ABSTRACT
Background
The survival advantage of radiotherapy for patients with stage IV classic Hodgkin lymphoma (HL) has not been adequately evaluated.
Methods
We analyzed patients with stage IV HL enrolled from the Surveillance, Epidemiology, and End Results (SEER) registry from January 2000 to December 2012. Propensity score (PS) analysis with 1:2 matching was performed to ensure well-balanced characteristics of the comparison groups. Kaplan-Meier and Cox proportional hazardous model were used to evaluate the overall survival (OS), cancer-specific survival (CSS), the hazards ratio (HR) and corresponding 95% confidence intervals (95% CI).
Results
Overall, for all patients with stage IV HL, receiving radiotherapy was associated with both significantly improved OS and CSS. Radiotherapy to any lesions could independently improve the OS and CSS by 30% to 36% in the multivariate analyses before and after PS matching (PSM), with the best improvement of 33% to 40% observed for patients with nodular sclerosis (P < 0.05) among all HL pathological types. In particular, radiotherapy, most likely to the residual site, was more pronouncedly associated with the improvement in survival for patients with stage IV HL who were young (age<45, P < .05) or without B symptoms (PInteraction for OS = 0.099, PInteraction for CSS = 0.255). For those patients without B symptoms, after PSM, the OS was improved by 65% (P = .021).
Conclusions
The large SEER results support that radiotherapy is associated with better survival of patients with stage IV HL.
Introduction
Classic Hodgkin lymphoma (HL) is a potentially curable disease. Radiotherapy (RT) was the first effective treatment for the management of HL.Citation1 However, advanced stage (stage III/IV) HL is less curable with RT alone. With the advent of effective systemic regimens with adriamycin, bleomycin, vincristine and dacarbazine (ABVD), improved cure rates were achieved, establishing chemotherapy as the treatment of choice for advanced HL.Citation2 However, for advanced HL after chemotherapy, disease relapse rates are still high.Citation3 Although RT has been shown to be efficacious in treating early-stage HL, its indications in advanced stage diseases, especially in patients with stage IV, have been controversial.
A growing body of evidence has suggested that there might be a benefit for consolidation RT in select patients with advanced HL.Citation4–Citation7 However, the role of RT in treating advanced HL has not been established because relevant randomized controlled clinical trials (RCTs) are sparse, with some not supporting a role of RT in advanced HL.Citation8–Citation11 Another reason why the use of RT is questioned is the concern about long-term toxicities such as heart disease and secondary malignancy, which are not evident until years after RT. This concern has been found to be associated with a decreasing use of consolidative RT from a peak of 47% in 2000 to a nadir of 32% in 2012 (P < .001) for early-stage HL over the last 15 years.Citation12 However, extended RT fields of the past have been abandoned. Modern advanced RT techniques enable treatment of lymphoma with much less radiation volumes and doses.Citation13 Therefore, omission of RT and abandonment of multimodality approaches in contemporary clinical practice might not be adequately evidence-based, and modern RT in advanced lymphomas warrants further evaluation, especially in phase III RCTs.
Nevertheless, RT may still be a valuable and not an outdated treatment option for advanced HL. Previous data from the surveilance, epidemiology, and end results (SEER) database showed that radiation therapy improved survival in patients with all stages of HL.Citation14–Citation16 Although Son et al.Citation16 have shown the effectiveness of RT in patients in stages III and IV, the study did not attempt to control confounding factors using methods such as propensity score matching (PSM) and only targeted people older than 18 years of age. Hence, with an aim to make up for the knowledge gap and to further examine the benefits of radiotherapy for stage IV patients, we analyzed the data of patients with stage IV HL from the SEER registry through conventional methods and a propensity score matching approach.
