ABSTRACT
Background
Colorectal cancer (CRC) is a leading cause of cancer-related death. Epithelial-mesenchymal transition (EMT) is a major process in tumor metastasis development. This systematic review aims to describe the role of long non-coding RNA (lncRNA) in EMT in CRC.
Methods
The electronic databases, PubMed, Cochrane, and EMBASE, were searched from January1990 to June 2019 to identify studies examining lncRNA and their role in mediating EMT in CRC. Studies examining clinical specimens and/or in vitro experiments were included.
Results
In 61 identified studies, 54 lncRNAs were increased in CRC compared to normal colorectal epithelium. Increased lncRNA expression was frequently associated with worse survival. Many lncRNAs mediate their effect through competitive endogenous RNA or transcription factor regulation. The ZEB1, 2/E-cadherin, Wnt/β-catenin signaling, and chromatin remodeling pathways are discussed in particular.
Conclusions
lncRNAs are major regulators of EMT and predictor adverse outcome in CRC patients. Future research must focus on delineating lncRNA function prior to potential clinical use.
Introduction
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the third leading cause of cancer-related death in the United States.Citation1 While the prognosis for patients with localized disease is good, with 5-year overall survival rates of approximately 90%, there is a significant decrease in the survival of patients with regional lymph node disease (70%) and distant metastasis (14%). Although screening for colon and rectal cancer has been shown to improve survival, many patients are still diagnosed with advanced stage disease at initial presentation.Citation2
Figure 1. Illustration of the different mechanisms through which lncRNAs can signal. (a) Competitive endogenous RNA function (ceRNA): the lncRNA acts as a functional decoy or “sponge” for other molecules, which prevents the other molecules from executing their function, such as in the case of lncRNA-miRNA sponging; (b) Enhancer function: the lncRNA can act as an enhancer or transcription factor-like molecule in cells to promote gene expression; (c) Guide function: the lncRNA can recruit proteins to a nuclear site,; for instance, to assist in chromatin remodeling; (d) Scaffold function: the lncRNA can act to bring proteins spatially close to each other to aid in ribonuclear protein formation; (e) Signal function: lncRNA acts as a signal for a molecule such as a transcription factor to promote or repress gene expression.
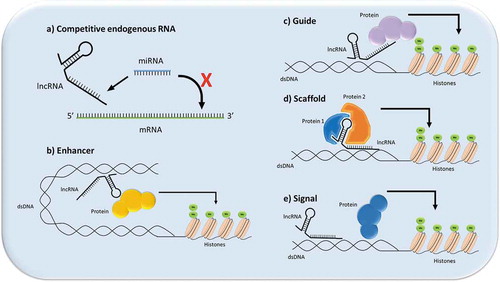
Table 1. In vitro data from selected studies.
Table 2. Clinical data from the selected studies.
Over the past 20 years, there has been significant research into the process of tumor metastasis. One of the critical signaling mechanisms in this process is epithelial-to-mesenchymal transition (EMT). EMT is a process whereby cells transition from an epithelial to a mesenchymal phenotype, through a series of changes in gene expression. Since the initial description by May et al, EMT has become a major focus of cancer research.Citation3 Additionally, it is a critical component of normal embryological development, involved in gastrulation, neural crest formation, and heart morphogenesis.Citation4
At a macroscopic level, cells shift from a locally proliferative and relatively static cell phenotype to a more motile and invasive cell phenotype, which is essential for cancer metastasis.Citation5,Citation6 At the cellular level, epithelial-like cells lose cell polarity, cell-to-cell adhesion, and gain a migratory and invasive phenotype leading to tumor metastasis.Citation7 Epithelial cells express a number of different cell surface markers, most notably E-cadherin, but also cytokeratins, occludins, and claudins among others.Citation8,Citation9 These markers are significant factors in cell-cell adhesion and apical-basal cellular polarity. In contrast, mesenchymal cells are characterized by the expression of markers including vimentin, N-cadherin, and fibronectin.Citation9 These markers are associated with front-rear polarity and stem cell characteristics.
During cancer development, mesenchymal cells are associated with resistance to apoptosis and possess an ability to invade through tissue.Citation6,Citation10 The mesenchymal characteristics that result from EMT activation enable tumor cells to undergo a number of the steps in tumor metastasis including; local invasion at the primary tumor site, blood vessel intravastation, extravasation into distant tissues, and enable survival at distant tissues.Citation7 Activation of EMT is also associated with chemotherapy drug resistance in in vitro and clinical patient studies.Citation11,Citation12
EMT is a complex process and is regulated through different signaling mechanisms. These factors include transcription factors, epigenetic modifiers, and non-coding RNAs. Overall, the EMT program is managed by a number of master regulatory transcription factors, namely; snail family transcriptional repressor 1 (SNAI1), TWIST-related protein 1 (TWIST), snail family transcriptional repressor 2 (SLUG), zinc finger E-box-binding homeobox -(ZEB)1, and ZEB2.Citation13,Citation14 However, these master transcription factors can be regulated by a number of different upstream regulators, which adds a significant layer of complexity.
Epigenetic regulation of EMT can occur through different processes in methylation and acetylation.Citation11,Citation15 Methylation at CpG islands of the promoter region of genes involved in EMT repression has previously been demonstrated.Citation16 Chromatin modifiers such as histone deacteylase 1/2 (HDAC1/2) and lysine-specific histone demethylase 1A (LSD1) have been implicated in EMT and are hypothesized to control the conversion between different intermediate EMT states.Citation17,Citation18 Treatment with a demethylating agent, in vivo, induces an epithelial-like phenotype in both naïve and chemoresistant cell lines.Citation19,Citation20
The field of microRNA, and more recently long non-coding RNA, represent a stimulating avenue of research for EMT. microRNAs (miR) have been clearly defined as regulators of EMT, the miR-200 family being the most notable.Citation11 The miR-200 family mediates EMT by decreasing the expression of ZEB1 and ZEB2 via post-transcriptional repression. In addition, ZEB1 and ZEB2 act on the promoter region of the miR-200 family to transcriptionally repress miR-200 family expression. Decreased expression of the miR-200 family leads to a mesenchymal phenotype, thereby promoting EMT.Citation21 TGF-β is a major regulator of EMT, inducing ZEB1 and SNAIL1 expression, which is also targeted by the miR-200 family to repress EMT.Citation21,Citation22 The miR-200 family has also been shown to induce proliferation through different signaling pathways such as the miR-200/RECK/SKP2, CDKN1B axis, and the miR-200/RASSF2/KRAS/ERK1,2 axis.Citation23,Citation24
lncRNAs are a recently described class of molecules that have roles in normal cellular function and tumorigenesis. They do not code for protein and are defined as being longer than 200 base pairs.Citation25 Similar to messenger RNA, lncRNA are transcribed by RNA polymerase II, but can mediate their action through a number of different mechanisms; competitive endogenous RNA (ceRNA), enhancer, scaffold, signal, and guide function ().Citation26,Citation27 Many lncRNAs are differentially expressed during EMTCitation28 and are involved in EMT activationCitation29-Citation31 and repression.Citation25,Citation32,Citation33
lncRNAs are critical mediators of cell signaling and play a major role in tumor signaling and metastasis. However, the expression of lncRNAs can vary widely across tumor types, suggesting differing roles of lncRNAs in different cancers.Citation34 While a number of reviews have demonstrated the role of lncRNAs in colorectal cancer and in other cancer types, the specific role of lncRNAs in EMT has not been described. Therefore, the aim of this systematic review is to describe in vitro and clinical studies that specifically examine the roles of lncRNAs in different EMT signaling pathways in colorectal cancer. This study also aims to identify current gaps in the literature and to identify future avenues of research and potential therapeutic targets.
