ABSTRACT
Previous studies have shown that DBDx, a combination consisting of dipyridamole, bestatin and dexamethasone is highly effective against several cancer xenografts in athymic mice. Here the therapeutic effects of DBDx and its combination with gemcitabine or capcitabine against human pancreatic cancer xenografts and the mechanism were studied. In vivo experiments performed in athymic mice showed that the antitumor efficacy of DBDx was much stronger than that of gemcitabine or capecitabine alone. Notably, the combination of DBDx and gemcitabine or capcitabine further enhanced the efficacy. In the case of DBDx (242 mg/kg) plus gemcitabine (100 mg/kg), tumor weight decreased about 97.7%, and tumor sizes were shrinking during the treatment. In the case of DBDx (242 mg/kg) plus capecitabine (718.7 mg/kg), tumor weight decreased about 94.9%. Moreover, DBDx and its combinations obviously prolonged theoverall survival of mice compared with gemcitabine or capcitabine alone. DBDx-based drug combination therapy showed no obvious systematic toxicity. The gene expression profile analysis showed that the genes changed by DBDx were related to immune system and tumor vasculature. The result of protein array showed that the changed proteins in the serum of treated mice were related to immune and inflammation system. These results show that DBDx-based drug combinations, a new strategy which integrates the use of low-cytotoxic drugs and cytotoxic chemotherapeutics, are highly effective regimens against human pancreatic cancer in athymic mice at well tolerated doses. DBDx-based drug combination therapy might provide new options for the treatment of pancreatic cancer.
Introduction
Pancreatic cancer is one of the most deadly malignancies worldwide, for which the five-year survival is only 6%.Citation1 Despite aggressive research and progress in new therapeutic approaches, there has been no significant improvement in the survival during the last decade.Citation2,Citation3 New therapies are urgently needed for this malignant disease. Recently, two combinations FOLFIRINOX (5-fluorouracil, irinotecan, folinic acid,and oxaliplatin) and nab-paclitaxel plus gemcitabine are proved to be more effective than gemcitabine alone, and extended the overall survival (OS) of patients from 6 months to nearly 1 year,Citation4,Citation5 while with significantly increased toxicities.Citation6,Citation7 Nevertheless, only 31.6% of patients showed response to FOLFIRINOX, and 23% to nab-paclitaxel plus gemcitabine. Here the therapeutic effects of a low-cytotoxic drug combination DBDx against human pancreatic cancer xenografts and its mechanism were studied. The therapeutic efficacy of DBDx plus gemcitabine or capecitabine was also evaluated.
DBDx is a highly effective anti-cancer drug combination consisting of dipyridamole, bestatin and dexamethasone.Citation8 Our previous work proved that the metabolic process, immune system and angiogenesis were affected by DBDx. DBDx was highly effective against human tumor xenografts including hepatocellular carcinoma, lung adenocarcinoma and epidermoid carcinoma xenografts. Both gemcitabine and capecitabine are nucleoside analogues that act on DNA synthesis. In clinical, gemcitabine monotherapy, and gemcitabine-based combination therapies are used for the treatment of advanced pancreatic cancer.Citation4,Citation9 Capecitabine is used in combination with gemicitabine or other chemotherapeutics to enhance dwthe therapeutic efficacy in pancreatic cancer.Citation10–Citation12 Our present research proves that DBDx effectively inhibits the growth of pancreatic cancer xenografts. When combined with gemcitabine or capecitabine, its antitumor activity is further improved.