Participants and methods
Study population and data sources
SEER encompasses population-based cancer registries covering approximately 28% of the US population and records basic demographics, tumor site, histology type, stage, grade, treatments received and so on.Citation17 SEER*stat software (version 8.3.4) was used to select patients from the SEER-18 database if they were diagnosed with HL in 2000–2012 (n = 29,580). This dataset was then limited to patients with classic HL histology (using lymphoma subtype recode/WHO 2008) and patients in stage IV (using the Ann Arbor Stage) (n = 5,223). We categorized patients into older (≥45 years old) and younger patients (<45 years old). Lower family income was defined as those with less than 5000 dollars per annum. Regions were defined by the Contract Health Services Delivery Areas (CHSDA): East represents Connecticut, Georgia, Kentucky, Louisiana, and New Jersey; Northern Plains represents Michigan and Iowa; Pacific Coast/Alaska represents Alaska, California, Hawaii and Washington; and Southwest represents New Mexico and Utah.
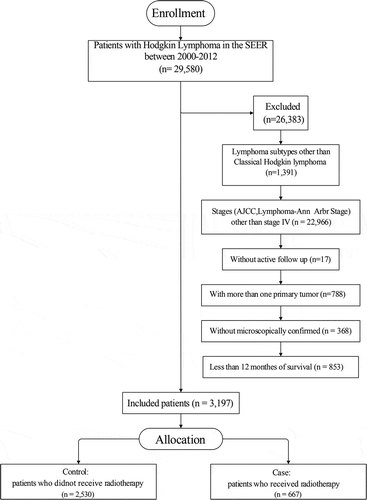
Demographics and treatment characteristics of pertinent patients were included in this analysis. Treatment-related variables coded in SEER reflect initial treatment only. No specific chemotherapy protocol could be ascertained. The lymphoma subtype was determined as lymphocyte-rich, mixed cellularity, lymphocyte-depleted, nodular sclerosis and classical HL not otherwise specified (NOS). Extranodal status was also determined. Included in our study were patients with one primary only, microscopically confirmed, and complete data of age, sex, race, marital status, region, income, radiation recode, with active follow-up and more than 12 months of survival. Patients with unknown vital status were excluded. These exclusions were not mutually exclusive. This resulted in a total of 3,197 patients in this study, including those who received radiotherapy (cases, n = 667) and those who did not receive any radiotherapy (controls, n = 2,530) ().
PSM
PSM is a tool to reduce the selection bias in nonrandomized studies. Multivariate logistic regression models were used to calculate propensity scores for each patient. Then, propensity 1:2 nearest neighbor matching, which matched patients in the group with RT and that without RT according to the propensity scores, was employed to reduce possible bias.Citation18 The covariate balance was examined by standardized deviation. Statistics were performed in R version 2.15.3 and SPSS 23 with PS matching 3.03 (IBM Corp, Armonk, NY, USA).
Statistical analysis
Categorical data were described with absolute frequency counts and percentages; continuous data were described with means (since the median survival has not yet been reached as of the date of analysis). Mann-Whitney U tests and Mantel-Haenszel chi-square tests were used to compare the distribution of demographic characteristics. The primary objectives were to evaluate the overall survival (OS) and cause-specific survival (CSS). OS was defined as the time from diagnosis to death of any cause; patients who were alive in the SEER-18 database were censored at the time of last follow-up. CSS was defined as the time from diagnosis to death that was marked as being related to Hodgkin lymphoma. Patients with any other causes of death or who were alive were censored at the time of death or last follow-up.
To test our hypothesis that OS and CSS were associated with RT in patients with HL in stage IV at diagnosis, our statistical analyses consisted of multiple steps. First, Kaplan-Meier curves were used to estimate OS and CSS for the RT (case) and No-RT cohorts (control). Then, we employed multivariable Cox regression analyses (MVA) to test the relationship between RT with OS and CSS. Significant variables in univariate analysis were entered into the multivariate models. Then, variables that remained significant were further entered into the final multivariate regression models. Variables that remained in the final model were selected to generate the propensity score for PSM, and were kept in the final Cox regression models after PSM. Clinically, for patients with stage IV HL, chemotherapy should be the standard of care. Patients who did not receive chemotherapy may suffer from severe comorbidity or had other special reasons. Thus, sensitivity analyses were conducted by inclusion and exclusion of this small group of patients who did not receive chemotherapy. And, we presented the results of patients who received chemotherapy in the manuscript.