Methods
This study was conducted using the “Preferred Reporting for Systematic Reviews and Meta-analysis” guidelines.Citation35,Citation36 The study methodology was registered with the PROSPERO database (Reference: 159776).
Study selection
The electronic PubMed (NCBI, Bethesda, MD), Cochrane Central Register of Controlled Trials (CENTRAL) (John Wiley & Sons, Hoboken, NJ), and EMBASE (Elsevier, Amsterdam, The Netherlands) databases were systematically searched for all studies relating to lncRNA and their role in EMT in colorectal adenocarcinoma. No restrictions were placed upon the language. The search was restricted from January 1st 1990 to June 31st 2019. The following search terms were used: “colorectal cancer,” “colorectal adenocarcinoma,” “colon cancer,” “rectal cancer,” “colon adenocarcinoma,” “rectal adenocarcinoma,” “lncRNA,” “long non-coding RNA,” “Epithelial-mesenchymal transition,” and “EMT.” The Boolean search terms “AND” and “OR” were used to define the search. Searches from all databases were collated and duplicate references removed.
Inclusion & exclusion criteria
Studies were included if they investigated the molecular signaling role of lncRNAs in EMT in colorectal cancer and/or investigated tumoral expression of lncRNAs in human clinical samples along with including related survival outcomes of patients with colorectal cancer. Studies were excluded if they did not specifically pertain to colorectal cancer, were focused on microRNA signaling, or were review papers.
Data extraction & outcomes
Two reviewers (SO’B and CB) extracted data from the selected studies. Titles and abstracts were screened for relevance by these two reviewers, and in the case of uncertain relevance to the review, the senior author reviewed the paper. Other significant articles from the references of the identified papers were included. The following data were extracted from each of the selected studies: authors, year of publication, journal of publication, main study hypothesis, use of human tumor tissue, colorectal cancer cell line selection and use, experimental techniques used, main study findings, and study limitations.
Results
One hundred and forty-nine studies were identified from the queried databases. Additional studies were manually identified from the references. The search process is illustrated in Figure S1. Ninety-five records remained after removal of duplicates. Thirty-four articles were excluded [reports on cancers other than CRC (n = 14), articles focusing on microRNA (n = 7), and review articles (n = 13)], leaving 61 studies for inclusion in the final analysis.Citation37–Citation97
In vitro studies
A composite figure of the lncRNAs and their associated individual signaling pathways is shown in Figure S2 and lncRNAs in which the signaling pathway is not fully explored are described in Figure S3.
Thirty-one distinct lncRNAs were found to regulate EMT through a number of different signaling cascades, some of the most common including the ZEB1, ZEB2/E-cadherin, vimentin pathway, Wnt/β-catenin pathway, JAK/STAT pathway, the mTOR pathway, and the MAPK/ERK pathway ().Citation37–Citation91
The majority (14) of the lncRNAs identified in this study mediate their effect through a ceRNA mechanism, by sponging a target miRNA (Figure S2 and ). In contrast, seven lncRNAs mediate their effect via a transcription-factor like mechanism, and one lncRNA acts through a scaffold mechanism to mediate an effect on EMT. Ten of the lncRNAs do not have the mechanism fully delineated and represent a significant area for further research (Figure S3).
Pathway affected
ZEB1, 2/E-cadherin, vimentin signaling
The ZEB1, 2/E-cadherin, vimentin axis is a well characterized signaling pathway critical to the process of EMT. Ten of the lncRNAs were regulators of this pathway (H19, SNHG6, SPRY4-IT1, CHRF, N-BLR, XIST, UICLM, B3GALT5-AS1, SNHG15) (),Citation37–Citation46,Citation50,Citation92 the majority of which (9/10) mediated their effect through a ceRNA mechanism. The other lncRNA mediated its effect through a transcription factor regulation mechanism (SNHG15).Citation47 The miR-200 family was the most common miRNA target of the lncRNAs examined (XIST, N-BLR, and H19).Citation37–Citation39,Citation41,Citation50,Citation92 As expected, the major transcription factors that regulate EMT were targeted by lncRNAs to regulate the process (ZEB1, ZEB2, TWIST, SNAIL, SLUG). Few studies have examined the interaction between lncRNAs that target the same molecule in a signaling cascade. For example, SNHG6 and SPRY1-IT1 both directly target miR-101, which acts on the transcription factor ZEB1.Citation43,Citation44 However, it is not clear what effect SNHG6 knockdown would have on the expression and other downstream functions of SPRY1-IT1. These examples indicate that a systems biology approach is needed for exploring the interaction between lncRNAs,
Wnt/β-catenin signaling
The Wnt/β-catenin signaling pathway is another critical mediator of EMT that is regulated by lncRNAs. Seven lncRNAs targeted the Wnt/β-catenin signaling cascade (H19, PlncRNA-1, TUG1, SLCO4A1-AS1, CYTOR, CTD903, lncTCF7).Citation49,Citation50,Citation52,Citation53,Citation60,Citation93,Citation98 Five of the lncRNAs converged on β-catenin to mediate their effect, and three of the lncRNAs mediated their effect through a ceRNA mechanism (H19, PlncRNA-1, TUG1).Citation49,Citation50,Citation60,Citation93,Citation98 SLCO4A1-AS1 and CYTOR regulated upstream molecules of β-catenin through a transcriptional factor mechanism.Citation51,Citation54 Both lncTCF7 and CTD903 have been shown to mediate EMT through Wnt/β-catenin signaling, but the exact mechanism of action was not fully examined.Citation52,Citation53
Interestingly, some identified lncRNAs mediate EMT through a number of different signaling cascades by different mechanisms. H19 is a well-characterized lncRNA that regulates Wnt/β-catenin signaling, ZEB1, 2/E-cadherin, vimentin signaling, and MAPK/ERK signaling through different ceRNA mechanisms.Citation38,Citation39,Citation50,Citation51 This demonstrates that lncRNAs can have complex roles in regulating a specific cellular characteristic, such as EMT. Although examining single signaling pathways is critical to understanding a lncRNA’s mechanism of action, it is important to examine lncRNAs in a network signaling approach, as it may help identify potential therapeutic targets.