Results
DBDx and its combination with gemcitabine significantly inhibits the growth of human pancreatic carcinoma xenograft
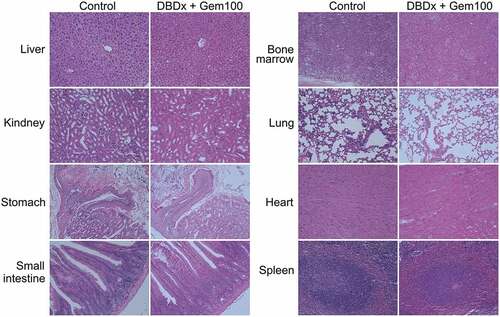
Previous study showed that DBDx could inhibit the growth of various human tumor xenografts.Citation8 Here, we focused on the most lethal pancreatic cancer and evaluated the antitumor activity of DBDx against human pancreatic carcinoma MIA PaCa-2 xenografts, and gemcitabine was used for comparison. In addition, the antitumor efficacy of DBDx plus gemcitabine was evaluated. Seven days after tumor implantation, drugs were given. DBDx was administered orally and gemcitabine intraperitoneally. Three days after the last administration, mice were sacrificed. The inhibitory rates of Gem50 (gemcitabine at 50 mg/kg), Gem100 (gemcitabine at 100 mg/kg), DBDx (242 mg/kg), DBDx + Gem50 and DBDx + Gem100 were 55.7%, 60.1%, 83.2%, 90.2%% and 95.1%, respectively, as evaluated by the tumor volume (). Evaluated by the tumor weights, the inhibitory rates were 48.9%, 50.7%, 87.4%, 91.5% and 97.7%, respectively (). As shown, the antitumor efficacy of DBDx was much stronger than that of gemcitabine. Notably, the combination of DBDx and gemcitabine further enhanced the efficacy; in the case of DBDx plus gemcitabine (100 mg/kg), tumor sizes were shrinking during the treatment. No significant body weight loss was observed in all groups ().Histopathological examination of mice treated with DBDx + Gem100 or saline was performed. No histopathological lesions were found in the small intestine, stomach, bone marrow and liver (). All of these results suggested that the doses of administrated drugs were tolerated.
Figure 1. Inhibition of the growth of human pancreatic carcinoma MIA PaCa-2 xenografts by DBDx and its combination with gemcitabine. (a) Tumor volume and body weight changes during the experiment. DBDx at 242 mg/kg and gemcitabine at 50 (GEM50) and 100 (GEM100) mg/kg were used; (b) At the end of the experiment, mice were sacrificed. Tumors were photographed and tumor weights were measured. (Dose: mg/kg). *P < .05, **P < .01 vs DBDx. Ctrl, control. Gem, gemcitabine.
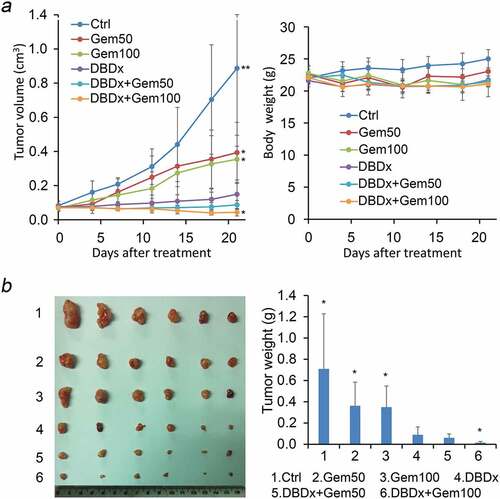
Figure 2. Histopathological examination of various organs from mice treated with saline or DBDx + Gem100. No toxicological damages were found in liver, kidney, stomach, intestine, bone marrow, lung, heart and spleen in DBDx-treated animals (H&E). (×200).
We further evaluated the therapeutic effects of DBDx plus gemcitabine against human pancreatic carcinoma AsPC-1 xenografts. Drugs were given as above. At the end of the experiment, Gem (100 mg/kg), DBDx (242 mg/kg) and DBDx + Gem inhibited the tumor growth by 60.2%, 75.3% and 90.2%, respectively, as evaluated by the tumor volume (). Evaluated by the tumor weights, the inhibitory rates were 60.7%, 80.1% and 93.3%, respectively (). The result further proved that DBDx showed much stronger antitumor efficacy than that of gemcitabine, and combination of DBDx and gemcitabine significantly enhanced the efficacy (). No significant body weight loss was observed in all groups ().