Age was used as the time scale for all models, with entry time defined as age at diagnosis and exit time defined as age at death, last follow-up, or December 31, 2012, whichever came first. Interaction tests were conducted in the polytomous model, testing the null hypothesis that the regression coefficients for the survival benefits of RT were the same between the two subgroups. In the analysis of the interaction of RT with B symptoms, patients with an unknown status of B symptoms were excluded. P-values of ≤ 0.05 (2-sided probability) were considered statistically significant. PInteraction ≤ 0.1 indicated that there was a statistically significant interaction, which means the efficacy of RT was different between the subgroups. All analyses were conducted using SPSS 23 (IBM Corp, Armonk, NY, USA), and R version 2.15.3. Kaplan-Meier survival curve were generated using on GraphPad Prism 7.
Results
Baseline characteristics
Distributions of characteristics are presented in for the study groups categorized by whether patients received RT (cases) or not (controls). Nearly 21% of patients received RT. Before PSM, the mean age of the patients was 38.0 years old (range, 3–91). The majority of patients were male (60.4%) and white (77.8%), had nodular sclerosis pathology (58.2%), and lived in Pacific Coast/Alaska region (48.1%). More patients had B symptoms (47.0%) than those without (21.5%). Compared with controls, cases were more likely to be younger, unmarried, receiving chemotherapy, receiving surgery, and without B symptoms (P < 0.05). Similarly, there were differences in the distribution of regions and histological types. It was interesting to note that the ratio of receiving RT in nodular sclerosis HL (26.4%) was higher than that of other types. The distributions of most demographical and clinical factors were well balanced between cases and controls after using a PS purely composed of two demographical factors (age and income) and one clinical factor (the presence of B symptoms). After PSM, cases were still 3.3 years younger than controls. However, the difference was insignificant between age groups (<45, ≧45; P = 0.096).
Table 1. Baseline characteristics of patients with stage IV HL, before and after PSM, SEER 2000–2012.
Univariate survival analysis
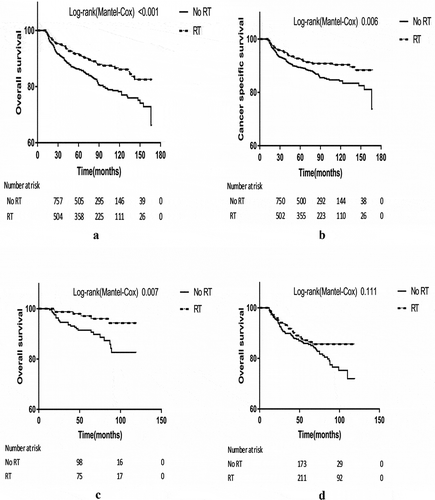
All baseline characteristics were included in univariate analyses between cases and controls in relation to both OS and CSS. The results of analyses by excluding or including those patients without or with unknown chemotherapy did not change appreciably. shows the 5-year survival rates and means of survival time for patients with stage IV HL who received chemotherapy before PSM. As expected, older age, lower family income, and B symptoms were associated with poorer OS and CSS. Although unmarried status was associated with better OS and CSS, this was mainly because unmarried subjects were younger (mean age, 28.3 years old vs. 48.4 years old). The nodular sclerosis subtype demonstrated the best survival time among all subtypes both in OS and CSS. Further pairwise comparisons found that patients with nodular sclerosis had a statistically better OS and CSS compared with patients with subtypes of mixed cellularity (POS = 0.001, PCSS = 0.008), lymphocyte-depleted (POS = 0.034, PCSS = 0.074), and classical HL NOS (POS<0.001, PCSS = 0.007) but not lymphocyte-rich (POS = 0.297, PCSS = 0.128). RT was found to be strongly associated with better survival for the overall patients (POS<0.001, PCSS<0.001) before (OS, 148.8 months vs. 134.8 months; CSS, 153.6 months vs. 145.4 months) and after PSM (OS, 150.0 months vs. 134.9 months; CSS, 154.2 months vs. 145.2 months; ,).