Epigenetic regulation- chromatin remodeling
Epigenetic modification of gene expression through chromatin remodeling, such as histone methylation and acetylation, has been shown to mediate EMT. Four lncRNAs identified in this study regulate EMT by targeting different core molecules associated with methylation, such as EZH2 (enhancer of zeste homolog 2) and DNMT3A (DNA methyltransferase 3 alpha).Citation55,Citation58,Citation59,Citation61 EZH2 is the enzymatic component of the polycomb repressive complex 2, which is critical in histone methylation. DNMT3A is an enzyme that transfers methyl groups to CpG islands, leading to gene repression. Two lncRNAs regulate EMT by targeting core molecules associated with acetylation, p300, and HDAC1 (histone deactylase 1).Citation59,Citation60 Four lncRNAs directly target miRNAs as their mechanism of action but, in contrast, SATB2-AS1 has a more complex signaling mechanism. It acts through a scaffold mechanism to recruit p300 and to acetylate both H3K27 and H3K9 at the promoter region of SATB2. In turn, SATB2 leads to the subsequent recruitment of HDAC1 to the promoter of SNAIL, thereby silencing SNAIL and inhibiting EMT.
Other signaling cascades
JAK-STAT3 signaling
Both BC200 and lncRNA AB073614 act through a transcriptional factor mechanism to reduce STAT3 phosphorylation in the JAK/STAT signaling cascade.Citation62,Citation94 This leads to the modulation of matrix metalloproteinases and induces EMT.
mTOR signaling
The mTOR pathway is a complex signaling family involved in EMT. ZFAS1 and UCA1 induce EMT through a ceRNA mechanism but target different molecules in the pathway.Citation63,Citation95 Through binding with miR-150, ZFAS1 targets VEGFA and subsequently Akt. Few studies examine upstream regulators of lncRNA, and interestingly, the authors demonstrate an upstream regulator of ZFAS1 through the SP1 transcription factor.Citation63 UCA1 directly binds miR-143, which targets mTOR to induce EMT. However, the authors demonstrate the complexity of the tumor microenvironment and its effect on lncRNA expression by co-culturing the cells with cancer-associated fibroblast-conditioned media.Citation95 This suggests that lncRNA expression can be changed through exogenous agents, indicating a potential avenue for research into their therapeutic modulation.
MAPK/ERK signaling
As previously mentioned, H19 acts through a ceRNA mechanism in both Wnt/β-catenin and in ZEB1, 2/E-cadherin, and vimentin signaling. H19 also directly binds miR-194, which targets FoxM1, a downstream target of the MAPK/ERK signaling pathway.Citation64 SNHG7 also affects MAPK/ERK signaling by targeting GALNT1, a downstream molecule in the MAPK/ERK signaling pathway.Citation65,Citation99 Three other lncRNAs (BANCR, NNT-AS1, SLCA25-AS1) mediate their effect on EMT through MAPK/ERK signaling, but further work is needed to identify their direct targets.Citation66–Citation68
TGF-β signaling
The TGF-β signaling superfamily is a complex mediator of EMT. Both PVT1 and LINC001133 mediate EMT through TGF-β signaling but further work is needed to identify their direct targets.Citation69,Citation70
Neuropilin 2 signaling
XIST targets neuropilin 2 signaling through a ceRNA mechanism with miR-486.Citation72 This study is limited as it does not explore downstream targets of NRP2, marking an area of future research.Citation100
OCT4 signaling
CRCMSL also functions through a ceRNA-like mechanism, but binds HMGB2 in the cellular cytoplasm and prevents its shuttling to the nucleus. This prevents the interaction between HMGB2 and OCT4 in the nucleus, promoting a mesenchymal phenotype.Citation75 This is a different mechanism compared other included studies that demonstrate lncRNAs bind miRNAs, and shows the complexity in studying lncRNAs.
Caspase signaling
Both lncRNA-ATB and LINC00959 mediate an effect on EMT through caspase signaling,Citation76,Citation77 but the exact signaling mechanism is unclear. However, it suggests an important avenue of investigation as caspase signaling is typically associated with apoptosis.
NOTCH signaling
FOXD2-AS1 mediates EMT through NOTCH signaling, but the exact mechanism requires further investigation.Citation79
NF-κB signaling
CYTOR acts through a transcriptional factor mechanism to regulate Wnt/β-catenin signaling; it can also act through a scaffold mechanism by mediating the interaction between NCL and Sam86 in the NF-κB signaling pathway.Citation78 In a hypoxia induction model, CPS1-IT1 induces HIF1-α signaling to mediate EMT.Citation73,Citation74
Function affected
Chemoresistance
Previous studies have demonstrated that a mesenchymal phenotype is associated with chemoresistance in colorectal cancer.Citation101,Citation102 Only three of the included studies examine lncRNAs regulating EMT in the context of chemoresistance.Citation41,Citation55,Citation68 N-BLR regulates EMT through miR-200 family signaling, thereby affecting the downstream expression of ZEB1 and ZEB2.Citation41 N-BLR also regulates chemoresistance through this mechanism. The miR-200 family is an extensively studied regulator of chemoresistance.Citation11 Similarly, MALAT1 is involved in CRC chemoresistance. MALAT1 expression is increased in a chemoresistant cell line model compared to a parental cell line, but this effect on chemoresistance is mediated through epigenetic regulation of EZH2, by directly binding miR-218.Citation55 In contrast, SLC25A25-AS1 is decreased in colorectal cancer compared to normal tissue, and knockdown leads to chemoresistance and increased proliferation, through MAPK/ERK signaling.Citation68 Many studies do not examine the role of lncRNAs in chemoresistance, and there is significant scope to examine their role as a potential modulator of this process to improve patient outcomes.
Cell proliferation
Thirty-one studies demonstrated that increased lncRNA expression was associated with increased cellular proliferation in vitro.Citation37,Citation38,Citation40–Citation45,Citation49,Citation50,Citation54,Citation58,Citation59,Citation61–Citation63,Citation65-Citation67,Citation69,Citation72,Citation73,Citation77,Citation79,Citation80,Citation83,Citation84,Citation86–Citation90,Citation94-Citation97
In contrast, decreased expression of four lncRNAs was associated with increased cellular proliferation.Citation46,Citation68,Citation75,Citation85
Migration and invasion
Thirty-nine studies demonstrated increased lncRNA expression was associated with increased cellular migration and invasion in vitro.Citation37–Citation45,Citation47-Citation66,Citation69,Citation72,Citation73,Citation76–Citation81,Citation83,Citation84,Citation86,Citation87,Citation89–Citation97 In contrast, decreased expression of four lncRNAs was associated with increased cellular migration and invasion.Citation46,Citation70,Citation75,Citation85
Tumor growth in vivo
Seven studies demonstrated increased lncRNA expression was associated with increased tumor growth in vivo.Citation39,Citation47,Citation65,Citation66,Citation74,Citation88,Citation90 In contrast, decreased expression of two lncRNAs was associated with increased tumor growth in vivo.Citation46,Citation75
Human studies
Fifty-three lncRNAs were increased in expression in cancer tissue, while 8 lncRNAs were decreased in expression when compared to normal adjacent colorectal epithelium (Table 2). Interestingly, a majority of studies examine lncRNAs that are increased in expression compared to normal colon epithelium, suggesting their role as biomarkers, but also underscoring their carcinogenic function. As is evident in , for the majority of presented lncRNA, increased expression is associated with an increase in tumor stage (higher incidence of metastatic disease), supporting their role in EMT.