Figure 3. In vivo antitumor activity of DBDx and gemcitabine against AsPC-1 xenografts and cytotoxcity of DBDx to different cell lines. (a) Tumor volume and body weight changes during the experiment. DBDx at 242 mg/kg and gemcitabine at 100 mg/kg were used; (b) At the end of the experiment, mice were sacrificed. Tumors were photographed and tumor weights were measured. **P < .01 vs DBDx. (c) Cytotoxcity of DBDx to different pancreatic cell lines (mean ± SD, n = 3).
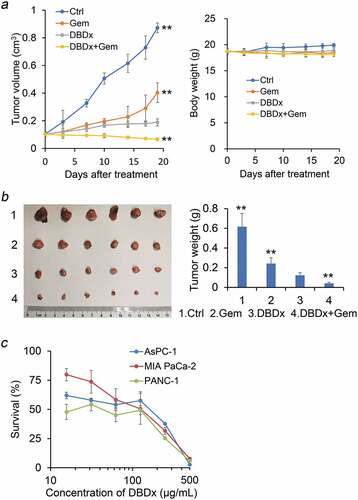
Then we evaluated the cytotoxicity of DBDx to various cultured human pancreatic cancer cell lines in vitro via MTT assay (). The IC50 values of DBDx to MIA PaCa-2, PANC-1 and AsPC-1 were 87.6 μg/mL, 48.9 μg/mL and 68.3 μg/mL, respectively.
DBDx plus capecitabine significantly inhibits the growth of human pancreatic carcinoma xenograft
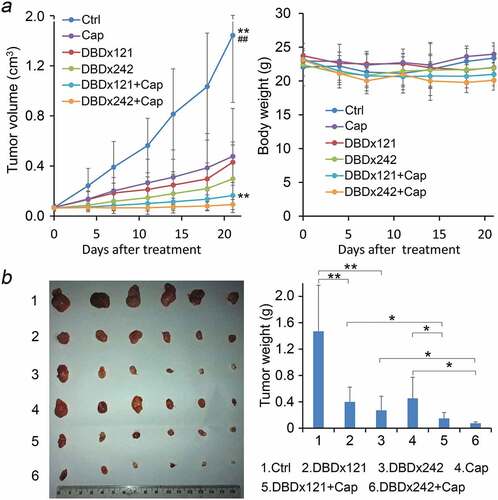
In clinic, capecitabine has been evaluated alone or in combination with gemcitabine for the treatment of advanced pancreatic cancer.Citation10,Citation11,Citation13We further evaluated the antitumor activity of DBDx plus capecitabine in MIA PaCa-2 xenograft model. Drugs were administrated as described above. Three days after the last administration, mice were sacrificed. DBDx at 121 and 242 mg/kg inhibited the tumor growth by 70.2% and 79.4%, respectively, and capecitabine at 718.7 mg/kg inhibited the tumor growth by 66.9% evaluated by the tumor volume (). When DBDx and capecitabine combined, the inhibitory rates were increased to 88.6% and 93.6%, which were obviously different from that of DBDx and capecitabine monotherapy. After dissection, the tumor weights were measured. According to the tumor weights, the inhibitory rates of DBDx(121), DBDx(242), Cap, DBDx(121) + Cap and DBDx(242) + Cap were 72.8%, 81.6%, 69.1%, 89.9% and 94.9% (), respectively. No significant body weight changes were observed during the treatment ().
Figure 4. Inhibition of the growth of human pancreatic carcinoma xenograft by DBDx plus capecitabine. (a) Tumor volume and body weight changes during the experiment. DBDx at 121 mg/kg (DBDx121) and 242 mg/kg (DBDx242) and capecitabine at 718.7 mg/kg were used. **P < .01 vs DBDx121, ## P < .01 vs DBDx242; (b) At the end of the experiment, mice were sacrificed. Tumors were photographed and tumor weights were measured. (Dose: mg/kg). *P < .05. Cap, capecitabine.
