Y, Sun G, Liu X, Jia X, Yu X. MicroRNA-325-3p inhibits cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma by down-regulation of aquaporin 5. Cell Mol Biol Lett. 2019;24:13.
- Shimasaki M, Kanazawa Y, Sato K, Tsuchiya H, Ueda Y. Aquaporin-1 and −5 are involved in the invasion and proliferation of soft tissue sarcomas. Pathol Res Pract. 2018;214:80–88.
- Cagini L, Balloni S, Ludovini V, Andolfi M, Matricardi A, Potenza R, Vannucci J, Siggillino A, Tofanetti FR, Bellezza G, et al. Variations in gene expression of lung macromolecules after induction chemotherapy for lung cancer. Eur J Cardio-thoracic Surg. 2017;52:1077–1082.
- Li Q, Yang T, Li D, Ding F, Bai G, Wang W, Sun H. Knockdown of aquaporin-5 sensitizes colorectal cancer cells to 5-fluorouracil via inhibition of the Wnt-beta-catenin signaling pathway. Biochem Cell Biol. 2018;96:572–579.
- Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett. 2013;341:9–15.
- Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5:17.
- Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Xiong B. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Onco Targets Ther. 2019;12:3051–3063.
- Quan Q, Zhong F, Wang X, Chen K, Guo L. PAR2 Inhibition Enhanced the Sensitivity of Colorectal Cancer Cells to 5-FU and Reduced EMT Signaling. Oncol Res. 2019;27:779–788.
- Wang W, Li Q, Yang T, Li D, Ding F, Sun H, Bai G. Anti-cancer effect of Aquaporin 5 silencing in colorectal cancer cells in association with inhibition of Wnt/beta-catenin pathway. Cytotechnology. 2018;70:615–624.
- Chen C, Ma T, Zhang C, Zhang H, Bai L, Kong L, Luo J. Down-regulation of aquaporin 5-mediated epithelial-mesenchymal transition and anti-metastatic effect by natural product Cairicoside E in colorectal cancer. Mol Carcinog. 2017;56:2692–2705.
- He Z, Dong W, Hu J, Ren X. AQP5 promotes hepatocellular carcinoma metastasis via NF-kappaB-regulated epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2017;490:343–348.