Association with overall survival and recurrence-free survival
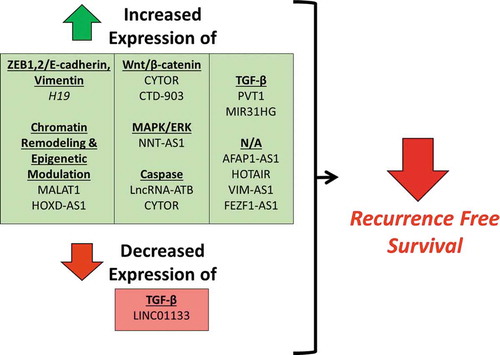
Differential expression of 35 lncRNAs was associated with worse overall survival in clinical patient samples (). This is in agreement with the process of EMT being associated with tumor metastasis. For 29 of these lncRNAs, increased expression was associated with worse overall survival, while decreased expression of six lncRNAs was associated with worse overall survival. Only 14 studies examined for differences in recurrence-free survival (). Thirteen studies demonstrated that increased lncRNA expression was associated with worse recurrence-free survival, with a single study demonstrating that decreased lncRNA expression was associated with worse recurrence-free survival. The clinical association of differential lncRNA expression supports the hypothesis that these lncRNAs have a major role in tumor signaling and in patient prognosis. However, the absence of this association does not rule out that a lncRNA has a role in tumor signaling. In fact, many of these studies demonstrate an association between lncRNA expression and clinical tumor stage or other prognostic indicators (Table 2). This suggests that a given lncRNA may have a role in a specific facet of EMT, but when examined alone, may not have an association with adverse clinical outcomes.
Figure 2. Differential expression of lncRNAs associated with worse overall survival.
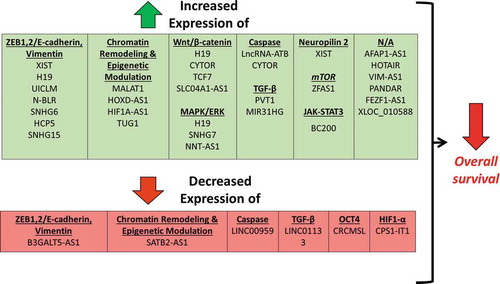
Figure 3. Differential expression of lncRNAs associated with worse recurrence-free survival.
Discussion
The aim of this systematic review was to describe the role of lncRNAs in the process of EMT in CRC and to discuss their mechanism of action as well as their interaction in particular signaling pathways. We also describe a number of areas of potential research regarding lncRNAs.
The majority of the studies identified lncRNAs, which are upregulated in CRC. These lncRNA were identified from screening sequencing data sets or had previously been associated with tumorigenesis and EMT in different types of cancer. Cancer cells, both in vivo and in vitro, were shown to phenotypically change their behavior following knock-down of these lncRNAs. Several investigators identified a mechanism partly mediated by a lncRNA-miRNA-mRNA mechanism or a competitive endogenous RNA-type mechanism. Additionally, lncRNAs were able to directly affect tumor-signaling pathways by interacting with key-signaling molecules. Finally, while knock down of various lncRNAs in an in vitro CRC model will lead to phenotypic changes, the responsible molecular mechanism has not been explored in a number of studies and provides a large area for further research.
Differential expression of lncRNAs within the tumor microenvironment is another interesting area of lncRNA investigation. The majority of studies identified in this review utilize whole tumor RNA isolation, which lends itself to contamination with stromal and white blood cell RNA. For this reason, investigators run the risk of biasing results.Citation103 Previous studies have demonstrated the heterogeneity of gene expression in colon cancer. This is particularly important in the study of EMT, as there is progressive change in gene expression, such as with the miR-200 family.Citation103 Laser capture microdissection allows for the selection of cancer cells without stromal or other “contamination.” However, the microenvironment represents an avenue of further investigation with recent studies examining the role of exosome-derived lncRNAs in tumor signaling.Citation104 For example, Jahangiri et al. demonstrated that co-culturing cancer-associated fibroblasts leads to increased UCA-1 expression, with a subsequent shift to a mesenchymal phenotype.Citation105
A number of studies did not explore the lncRNA mechanism of action in detail. As lncRNAs have multiple potential mechanisms of action and often act through multiple mechanisms at once, these can be difficult to delineate. Some authors have suggested mechanism discovery pipelines to attempt to refine this process.Citation27 In particular, the development of RNA-RNA binding and RNA-protein binding bioinformatics algorithms may assist in describing potential mechanisms of action.Citation106 Others have created bioinformatics algorithms to predict lncRNA-miRNA binding, one of the most commonly described mechanisms of action.Citation107 Combining these bioinformatics resources with exploratory studies may provide novel areas of research. Such a systems biology approach lends itself to the discovery of new lncRNAs and their mechanisms of action.
While the majority of studies included in this review group colon and rectal adenocarcinoma together, there are significant molecular and clinical differences between these two types of large bowel cancers. Right-sided colon adenocarcinoma tumors are more likely to be highly microsatellite instable tumors, whereas left-sided colon adenocarcinomas are more likely to be chromosomal instable tumors.Citation108 BRAF mutations more commonly occur in colon adenocarcinomas, and rectal adenocarcinomas typically have APC and TP53 mutations.Citation109 Clinically, rectal cancer has a tendency toward pulmonary metastases, whereas colon cancer has a tendency to develop liver metastases.Citation110,Citation111 Typical adjuvant chemotherapy strategies for colon cancer include fluorouracil-based therapies, but for high microsatellite instable tumors, there does not appear to be a survival benefit for such treatment.Citation112 As there are such clear differences between colon and rectal cancer, it is important for investigators to report on differences in lncRNA expression between left-sided, right-sided, and rectal cancers, as well as to report on specific details of the cancer as above.
There are some limitations to this study. Although a large number of lncRNAs are associated with adverse clinical outcomes, and are shown to mediate an effect on EMT-related genes, their mechanism is not fully explored. Therefore, the effect of these lncRNAs on EMT is difficult to fully summarize. While EMT is a critical component of tumor metastasis, this study focuses only on EMT and does not go into detail on the effect of lncRNAs on other hallmarks of carcinogenesis. Few studies delineate between lncRNA expression in the tumor microenvironment compared to expression directly from tumor cells themselves. This limits our ability to confirm whether the expression is increased in colon cancer cells themselves. Some of the included studies examining lncRNA expression in clinical samples are small and should be confirmed in larger data sets.
lncRNAs play a major role in tumor signaling and in EMT in CRC. This review describes clinical studies and in vitro mechanisms of the lncRNAs where available. Many lncRNAs, however, have multiple mechanisms of action, and further research is needed. Tumoral lncRNA expression may have a prognostic role in the clinical setting, which, therefore, warrants further work and validation in independent clinical samples.