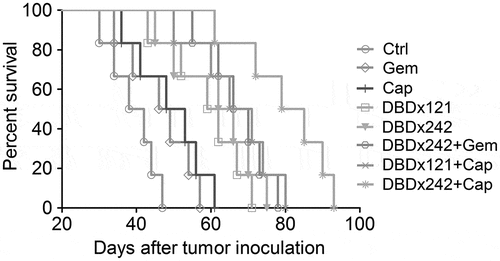
DBDx and its combinations prolong the median survival time of tumor-bearing mice
In another independent experiment, the median survival time of the tumor-bearing mice treated with DBDx, gemcitabine, capecitabine, DBDx plus gemcitabine, and DBDx plus capecitabine were evaluated. Seven days after MIA PaCa-2 tumor implantation, drugs were given as above. Then, the survival time was recorded. As showed in , the median survival time of Control, Gemcitabine, Capecitabine, DBDx121 and DBDx242 therapy were 40, 47.5, 50.5, 60.5 and 64 days, respectively. For gemcitabine, as combined with DBDx at 242 mg/kg, the median survival time was extended to 68 days. For capecitabine, as combined with DBDx at 121 or 242 mg/kg, the median survival time were extended to 68 and 82 days, respectively. The results indicated that DBDx at 121 or 242 mg/kg significantly increased the median survival time of mice; notably, DBDx plus capecitabine presented further prolongation.
Effects of DBDx on the gene expression profile
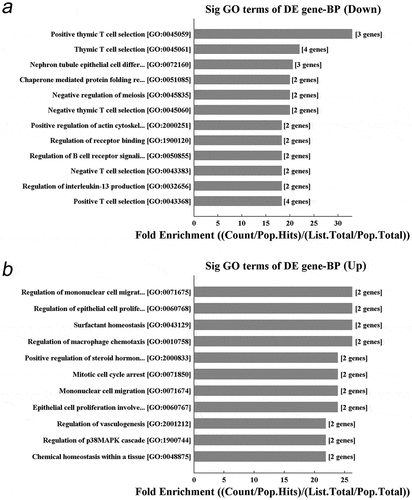
The differentially expressed genes in MIA PaCa-2 xenograft after DBDx treatment was analyzed using the whole human genome microarray. The result showed that 255 genes were increased and 212 genes were decreased by more than 2-fold between DBDx-treated and control group. In the top ten significant decreased terms of GO biological process, 5 were related to T cell selection, 1 was related to B cell, 1 was related to interleukin-13 (). In the top ten increased terms (), were related to epithelial cell and vasculogenesis, 2 were related to mononuclear cell migration, 1 was related to macrophage chemotaxis. These results demonstrated that DBDx mainly affects the immune system and tumor vasculature.
Figure 6. Significant GO biological process of the differentially expressed genes after DBDx treatment. (a) The top ten down-regulated terms in GO biological process classification; (b) The top ten up-regulated terms in GO cellular component classification. BP, biological process. DE, differentially expressed.
Protein array analysis after DBDx treatment
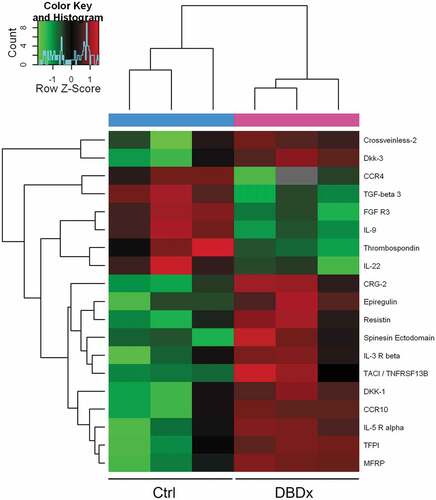
To further investigate the antitumor mechanism of DBDx, a total of 308 proteins in the sera of mice after DBDx treatment were measured using protein array analysis. Mice bearing MIA PaCa-2 xenograft were treated with DBDx or saline as above. At the end of the experiments, sera were collected and analyzed. The differentially expressed proteins between two groups are listed in (P < .05). The results revealed that 13 proteins were up-regulated and 6 proteins were down-regulated at least 2-fold in DBDx-treated group, as compared with the control. More specifically, CRG-2 was increased by 7.6-fold, while TGF-beta 3 and CCR4 were decreased by 10-fold and 5.3-fold, respectively. CRG-2, also known as CXCL10, is chemotactic for monocytes and T-lymphocytes.Citation14,Citation15 TGF-beta 3 and CCR4 are crucial for maintaining immunological homeostasis.Citation16,Citation17
Table 1. Cytokine levels in the sera of DBDx-treated mice relative to the control group.