Table 2. Univariate analysis of OS and CSS in patients with stage IV HL who received chemotherapy, before PSM, SEER 2000–2012.
Figure 2. (2a) Kaplan-Meier survival curve of OS in patients with stage IV HL after PSM. (2b) Kaplan-Meier survival curve of CSS in patients with stage IV HL after PSM. (2c) Kaplan-Meier survival curve of OS in stage IV HL patients without B symptoms after PSM. (2d) Kaplan-Meier survival curve of OS in stage IV HL patients with B symptoms after PSM.
Multivariate survival analysis
Before PSM, the variables that remained statistically significant in the multivariate model were “age”, “income”, “B symptoms”, and “RT”. shows the results of the final multivariate model after PSM. Being young (<45 years old), having a higher family income and without B symptoms were associated with improved OS and CSS (P < .05). RT was significantly associated with improving OS and CSS for patients with stage IV HL, either before PSM [HROS (95% CI) = 0.64 (0.50–0.83), POS = 0.001; HRCSS (95% CI) = 0.70 (0.52–0.94), PCSS = 0.016] or after PSM [HROS (95% CI) = 0.68 (0.51–0.89), POS = 0.006; HRCSS (95% CI) = 0.69 (0.50–0.95), PCSS = 0.022].
Table 3. Multivariate analysis of OS and CSS in patients with stage IV HL who received chemotherapy, after PSM,1 SEER 2000–2012.
Effect of radiotherapy on OS and CSS by different groups
presents the effect of RT on OS and CSS of patients with stage IV HL by subgroups, after PSM. In general, the benefit on OS and CSS from RT was seen across all subtypes of patients with stage IV HL. Although some of the benefits were not statistically significant. After PSM, numerically, RT was found to be more significantly associated with increased survival of patients who were young, with a lower family income and without B symptoms. And significant heterogeneities for effect of RT were only observed between patients with B symptoms and those without (PInteraction for OS <0.10). For patients with stage IV HL who did not suffer from B symptoms, RT was more strikingly related to improved survival by 50%-65%, whereas only improved the survival of patients suffering from B symptoms by 14%-25%. , show the survival curves of OS in patients without and with B symptoms, respectively.
Table 4. Effect of radiotherapy on OS and CSS by different subgroups in patients with stage IV HL, after PSM, SEER 2000–2012.
Discussion
To our knowledge, the present study is the first SEER analysis using PSM to assess the role of RT in the treatment of stage IV HL. In this study, we found that RT, in general, could offer a survival benefit for patients with stage IV HL in multivariate regression analysis both before and after PSM analysis. The beneficial effect of the survival seen from the large SEER database highlighted the importance of RT in the management of stage IV HL.
The role of RT for early-stage HL in improving survival has been well established.Citation19–Citation21 However, for advanced-stage HL and stage IV HL in particular, the effectiveness of RT has yet to be defined. Studies of consolidation RT for advanced HL have initially provided conflicting results, with some supporting the effect of RTCitation4-Citation7,Citation22,Citation23 and some denying it.Citation8–Citation11 More recent studies supported the effectiveness of this strategy.Citation7,Citation23
As Bates et al.Citation14 rightly pointed out, although both stage III and IV HL represented disseminated disease, patients with stage III HL may have disease distributions and burdens more amenable to RT. They found significantly low HRs for those patients who received RT, showing as 0.34 (P < .001) and 0.32 (P < .001) for OS and CSS, respectively.Citation14 And the corresponding figures from our study were 0.64 (P = .001) and 0.70 (P = .016), respectively, for patients with stage IV HL. The improvement in survival decreased in magnitude as the disease became more extensive, and the benefit of RT was more difficult to observe. Due to this decreased magnitude of survival benefit, for stage IV HL patients, it was difficult for previous studies with relatively inadequate sample size to demonstrate a significant prolonged survival which was associated with RT.