Author contributions
SOB and CB performed the literature search, SOB, CB, JH, CF, KS, MP, and JB extracted and verified the study data, SOB, CB, JH, CF, KS, MP, and JB synthesized study data and created figures and tables. SOB, CF, and SG wrote the paper and all authors were involved in final draft changes. SOB and SG developed the study and were in charge of overall direction and planning.
Disclosure of Potential Conflicts of Interest
Susan Galandiuk receives a stipend as Editor-in-Chief of the journal “Diseases of the Colon and Rectum.” The other authors do not have conflicts of interest.
Supplemental Material
Download Zip (843.8 KB)Supplementary data:
Supplemental material for this article can be accessed here.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi:10.3322/caac.21442.
- Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904–910. doi:10.7326/0003-4819-123-12-199512150-00002.
- Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–339. doi:10.1083/jcb.95.1.333.
- Kim DH, Xing T, Yang Z, Dudek R, Lu Q, Chen Y-H. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: a comprehensive overview. J Clin Med. 2017;7:1. doi:10.3390/jcm7010001.
- Nistico P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4:a011908.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi:10.1172/JCI39104.
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi:10.1038/nrm1835.
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437.
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi:10.1016/j.cell.2009.11.007.
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi:10.1016/j.cell.2008.03.027.
- O’Brien SJ, Carter JV, Burton JF, Oxford BG, Schmidt MN, Hallion JC, Galanduik S. The role of the miR-200 family in epithelial–mesenchymal transition in colorectal cancer: a systematic review. Int J Cancer. 2018;142:2501–2511.
- Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi:10.1038/nrclinonc.2017.44.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi:10.1038/nrm3758.
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi:10.1038/nrc2131.
- Lin Y, Dong C, Zhou BP. Epigenetic regulation of EMT: the snail story. Curr Pharm Des. 2014;20:1698–1705. doi:10.2174/13816128113199990512.
- Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene. 2012;31:2062–2074. doi:10.1038/onc.2011.383.
- Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi:10.1128/MCB.24.1.306-319.2004.
- Lin Y, Wu Y, Li J, Dong C, Ye X, Chi YI, Evers BM, Zhou BP. The SNAG domain of Snail1 functions as a molecular hook for recruiting lysine-specific demethylase 1. Embo J. 2010;29:1803–1816. doi:10.1038/emboj.2010.63.
- Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62(9):1315–1326. doi:10.1136/gutjnl-2011-301846.
- Tanaka S, Hosokawa M, Ueda K, Iwakawa S. Effects of decitabine on invasion and exosomal expression of miR-200c and miR-141 in oxaliplatin-resistant colorectal cancer cells. Biol Pharm Bull. 2015;38:1272–1279. doi:10.1248/bpb.b15-00129.
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9(6):582–589. doi:10.1038/embor.2008.74.
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi:10.1038/cr.2009.5.
- Carter JV, O’Brien SJ, Burton JF, Oxford BG, Stephen V, Hallion J, Bishop C, Galbraith NJ, Eichenberger MR, Sarojini H, et al. The microRNA‑200 family acts as an oncogene in colorectal cancer by inhibiting the tumor suppressor RASSF2. Oncol Lett. 2019;18:3994–4007. doi:10.3892/ol.2019.10753.
- Pan Y, Liang H, Chen W, Zhang H, Wang N, Wang F, Zhang S, Liu Y, Zhao C, Yan X. microRNA-200b and microRNA-200c promote colorectal cancer cell proliferation via targeting the reversion-inducing cysteine-rich protein with kazal motifs. RNA Biol. 2015;12(3):276–289. doi:10.1080/15476286.2015.1017208.
- Sun Y, Daemen A, Hatzivassiliou G, Arnott D, Wilson C, Zhuang G, Gao M, Liu P, Boudreau A, Johnson L. Metabolic and transcriptional profiling reveals pyruvate dehydrogenase kinase 4 as a mediator of epithelial-mesenchymal transition and drug resistance in tumor cells. Cancer Metabol. 2014;2(1):20. doi:10.1186/2049-3002-2-20.
- Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi:10.1016/j.molcel.2011.08.018.
- Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi:10.1146/annurev-biochem-051410-92902.
- Richards EJ, Zhang G, Li ZP, Permuth-Wey J, Challa S, Li Y, Kong W, Dan S, Bui MM, Coppola D, et al. Long non-coding RNAs (LncRNA) regulated by transforming growth factor (TGF) beta: lncRNA-hit-mediated TGFbeta-induced epithelial to mesenchymal transition in mammary epithelia. J Biol Chem. 2015;290:6857–6867. doi:10.1074/jbc.M114.610915.
- Flockhart RJ, Webster DE, Qu K, Mascarenhas N, Kovalski J, Kretz M, Khavari PA. BRAF V600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. 2012;22:1006–1014. doi:10.1101/gr.140061.112.
- Guo F, Parker Kerrigan BC, Yang D, Hu L, Shmulevich I, Sood AK, Xue F, Zhang W. Post-transcriptional regulatory network of epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions. J Hematol Oncol. 2014;7(1):19. doi:10.1186/1756-8722-7-19.
- Lou KX, Li ZH, Wang P, Liu Z, Chen Y, Wang XL, Cui H-X. Long non-coding RNA BANCR indicates poor prognosis for breast cancer and promotes cell proliferation and invasion. Eur Rev Med Pharmacol Sci. 2018;22:1358–1365. doi:10.26355/eurrev_201803_14479.
- Huang JF, Guo YJ, Zhao CX, Yuan SX, Wang Y, Tang GN, Zhou W-P, Sun S-H. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology (Baltimore, Md). 2013;57:1882–1892. doi:10.1002/hep.26195.
- Lv D, Wang Y, Zhang Y, Cui P, Xu Y. Downregulated long non-coding RNA DREH promotes cell proliferation in hepatitis B virus-associated hepatocellular carcinoma. Oncol Lett. 2017;14:2025–2032. doi:10.3892/ol.2017.6436.
- Li S, Li B, Zheng Y, Li M, Shi L, Pu X. Exploring functions of long noncoding RNAs across multiple cancers through co-expression network. Sci Rep. 2017;7:754. doi:10.1038/s41598-017-00856-8.
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097.
- Mahid SS, Hornung CA, Minor KS, Turina M, Galandiuk S. Systematic reviews and meta-analysis for the surgeon scientist. BJS. 2006;93:1315–1324. doi:10.1002/bjs.5596.
- Chen DL, Chen LZ, Lu YX, Zhang DS, Zeng ZL, Pan ZZ, Huang P, Wang F-H, Li Y-H, Ju H-Q, et al. Long noncoding RNA XIST expedites metastasis and modulates epithelial-mesenchymal transition in colorectal cancer. Cell Death Dis. 2017;8:e3011. doi:10.1038/cddis.2017.421.
- Chen SW, Zhu J, Ma J, Zhang JL, Zuo S, Chen GW, Wang X, Pan Y-S, Liu Y-C, Wang P-Y, et al. Overexpression of long non-coding RNA H19 is associated with unfavorable prognosis in patients with colorectal cancer and increased proliferation and migration in colon cancer cells. Oncol Lett. 2017;14:2446–2452. doi:10.3892/ol.2017.6390.
- Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao L-J, Wan DCC, Zhang J-F, et al. The LncRNA H19 promotes epithelial to mesenchymal transition by functioning as MiRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513–22525. doi:10.18632/oncotarget.4154.
- Chen DL, Lu YX, Zhang JX, Wei XL, Wang F, Zeng ZL, Pan -Z-Z, Yuan Y-F, Wang F-H, Pelicano H, et al. Long non-coding RNA UICLM promotes colorectal cancer liver metastasis by acting as a ceRNA for microRNA-215 to regulate ZEB2 expression. Theranostics. 2017;7:4836–4849. doi:10.7150/thno.20942.
- Rigoutsos I, Lee SK, Nam SY, Anfossi S, Pasculli B, Pichler M, Jing Y, Rodriguez-Aguayo C, Telonis AG, Rossi S, et al. N-BLR, a primate-specific non-coding transcript leads to colorectal cancer invasion and migration. Genome Biol. 2017;18(1):98. doi:10.1186/s13059-017-1224-0.
- Shen F, Cai W-S, Feng Z, Chen J-W, Feng J-H, Liu Q-C, Fang Y-P, Li K-P, Xiao H-Q, Cao J, et al. Long non-coding RNA SPRY4-IT1 pormotes colorectal cancer metastasis by regulate epithelial-mesenchymal transition. Oncotarget. 2017;8(9):14479–14486. doi:10.18632/oncotarget.10407.
- Jin J, Chu Z, Ma P, Meng Y, Yang Y. Long non-coding RNA SPRY4-IT1 promotes proliferation and invasion by acting as a ceRNA of miR-101-3p in colorectal cancer cells. Tumour Biol. 2017;39:1010428317716250. doi:10.1177/1010428317716250.
- Wang X, Lai Q, He J, Li Q, Ding J, Lan Z, Gu C, Yan Q, Fang Y, Zhao X, et al. LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-beta/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int J Med Sci. 2019;16:51–59. doi:10.7150/ijms.27359.
- Yang C, Sun J, Liu W, Yang Y, Chu Z, Yang T, Gui Y, Wang D. Long noncoding RNA HCP5 contributes to epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation and interacting with miR-139-5p. Am J Transl Res. 2019;11:953–963.
- Wang L, Wei Z, Wu K, Dai W, Zhang C, Peng J, He Y. Long noncoding RNA B3GALT5-AS1 suppresses colon cancer liver metastasis via repressing microRNA-203. Aging. 2018;10:3662–3682. doi:10.18632/aging.101628.
- Jiang H, Li T, Qu Y, Wang X, Li B, Song J, Sun X, Tang Y, Wan J, Yu Y. Long non-coding RNA SNHG15 interacts with and stabilizes transcription factor slug and promotes colon cancer progression. Cancer Lett. 2018;425:78–87. doi:10.1016/j.canlet.2018.03.038.
- Tao Y, Han T, Zhang T, Ma C, Sun C. LncRNA CHRF-induced miR-489 loss promotes metastasis of colorectal cancer via TWIST1/EMT signaling pathway. Oncotarget. 2017;8:36410–36422. doi:10.18632/oncotarget.16850.
- Jia GQ, Zhang MM, Wang K, Zhao GP, Pang MH, Chen ZY. Long non-coding RNA PlncRNA-1 promotes cell proliferation and hepatic metastasis in colorectal cancer. J Cell Biochem. 2018. doi:10.1002/jcb.27031.
- Ding D, Li C, Zhao T, Li D, Yang L, Zhang B. LncRNA H19/miR-29b-3p/PGRN axis promoted epithelial-mesenchymal transition of colorectal cancer cells by acting on wnt signaling. Mol Cells. 2018;41:423–435. doi:10.14348/molcells.2018.2258.
- Yue B, Liu C, Sun H, Liu M, Song C, Cui R, Qiu S, Zhong M. A positive feed-forward loop between LncRNA-CYTOR and Wnt/β-catenin signaling promotes metastasis of colon cancer. Mol Ther. 2018;26:1287–1298. doi:10.1016/j.ymthe.2018.02.024.
- Yuan Z, Yu X, Ni B, Chen D, Yang Z, Huang J, Wang J, Chen D, Wang L. Overexpression of long non-coding RNA-CTD903 inhibits colorectal cancer invasion and migration by repressing Wnt/β-catenin signaling and predicts favorable prognosis. Int J Oncol. 2016;48(6):2675–2685. doi:10.3892/ijo.2016.3447.
- Li T, Zhu J, Wang X, Chen G, Sun L, Zuo S, Zhang J, Chen S, Ma J, Yao Z. Long non-coding RNA lncTCF7 activates the Wnt/beta-catenin pathway to promote metastasis and invasion in colorectal cancer. Oncol Lett. 2017;14:7384–7390. doi:10.3892/ol.2017.7154.
- Yu J, Han Z, Sun Z, Wang Y, Zheng M, Song C. LncRNA SLCO4A1-AS1 facilitates growth and metastasis of colorectal cancer through beta-catenin-dependent Wnt pathway. J Exp Clin Cancer Res. 2018;37:222. doi:10.1186/s13046-018-0896-y.
- Li P, Zhang X, Wang H, Wang L, Liu T, Du L, Yang Y, Wang C. MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol Cancer Ther. 2017;16:739–751. doi:10.1158/1535-7163.MCT-16-0591.
- Xiong Y, Wang J, Zhu H, Liu L, Jiang Y. Chronic oxymatrine treatment induces resistance and epithelial-mesenchymal transition through targeting the long non-coding RNA MALAT1 in colorectal cancer cells. Oncol Rep. 2018;39:967–976. doi:10.3892/or.2018.6204.
- Liu X, Cui L, Hua D. Long non-coding RNA XIST regulates miR-137-EZH2 axis to promote tumor metastasis in colorectal cancer. Oncol Res. 2018;27:99–106. doi:10.3727/096504018X15195193936573.
- Li X, Zhao X, Yang B, Li Y, Liu T, Pang L, Fan Z, Ma W, Liu Z, Li Z. Long non-coding RNA HOXD-AS1 promotes tumor progression and predicts poor prognosis in colorectal cancer. Int J Oncol. 2018;53:21–32. doi:10.3892/ijo.2018.4400.
- Lin J, Shi Z, Yu Z, He Z. LncRNA HIF1A-AS2 positively affects the progression and EMT formation of colorectal cancer through regulating miR-129-5p and DNMT3A. Biomed Pharmacother. 2018;98:433–439. doi:10.1016/j.biopha.2017.12.058.
- Sun J, Ding C, Yang Z, Liu T, Zhang X, Zhao C, Wang J. The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial-mesenchymal transition. J Transl Med. 2016;14(1):42. doi:10.1186/s12967-016-0786-z.
- Wang YQ, Jiang DM, Hu SS, Zhao L, Wang L, Yang MH, Ai M-L, Jiang H-J, Han Y, Ding Y-Q. SATB2-AS1 suppresses colorectal carcinoma aggressiveness by inhibiting SATB2-dependent snail transcription and epithelial–mesenchymal transition. Cancer Res. 2019. doi:10.1158/0008-5472.CAN-18-2900.