According to the similarity in abundance of these 19 proteins, samples (3 repeats/group) were classified with an unsupervised clustering algorithm, and clustered into two groups containing DBDx-treated mice or saline-treated mice (). It indicates that the protein levels in the sera are obviously different between DBDx-treated and saline-treated mice.
Discussion
Drug combination is widely used in cancer treatment, whereas gemcitabine monotherapy remained the first-line treatment for pancreatic cancer since 1997 after failure of a series of gemcitabine-based combinations.Citation18,Citation19 Until recently, two combinations, FOLFIRINOX regimens and gemcitabine plus nab-paclitaxel, are proved to be more effective than gemcitabine alone. In this paper, we report a new modality of drug combination for pancreatic cancer treatment, DBDx therapy and DBDx-based combination therapy. Both DBDx therapy and DBDx-based combination therapy are highly effective against human pancreatic carcinoma xenografts in athymic mice. The therapeutic efficacy was more potent than that of gemcitabine or capcitabine alone. In the present study, DBDx was evaluated as monotherapy; actually DBDx is a multi-functional drug combination consisting of dipyridamole, bestatin and dexamethasone. All of the three components of DBDx are currently used in clinic, though they are not listed as classic anticancer chemotherapeutics. It is of particular interest that the alternative drug combination DBDx displays potent efficacy against pancreatic cancer in experimental therapy.
As the first-line treatment for patients with pancreatic cancer, gemcitabine just showed a marginal improvement on the OS of patients.Citation9,Citation20 In our experiment, gemcitabine showed limited effect on median survival time relative to control group (47.5 vs 40 days). Owing to the recent advancements, the OS of patients was extended from 6 months to nearly 1 year.Citation6,Citation7,Citation21 In pancreatic cancer xenograft model, DBDx therapy, especially DBDx-based combination substantially improved the median survival time of mice. DBDx242 plus capecitabine prolonged the median survival time of mice by over 2-fold.
DBDx is a low-cytotoxic drug combination. Most of clinically used chemotherapeutics for pancreatic cancer are cytotoxic agents. The gemcitabine-induced side effects may include vomiting, liver problems, bone marrow suppression, etc. FOLFIRINOX has adverse effects of blood and gastrointestinal toxicity.Citation6,Citation22 Notably, DBDx showed no obvious cytotoxicity to bone marrow, small intestine, stomach and liver. Its low cytotoxicity was also proved by in vitro MTT assay.
Recently, immunosuppression related signaling and inflammation has emerged as potential targets for pancreatic adenocarcinoma.Citation23 As known, the components of DBDx includes dipyridamole, bestatin and dexamethasone. Dexamethasone is used as an anti-inflammatory drug and can suppress angiogenesis.Citation24,Citation25 Bestatin is an immunomodulator. As an aminopeptidase inhibitor, bestatin can also inhibit angiogenesis.Citation26 Dipyridamole is an anti-thrombotic agent; in addition, it can reduce the immune cell infiltration in primary tumors, and the inflammatory cytokines levels in the sera of the treated mice.Citation27 In the present study, gene expression profile analysis showed that DBDx treatment induced the changes of genes related to the immune and inflammation system and tumor vasculature. Protein array analysis indicated that the changed proteins in the sera of treated mice were related to immune and inflammation system. Our previous study performed in human hepatocellular carcinoma xenograft model also proved that DBDx can modulate the tumor microenvironment.Citation8 Thinking of its low cytotoxicity, DBDx may exert its activity against human pancreatic cancer xenografts via modulating immune system and tumor vasculature, not by killing tumor cells directly. In the future study, we will perform the ‘omics’ analyses validation via qPCR for transcriptome and western blot for proteome using a mouse xenograft model, and further study the molecular pathway affected by DBDx.