Of note, using old chemotherapy regimens, outdated diagnostic/staging tools, and old two-dimensional radiotherapy (2D-RT) techniques in early studies might negatively affect the usefulness of radiotherapy for HL. The extended RT fields brought unacceptable long-term side effects for patients with HL in the past.Citation24,Citation25 Modern advanced imaging and conformal RT techniques, especially the modern standard practice of involved site radiation therapy (ISRT), enable treatments with much less radiation volumes and doses to normal tissues and consequently lower risks of long-term complications,Citation13 especially in reducing the incidence of the second tumor.Citation26,Citation27 A study of the SEER database for early-stage HL also showed that the addition of RT did not increase the development of secondary malignancies, but had a survival benefit.Citation28 Additionally, whether RT to residual lesions and/or initial volume was beneficial for patients with advanced HL also depends on the chemotherapy regimen.Citation29 Therefore, old findings cannot be extrapolated directly into the current practice in which patients received platinum-based chemotherapy and newer RT modalities. Moreover, radiation modalities such as protonCitation30 could be developed in treating HL, due to their remarkable physical and biophysical advantages. In conclusion, as with advancements in technology, an increasing number of sites of advanced HL would be effectively treated by RT.
Since its indications in advanced stage diseases have been controversial, the use of RT was decided by the treating physician. Clinically, patients with stage IV HL were probably the most unfavorable patients for receiving RT. However, patients with bulky disease,Citation31 incomplete or uncertain completed remission or patients treated on brief chemotherapy programs were more likely to benefit from ISRT.Citation32 Additionally, specific initial lesion sites, such as stage III patients with disease above the diaphragm,Citation33 seemed to benefit from RT in particular. And similar results are likely to be found in patients with stage IV HL. The UK Lymphoma Group trialCitation34 specified that RT should be considered for original bulk disease and for residual masses. In these settings, consolidation RT is highly efficacious at achieving local disease control and might improve OS. Therefore, it is reasonable to expect that the added survival benefit of RT for stage IV HL in our study might at least partially be attributed to RT rather than other favorable immeasurable confounding factors. Furthermore, we found that the survival benefit from RT was, to some extent, conditioned upon age, family income, histological type (nodular sclerosis or others), and existence of B symptoms. Previous studiesCitation14,Citation15 have come to similar conclusions, which reinforced our findings. In particular, we found that the presence or absence of B symptoms in patients with stage IV HL affected the patients’ responses to RT (PInteraction for OS = 0.099). This might be useful for clinical decision making on the use of RT, and thus warrants further study. Future RCTs are needed and shall focus on the application of RT in stage IV HL to find suitable patients for RT in a PET era.Citation35
Unlike RCTs, SEER registry data usually have high completeness and represent the real-world patient population. RCTs are prone to a selection bias by recruiting a specific group of patients of interest, thus limiting the generalizability of the findings. Compared with previous SEER analyses for advanced HL, we observed a longer 5-year OS of patients with stage IV HL who received RT than the study of Son CH et al.,Citation16 possibly because they excluded patients who survived less than 6 months while we excluded patients who survived less than 12 months. Thus, our analysis might suffer less from immortal bias.Citation36 In addition, all of the previous studiesCitation17-Citation19 failed to use methods such as PSM to control immeasurable confounders inherent to the registry data. None of the previous studies conducted sensitivity analyses by inclusion and exclusion of patients who did not receive chemotherapy. Thus, interpretations of their results were somehow challenged.