- Wu K, Xu K, Liu K, Huang J, Chen J, Zhang J, Zhang N. Long noncoding RNA BC200 regulates cell growth and invasion in colon cancer. Int J Biochem Cell Biol. 2018;99:219–225. doi:10.1016/j.biocel.2018.04.001.
- Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T, He B, Pan Y, Sun H, Wang S. SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 2018;9(10):982. doi:10.1038/s41419-018-0962-6.
- Li CF, Li YC, Wang Y, Sun LB. The effect of LncRNA H19/miR-194-5p axis on the epithelial-mesenchymal transition of colorectal adenocarcinoma. Cell Physiol Biochem. 2018;50:196–213. doi:10.1159/000493968.
- Shan Y, Ma J, Pan Y, Hu J, Liu B, Jia L. LncRNA SNHG7 sponges miR-216b to promote proliferation and liver metastasis of colorectal cancer through upregulating GALNT1. Cell Death Dis. 2018;9:722. doi:10.1038/s41419-018-0759-7.
- Guo Q, Zhao Y, Chen J, Hu J, Wang S, Zhang D, Sun Y. BRAF-activated long non-coding RNA contributes to colorectal cancer migration by inducing epithelial-mesenchymal transition. Oncol Lett. 2014;8:869–875. doi:10.3892/ol.2014.2154.
- Wang Q, Yang L, Hu X, Jiang Y, Hu Y, Liu Z, Liu J, Wen T, Ma Y, An G. Upregulated NNT-AS1, a long noncoding RNA, contributes to proliferation and migration of colorectal cancer cells in vitro and in vivo. Oncotarget. 2017;8(2):3441–3453. doi:10.18632/oncotarget.13840.
- Li Y, Huang S, Li Y, Zhang W, He K, Zhao M, Lin H, Li D, Zhang H, Zheng Z, et al. Decreased expression of LncRNA SLC25A25-AS1 promotes proliferation, chemoresistance, and EMT in colorectal cancer cells. Tumour Biol. 2016;37(10):14205–14215. doi:10.1007/s13277-016-5254-0.
- Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110(1):164–171. doi:10.1038/bjc.2013.698.
- Kong J, Sun W, Li C, Wan L, Wang S, Wu Y, Xu E, Zhang H, Lai M. Long non-coding RNA LINC01133 inhibits epithelial–mesenchymal transition and metastasis in colorectal cancer by interacting with SRSF6. Cancer Lett. 2016;380(2):476–484. doi:10.1016/j.canlet.2016.07.015.
- Eide PW, Eilertsen IA, Sveen A, Lothe RA. Long noncoding RNA MIR31HG is a bona fide prognostic marker with colorectal cancer cell-intrinsic properties. Int J Cancer. 2019;144:2843–53.
- Liu A, Liu L, Lu H. LncRNA XIST facilitates proliferation and epithelial-mesenchymal transition of colorectal cancer cells through targeting miR-486-5p and promoting neuropilin-2. J Cell Physiol. 2019;234:13747–61.
- Zhang W, Yuan W, Song J, Wang S, Gu X. LncRna CPS1-IT1 suppresses cell proliferation, invasion and metastasis in colorectal cancer. Cell Physiol Biochem. 2017;44:567–580. doi:10.1159/000485091.
- Zhang W, Yuan W, Song J, Wang S, Gu X. LncRNA CPS1-IT1 suppresses EMT and metastasis of colorectal cancer by inhibiting hypoxia-induced autophagy through inactivation of HIF-1α. Biochimie. 2018;144:21–27. doi:10.1016/j.biochi.2017.10.002.
- Han Q, Xu L, Lin W, Yao X, Jiang M, Zhou R, Sun X, Zhoa L. Long noncoding RNA CRCMSL suppresses tumor invasive and metastasis in colorectal carcinoma through nucleocytoplasmic shuttling of HMGB2. Oncogene. 2019;38:3019–32.
- Yue B, Qiu S, Zhao S, Liu C, Zhang D, Yu F, Peng Z, Yan D. LncRNA-ATB mediated E-cadherin repression promotes the progression of colon cancer and predicts poor prognosis. J Gastroenterol Hepatol. 2016;31(3):595–603. doi:10.1111/jgh.13206.
- Sun Z-Q, Chen C, Zhou Q-B, Liu J-B, Yang S-X, Li Z, Ou C-L, Sun X-T, Wang G-X, Song J-M. Long non-coding RNA LINC00959 predicts colorectal cancer patient prognosis and inhibits tumor progression. Oncotarget. 2017;8(57):97052–97060. doi:10.18632/oncotarget.21171.
- Wang X, Yu H, Sun W, Kong J, Zhang L, Tang J, Wang J, Xu E, Lai M, Zhang H, et al. The long non-coding RNA CYTOR drives colorectal cancer progression by interacting with NCL and Sam68. Mol Cancer. 2018;17(1):110. doi:10.1186/s12943-018-0860-7.
- Yang X, Duan B, Zhou X. Long non-coding RNA FOXD2-AS1 functions as a tumor promoter in colorectal cancer by regulating EMT and notch signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21:3586–3591.
- Han X, Wang L, Ning Y, Li S, Wang Z. Long non-coding RNA AFAP1-AS1 facilitates tumor growth and promotes metastasis in colorectal cancer. Biol Res. 2016;49:36. doi:10.1186/s40659-016-0094-3.
- Bo H, Fan L, Li J, Liu Z, Zhang S, Shi L, Guo C, Li X, Liao Q, Zhang W, et al. High expression of lncRNA AFAP1-AS1 promotes the progression of colon cancer and predicts poor prognosis. J Cancer. 2018;9(24):4677–4683. doi:10.7150/jca.26461.
- Ye Z, Zhou M, Tian B, Wu B, Li J. Expression of lncRNA-CCAT1, E-cadherin and N-cadherin in colorectal cancer and its clinical significance. Int J Clin Exp Med. 2015;8:3707–3715.
- Wu ZH, Wang XL, Tang HM, Jiang T, Chen J, Lu S, Qui GQ, Peng ZH, Yan DW. Long non-coding RNA HOTAIR is a powerful predictor of metastasis and poor prognosis and is associated with epithelial-mesenchymal transition in colon cancer. Oncol Rep. 2014;32:395–402. doi:10.3892/or.2014.3186.
- Tong G, Wu X, Cheng B, Li L, Li X, Li Z, Nong Q, Chen X, Liu Y, Wang S, et al. Knockdown of HOXA-AS2 suppresses proliferation and induces apoptosis in colorectal cancer. Am J Transl Res. 2017;9:4545–4552.
- Rezanejad Bardaji H, Asadi MH, Yaghoobi MM. Long noncoding RNA VIM-AS1 promotes colorectal cancer progression and metastasis by inducing EMT. Eur J Cell Biol. 2018;97:279–288. doi:10.1016/j.ejcb.2018.04.004.