Conclusively, the research demonstrated that DBDx therapy and DBDx-based combination therapy are more effective than gemcitabine or capecitabine alone in inhibition of pancreatic cancer xenograft growth. Furthermore, it also confers a meaningful survival advantage over gemcitabine or capecitabine monotherapy. In China, pancreatic cancer is the sixth leading cause of cancer-related death, and accounted for over 79,400 deaths in 2015.Citation28 DBDx therapy, especially DBDx-based combination therapy that integrates the use of low-cytotoxic drugs and cytotoxic chemotherapeutics, might provide new options for patients with pancreatic cancer.
Materials and methods
Materials
The following reagents were used: dexamethasone and dipyridamole from National Institutes for Food and Drug Control (Beijing, China), bestatin from Apeloa Kangyu (China), gemcitabine (Gem) from Lilly (France), and capecitabine (Cap) from Roche (Switzerland). DBDx was prepared according to previous report.Citation8 All chemicals used were of analytical grade.
Cells culture and cytotoxicity assay
Human pancreatic carcinoma AsPC-1, PANC-1 and MIA PaCa-2 cell lines were purchased from the Cell Center of the Institute of Basic Medical Sciences, Peking Union Medical College, and cultured in RPMI-1640 medium (Gibco BRL Inc.) with 10% fetal bovine serum (Gibco BRL Inc.), 2 mM glutamine and streptomycin/penicillin. All cell lines were authenticated by short-tandem repeat (STR) profiling. MTT assay was used to evaluate the cytotoxicity of drugs as previously reported.Citation29 Briefly, tumor cells were incubated with drugs for 48 h, then MTT was added. Three replicates of each sample were used.
In vivo therapy study
Female NIH (nu/nu) athymic mice (ages 4–6 weeks, 18–22 g) were selected and inoculated subcutaneously (s.c.) with MIA PaCa-2 cells (1 × 107) or AsPC-1 cells (1 × 107) in the right armpit. Three weeks later, tumors tissue was extracted and transplanted s.c. into new mice. When tumor volume reached about 100 mm3, mice were divided randomly with 6 mice per group. DBDx, Cap or saline was administrated orally and Gem intraperitoneally, once/day, 5 days/week for 3 weeks. Every 3–4 days, the therapeutic effects were checked by measuring tumor size (the longitudinal diameter a, and the perpendicular diameter b) and body weight. Tumor size was calculated according to the formula: tumor volume = ab2/2. When the experiment ended, tumors were surgically extracted and the weights were measured. The inhibitory rates of tumor growth were evaluated by tumor volume or tumor weights, respectively. Statistical analysis was performed with Excel (Microsoft Office Standard 2013). A two tailed unpaired t test was employed. A p value less than 0.05 was considered as statistical significance. Histopathologic examination was performed with H&E (hematoxylin and eosin) staining and observed using a light microscope (Leica Inc.).
Microarrays and gene expression analysis
Mice bearing MIA PaCa-2 xenografts were administrated orally with saline or DBDx at 242 mg/kg, once/day, 5 days/week for 3 weeks. Three days after the last administration, tumors were extracted from mice for gene expression analysis. The experiment was conducted using the Whole Human Genome Oligo Microarray (Agilent Technology) following the method of Agilent One-Color Microarray-Based Gene Expression Analysis in KangChen Bio-tech Inc. (Shanghai, China). Briefly, total RNA was extracted from tumor, amplified and labeled with Cy3-UTP. After hybrization, the arrays were washed, fixed and scanned using the Agilent DNA Microarray Scanner. Agilent Feature Extraction software (version 11.0.1.1) and the GeneSpring GX v12.1 software package were used to analyze the array data. Genes with twofold change were analyzed by Gene ontology (GO) biological process analysis (topGO, Adrian Alexa and Jorg Rahnenfuhrer). The microarray data were accessible through Gene Expression Omnibus (accession number: GSE123521).