Nevertheless, we acknowledge several limitations to this study. First, the possibility of bias was a concern. We used the PSM to reduce the bias caused by the imbalanced distribution of measured covariates. However, unmeasured factors are unavoidable. Then, RT in the SEER database is defined as using RT during the first course of cancer-directed therapy with no information on dose and intended target. Although it would be preferable to obtain more details about RT, the aim of the current study is to describe the role of RT in stage IV HL from a qualitative, rather than quantitative, perspective. In the current analysis, we did not intend to demonstrate the types, dose, timing, intent or methods that should be used in stage IV HL. Finally, the SEER registry does not provide any data on risk factors for HL, which may have impacted survival. Although the SEER-Medicare database reports the comorbidity, detailed radiation information and second-line chemotherapy, those patients are limited to an older population (>65 years of age), which is not relevant for HL.
Conclusion
In conclusion, we performed multivariable analyses, PSM analyses and sensitivity analyses, and the results of OS and CSS did not change appreciably and remained stable. With various methods used to control bias, our results seemed more solid and valid than previous studies. The present study from the large SEER database, supports that RT in addition to chemotherapy might be beneficial for the survival of patients with stage IV HL, especially for patients without B symptoms. Although well-designed phase III RCTs are warranted to ascertain the value of RT in this setting, it is prudent to routinely select suitable patients for radiation therapy in stage IV HL.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Evens AM, Hutchings M, Diehl V. Treatment of Hodgkin lymphoma: the past, present, and future. Nat Clin Pract Oncol. 2008;5(9):543–556. doi:10.1038/ncponc1186.
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi:10.3322/caac.21438.
- Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, Canellos GP, Peterson BA. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: report of an intergroup trial. J Clin Oncol. 2003;21(4):607–614. doi:10.1200/JCO.2003.12.086.
- Yahalom J, Ryu J, Straus DJ, Gaynor JJ, Myers J, Caravelli J, Clarkson BD, Fuks Z. Impact of adjuvant radiation on the patterns and rate of relapse in advanced-stage Hodgkin’s disease treated with alternating chemotherapy combinations. J Clin Oncol. 1991;9(12):2193–2201. doi:10.1200/JCO.1991.9.12.2193.
- Fabian CJ, Mansfield CM, Dahlberg S, Jones SE, Miller TP, Van SE, Grozea PN, Morrison FS, Jr CC, Fisher RI. Low-dose involved field radiation after chemotherapy in advanced Hodgkin disease. A Southwest Oncology Group randomized study. Ann Intern Med. 1994;120(11):903–912. doi:10.7326/0003-4819-120-11-199406010-00002.
- Laskar S, Gupta T, Vimal S, Muckaden MA, Saikia TK, Pai SK, Naresh KN, Dinshaw KA. Consolidation radiation after complete remission in Hodgkin’s disease following six cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine chemotherapy: is there a need? J Clin Oncol. 2004;22(1):62–68. doi:10.1200/JCO.2004.01.021.
- Johnson PW, Sydes MR, Hancock BW, Cullen M, Radford JA, Stenning SP. Consolidation radiotherapy in patients with advanced Hodgkin’s lymphoma: survival data from the UKLG LY09 randomized controlled trial (ISRCTN97144519). J Clin Oncol. 2010;28(20):3352–3359. doi:10.1200/JCO.2009.26.0323.
- Diehl V, Loeffler M, Pfreundschuh M, Ruehl U, Hasenclever D, Nisters-Backes H, Sieber M, Smith K, Tesch H, Geilen W, et al. Further chemotherapy versus low-dose involved-field radiotherapy as consolidation of complete remission after six cycles of alternating chemotherapy in patients with advance Hodgkin’s disease. German Hodgkins’ Study Group (GHSG). Ann Oncol. 1995;6(9):901–910. doi:10.1093/oxfordjournals.annonc.a059357.