- Gu ZG, Shen GH, Lang JH, Huang WX, Qian ZH, Qiu J. Effects of long non-coding RNA URHC on proliferation, apoptosis and invasion of colorectal cancer cells. Eur Rev Med Pharmacol Sci. 2018;22:1658–1664. doi:10.26355/eurrev_201803_14577.
- Zhou J, Li X, Wu M, Lin C, Guo Y, Tian B. Knockdown of long noncoding RNA GHET1 inhibits cell proliferation and invasion of colorectal cancer. Oncol Res. 2016;23:303–309. doi:10.3727/096504016X14567549091305.
- Li J, Lian Y, Yan C, Cai Z, Ding J, Ma Z, Peng P, Wang K. Long non-coding RNA FOXP4-AS1 is an unfavourable prognostic factor and regulates proliferation and apoptosis in colorectal cancer. Cell Prolif. 2016;50:e12312. doi:10.1111/cpr.12312.
- Lu M, Liu Z, Li B, Wang G, Li D, Zhu Y. The high expression of long non-coding RNA PANDAR indicates a poor prognosis for colorectal cancer and promotes metastasis by EMT pathway. J Cancer Res Clin Oncol. 2017;143:71–81. doi:10.1007/s00432-016-2252-y.
- Chen N, Guo D, Xu Q, Yang M, Wang D, Peng M, Ding Y, Wang S, Zhou J. Long non-coding RNA FEZF1-AS1 facilitates cell proliferation and migration in colorectal carcinoma. Oncotarget. 2016;7:11271–11283. doi:10.18632/oncotarget.7168.
- Li Y, Zhao L, Zhang Y, Guan L, Zhang H, Zhou H, Gao T, Miao P, Sun M. Downregulation of the long non-coding RNA XLOC-010588 inhibits the invasion and migration of colorectal cancer. Oncol Rep. 2018;39:1619–1630. doi:10.3892/or.2018.6260.
- Rokavec M, Horst D, Hermeking H. Cellular model of colon cancer progression reveals signatures of mRNAs, miRNA, lncRNAs, and epigenetic modifications associated with metastasis. Cancer Res. 2017;77:1854–1867. doi:10.1158/0008-5472.CAN-16-3236.
- Sun J, Hu J, Wang G, Yang Z, Zhao C, Zhang X, Wang J. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res. 2018;37(1):106. doi:10.1186/s13046-018-0771-x.
- Xue J, Liao L, Yin F, Kuang H, Zhou X, Wang Y. LncRNA AB073614 induces epithelial- mesenchymal transition of colorectal cancer cells via regulating the JAK/STAT3 pathway. Cancer Biomarkers. 2018;21(4):849–858. doi:10.3233/CBM-170780.
- Jahangiri B, Khalaj-Kondori M, Asadollahi E, Sadeghizadeh M. Cancer-associated fibroblasts enhance cell proliferation and metastasis of colorectal cancer SW480 cells by provoking long noncoding RNA UCA1. J Cell Commun Signal. 2019;13:53–64. doi:10.1007/s12079-018-0471-5.
- Wu XL, Lu RY, Wang LK, Wang YY, Dai YJ, Wang CY, Zhang N. Long noncoding RNA HOTAIR silencing inhibits invasion and proliferation of human colon cancer LoVo cells via regulating IGF2BP2. J Cell Biochem. 2018.
- Dou J, Ni Y, He X, Wu D, Li M, Wu S, Zhang R, Guo M, Zhao F. Decreasing lncRNA HOTAIR expression inhibits human colorectal cancer stem cells. Am J Transl Res. 2016;8:98–108.
- Wang L, Zhao Z, Feng W, Ye Z, Dai W, Zhang C, Peng J, Wu K. Long non-coding RNA TUG1 promotes colorectal cancer metastasis via EMT pathway. Oncotarget. 2016;7:51713–51719. doi:10.18632/oncotarget.10563.
- Li C, Du Y, Yang Z, He L, Wang Y, Hao L, Ding M, Yan R, Wang J, Fan Z. GALNT1-mediated glycosylation and activation of sonic hedgehog signaling maintains the self-renewal and tumor-initiating capacity of bladder cancer stem cells. Cancer Res. 2016;76:1273–1283. doi:10.1158/0008-5472.CAN-15-2309.
- Grandclement C, Borg C. Neuropilins: a new target for cancer therapy. Cancers. 2011;3:1899–1928. doi:10.3390/cancers3021899.
- Senfter D, Holzner S, Kalipciyan M, Staribacher A, Walzl A, Huttary N, Krieger S, Brenner S, Jäger W, Krupitza G. Loss of miR-200 family in 5-fluorouracil resistant colon cancer drives lymphendothelial invasiveness in vitro. Hum Mol Genet. 2015;24:3689–3698. doi:10.1093/hmg/ddv113.
- Tanaka S, Hosokawa M, Yonezawa T, Hayashi W, Ueda K, Iwakawa S. Induction of epithelial-mesenchymal transition and down-regulation of miR-200c and miR-141 in oxaliplatin-resistant colorectal cancer cells. Biol Pharm Bull. 2015;38:435–440. doi:10.1248/bpb.b14-00695.
- Bojmar L, Karlsson E, Ellegard S, Olsson H, Bjornsson B, Hallbook O, Larsson M, Stål O, Sandström P. The role of microRNA-200 in progression of human colorectal and breast cancer. PloS One. 2013;8:e84815. doi:10.1371/journal.pone.0084815.
- Sun Z, Yang S, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Xu J, Xia K, Chang Y. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer. 2018;17:82. doi:10.1186/s12943-018-0831-z.
- Jahangiri B, Khalaj-kondori M, Asadollahi E, Sadeghizadeh M. Cancer-associated fibroblasts enhance cell proliferation and metastasis of colorectal cancer SW480 cells by provoking long noncoding RNA UCA1. J Cell Commun Signal. 2019;13:53–64.
- Gawronski AR, Uhl M, Zhang Y, Lin YY, Niknafs YS, Ramnarine VR, Malik R, Feng F, Chinnaiyan AM, Collins CC. MechRNA: prediction of lncRNA mechanisms from RNA-RNA and RNA-protein interactions. Bioinformatics (Oxford, England). 2018;34:3101–3110. doi:10.1093/bioinformatics/bty208.
- Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P, Floros E, Dalamagas T. DIANA-LncBase v2: indexing microRNA targets on non-coding transcripts. Nucleic Acids Res. 2016;44(D1):D231–D8. doi:10.1093/nar/gkv1270.
- Hugen N, Brown G, Glynne-Jones R, de Wilt JH, Nagtegaal ID. Advances in the care of patients with mucinous colorectal cancer. Nat Rev Clin Oncol. 2016;13:361–369. doi:10.1038/nrclinonc.2015.140.
- Hong TS, Clark JW, Haigis KM. Cancers of the colon and rectum: identical or fraternal twins? Cancer Discov. 2012;2:117–121. doi:10.1158/2159-8290.CD-11-0315.
- Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi:10.1038/srep29765.
- O’Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi:10.1056/NEJM199408253310803.
- Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi:10.1056/NEJMoa022289.