Protein array analysis
Mice bearing MIA PaCa-2 xenografts were administrated orally with saline or DBDx at 242 mg/kg once/day, 5 days/week for 3 weeks. Three days after the last administration, sera from treated or control mice were collected and used for protein array analysis. Three mice per group were used. The experiment was performed in KangChen Bio-tech Inc. (Shanghai, China) using antibody arrays (Mouse L-308 Array, Glass; Ray Biotech, USA) according to the manufacturer’s instructions.
Ethics approval
Animal experiments were conducted after approved by the Committee on the Ethics of Animal Experiments of the Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences (IMBF20060302). The study protocols were performed following the Regulation for the Management of Laboratory Animals of the Ministry of Science and Technology of China.
Authors contributions
XJL designed and performed the experiments; YBZ performed the experiments, analyzed the data, and wrote the manuscript; MRZ and YL performed the experiments; YSZ supervised the work and revised the manuscript.
Disclosure of interest
The authors report no conflict of interest.
Additional information
Funding
References
- Ilic M, Ilic I. 2016. Epidemiology of pancreatic cancer. World J Gastroenterol. 22:9694–9705. doi:10.3748/wjg.v22.i44.9694.
- Mohammed A, Janakiram NB, Madka V, Li M, Asch AS, Rao CV. 2018. Current challenges and opportunities for chemoprevention of pancreatic cancer. Curr Med Chem. 25:2535–2544. doi:10.2174/0929867324666170209104453.
- Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, Cruceru ML, Albulescu R. 2014. Cancer stem cells: involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics. World J Gastroenterol. 20:10790–10801. doi:10.3748/wjg.v20.i31.10790.
- Teague A, Lim KH, Wang-Gillam A. 2015. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol. 7:68–84. doi:10.1177/1758834014564775.
- Bittoni A, Andrikou K, Lanese A, Santoni M, Pellei C, Faloppi L, Del Prete M, Giampieri R, Cascinu S. 2015. Treatment of pancreatic cancer. Actuality and perspective. Recenti Prog Med. 106:208–216. doi:10.1701/1868.20404.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. 2011. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. doi:10.1056/NEJMoa1011923.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. 2013. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 369:1691–1703. doi:10.1056/NEJMoa1304369.
- Liu XJ, Zheng YB, Li Y, Wu SY, Zhen YS. 2014. A multifunctional drug combination shows highly potent therapeutic efficacy against human cancer xenografts in athymic mice. PLoS One. 9:e115790. doi:10.1371/journal.pone.0115790.
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. 1997. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 15:2403–2413. doi:10.1200/JCO.1997.15.6.2403.
- Sidaway P. 2017. Pancreatic cancer: addition of capecitabine prolongs overall survival. Nat Rev Clin Oncol. 14:198. doi:10.1038/nrclinonc.2017.21.
- Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, Dunn J, Tudur-Smith C, West J, et al. 2009. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 27:5513–5518. doi:10.1200/JCO.2009.24.2446.
- Chung KH, Ryu JK, Son JH, Lee JW, Jang DK, Lee SH, Kim YT. 2017. Efficacy of capecitabine plus oxaliplatin combination chemotherapy for advanced pancreatic cancer after failure of first-Line gemcitabine-based therapy. Gut Liver. 11:298–305. doi:10.5009/gnl16307.
- Cartwright TH, Cohn A, Varkey JA, Chen YM, Szatrowski TP, Cox JV, Schulz JJ. 2002. Phase II study of oral capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol. 20:160–164. doi:10.1200/JCO.2002.20.1.160.