- Ferme C, Sebban C, Hennequin C, Diviné M, Lederlin P, Gabarre J, Ferrant A, Caillot D, Bordessoule D, Brice P, et al.. Comparison of chemotherapy to radiotherapy as consolidation of complete or good partial response after six cycles of chemotherapy for patients with advanced Hodgkin’s disease: results of the groupe d’etudes des lymphomes de l’Adulte H89 trial. Blood. 2000;95(7):2246–2252.
- Aleman BM, Raemaekers JM, Tirelli U, Bortolus R, Veer MB, Lybeert ML, Keuning JJ, Carde P, Girinsky T, Maazen RW, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348(24):2396–2406. doi:10.1056/NEJMoa022628.
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, Eich HT, Mueller-Hermelink HK, Kanz L, Greil R, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin study group. J Clin Oncol. 2011;29(32):4234–4242. doi:10.1200/JCO.2010.33.9549.
- Vargo JA, Gill BS, Balasubramani GK, Beriwal S. Treatment selection and survival outcomes in early-stage diffuse large B-cell lymphoma: do we still need consolidative radiotherapy? J Clin Oncol. 2015;33(32):3710–3717. doi:10.1200/JCO.2015.61.7654.
- Specht L, Yahalom J, Illidge T, Berthelsen AK, Constine LS, Eich HT, Girinsky T, Hoppe RT, Mauch P, Mikhaeel NG, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854–862. doi:10.1016/j.ijrobp.2013.05.005.
- Bates JE, Dhakal S, Mazloom A, Casulo C, Constine LS. Benefit from the inclusion of radiation therapy in the treatment of patients with stage III classical Hodgkin lymphoma: a propensity matched analysis of the surveillance, epidemiology, and end results database. Radiother Oncol. 2017;124(2):325–330. doi:10.1016/j.radonc.2017.07.016.
- Master S, Koshy N, Wilkinson B, Rosen L, Mills G, Mansour R, Shi R. Effect of radiation therapy on survival in hodgkin’s lymphoma: a SEER data analysis. Anticancer Res. 2017;37(6):3035–3043. doi:10.21873/anticanres.11658.
- Son CH, Chiu BC, Koshy M. Patterns of care and survival outcomes examining radiation therapy for advanced Hodgkin lymphoma. Leuk Lymphoma. 2017;58(2):343–347. doi:10.1080/10428194.2016.1193856.
- Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121.
- Cook RD, Weisberg S. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi:10.1093/biomet/70.1.41.
- Ad V B, Paltiel O, Glatstein E. Radiotherapy for early-stage Hodgkin’s lymphoma: a 21st century perspective and review of multiple randomized clinical trials. Int J Radiat Oncol Biol Phys. 2008;72(5):1472–1479. doi:10.1016/j.ijrobp.2008.08.026.
- Filippi AR, Franco P, Ciammella P. Role of modern radiation therapy in early stage Hodgkin’s lymphoma: A young radiation oncologists’ perspective. Rep Pract Oncol Radiother. 2012;17(5):246–250. doi:10.1016/j.rpor.2012.05.003.
- Shrestha R, Nee J, Durbin EB, Zia M, Ramlal R, Monohan G, Herzig RH, Fleischman R, Hildebrandt G, Saeed H, et al. Chemotherapy and radiation improve survival in early stage classical Hodgkin lymphoma: a statewide cancer registry analysis. Hemato Onco. 2017;35(S2):315–316. doi:10.1002/hon.2439_55.
- Aleman BM, Raemaekers JM, Tomisic R, Baaijens MH, Bortolus R, Lybeert M, Maazen R, Girinsky T, Demeestere G, Lugtenburg P, et al. Involved-field radiotherapy for patients in partial remission after chemotherapy for advanced Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2007;67(1):19–30. doi:10.1016/j.ijrobp.2006.08.041.
- Engert A, Haverkamp H, Kobe C, Jana M, Renner C, Ho A, Zijlstra J, Král Z, Fuchs M, Hallek M, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–1799. doi:10.1016/S0140-6736(11)61940-5.