- van den Borne P, Quax PH, Hoefer IE, Pasterkamp G. 2014. The multifaceted functions of CXCL10 in cardiovascular disease. Biomed Res Int. 2014:893106. doi:10.1155/2014/893106.
- Lee EY, Lee ZH, Song YW. 2009. CXCL10 and autoimmune diseases. Autoimmun Rev. 8:379–383. doi:10.1016/j.autrev.2008.12.002.
- Komai T, Okamura T, Yamamoto K, Fujio K. 2016. The effects of TGF-βs on immune responses. Nihon Rinsho Meneki Gakkai Kaishi. 39:51–58. doi:10.2177/jsci.39.51.
- Hao S, Han X, Wang D, Yang Y, Li Q, Li X, Qiu CH. 2016. Critical role of CCL22/CCR4 axis in the maintenance of immune homeostasis during apoptotic cell clearance by splenic CD8α(+) CD103(+) dendritic cells. Immunology. 148:174–186. doi:10.1111/imm.12596.
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. 2002. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern cooperative oncology group trial E2297. J Clin Oncol. 20:3270–3275. doi:10.1200/JCO.2002.11.149.
- Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, et al. 2006. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 24:3946–3952. doi:10.1200/JCO.2005.05.1490.
- Zhang YD, Yang Q, Jiang ZM, Ma W, Zhou SW, Xie DR. Overall survival of patients with advanced pancreatic cancer improved with an increase in second-line chemotherapy after gemcitabine-based therapy. JOP. 2011;12:131–137.
- Oberstein PE, Olive KP. 2013. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 6:321–337. doi:10.1177/1756283X13478680.
- Hoshi N, Kofunato Y, Yashima R, Shimura T, Takenoshita S. Treating side effects of FOLFIRINOX–A study of the effect of Hange-Shashin-To on preventing diarrhea. Gan To Kagaku Ryoho. 2015;42:2364–2366.
- Kimbara S, Kondo S. 2016. Immune checkpoint and inflammation as therapeutic targets in pancreatic carcinoma. World J Gastroenterol. 22:7440–7452. doi:10.3748/wjg.v22.i33.7440.
- Yano A, Fujii Y, Iwai A, Kageyama Y, Kihara K. 2006. Glucocorticoids suppress tumor angiogenesis and in vivo growth of prostate cancer cells. Clin Cancer Res. 12:3003–3009. doi:10.1016/j.urolonc.2006.12.007.
- Fan Z, Sehm T, Rauh M, Buchfelder M, Eyupoglu IY, Savaskan NE. 2014. Dexamethasone alleviates tumor associated brain damage and angiogenesis. PLoS One. 9:e93264. doi:10.1371/journal.pone.0093264.
- Aozuka Y, Koizumi K, Saitoh Y, Ueda Y, Sakurai H, Saiki I. 2004. Anti-tumor angiogenesis effect of aminopeptidase inhibitor bestatin against B16–BL6 melanoma cells orthotopically implanted into syngeneic mice. Cancer Lett. 216:35–42. doi:10.1016/j.canlet.2004.06.050.
- Spano D, Marshall JC, Marino N, De Martino D, Romano A, Scoppettuolo MN, Bello AM, Di Dato V, Navas L, De Vita G, et al. 2013. Dipyridamole prevents triple-negative breast-cancer progression. Clin Exp Metastasis. 30:47–68. doi:10.1007/s10585-012-9506-0.
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. 2016. Cancer statistics in China, 2015. CA Cancer J Clin. 66:115–132. doi:10.3322/caac.21338.
- Zheng YB, Gong JH, Liu XJ, Li Y, Zhen YS. 2017. A CD13-targeting peptide integrated protein inhibits human liver cancer growth by killing cancer stem cells and suppressing angiogenesis. Mol Carcinog. 56:1395–1404. doi:10.1002/mc.22600.