- Dores GM, Metayer C, Curtis RE, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, Leeuwen E, Holowaty EJ, et al. Second malignant neoplasms among long-term survivors of Hodgkin’s disease: a population-based evaluation over 25 years. J Clin Oncol. 2002;20(16):3484–3494. doi:10.1200/JCO.2002.09.038.
- Aleman BM, Belt-Dusebout AW, De Bruin ML, Veer MB, Baaijens MHA, Boer JP, Hart AAM, Klokman WJ, Kuenen MA, Ouwens GM, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–1886. doi:10.1182/blood-2006-07-034405.
- Koh ES, Tran TH, Heydarian M, Sachs RK, Tsang RW, Brenner DJ, Pintilie M, Xu T, Chung J, Paul N, et al. A comparison of mantle versus involved-field radiotherapy for Hodgkin’s lymphoma: reduction in normal tissue dose and second cancer risk. Radiat Oncol. 2007;2:13. doi:10.1186/1748-717X-2-13.
- Maraldo MV, Brodin NP, Aznar MC, Vogelius IR, Rosenschold PM, Petersen PM, Specht L. Estimated risk of cardiovascular disease and secondary cancers with modern highly conformal radiotherapy for early-stage mediastinal Hodgkin lymphoma. Ann Oncol. 2013;24(8):2113–2118. doi:10.1093/annonc/mdt156.
- Koshy M, Rich SE, Mahmood U, Kwok Y. Declining use of radiotherapy in stage I and II Hodgkin’s disease and its effect on survival and secondary malignancies. Int J Radiat Oncol Biol Phys. 2012;82(2):619–625. doi:10.1016/j.ijrobp.2010.10.069.
- Chisesi T, Bellei M, Luminari S, Montanini A, Marcheselli L, Levis A, Gobbi P, Vitolo U, Stelitano C, Pavone V, et al. Long-term follow-up analysis of HD9601 trial comparing ABVD versus Stanford V versus MOPP/EBV/CAD in patients with newly diagnosed advanced-stage Hodgkin’s lymphoma: a study from the Intergruppo Italiano Linfomi. J Clin Oncol. 2011;29(32):4227–4233. doi:10.1200/JCO.2010.30.9799.
- Hoppe BS, Flampouri S, Su Z, Morris CG, Latif N, Dang NH, Lynch J, Li Z, Mendenhall NP. Consolidative involved-node proton therapy for stage IA-IIIB mediastinal Hodgkin lymphoma: preliminary dosimetric outcomes from a phase II study. Int J Radiat Oncol Biol Phys. 2012;83(1):260–267. doi:10.1016/j.ijrobp.2011.06.1959.
- Horning SJ, Williams J, Bartlett NL, Bennett JM, Hoppe RT, Neuberg D, Cassileth P. Assessment of the stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin’s disease: eastern cooperative oncology group pilot study E1492. J Clin Oncol. 2000;18(5):972–980. doi:10.1200/JCO.2000.18.5.972.
- Yahalom J. Role of radiation therapy in Hodgkin’s lymphoma. Cancer J. 2009;15(2):155–160. doi:10.1097/PPO.0b013e3181a1437a.
- Phan J, Mazloom A, Abboud M, Salehpour M, Reed V, Zreik T, Shihadeh F, Fisher C, Wogan C, Dabaja B. Consolidative radiation therapy for stage III Hodgkin lymphoma in patients who achieve complete response after ABVD chemotherapy. Am J Clin Oncol. 2011;34(5):499–505. doi:10.1097/COC.0b013e3181f477a8.
- Johnson PW, Radford JA, Cullen MH, Sydes, Matthew R, Walewski J, Jack AS, Maclennan KA, Stenning SP, Clawson S, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom lymphoma group LY09 trial (ISRCTN97144519). J Clin Oncol. 2005;23(36):9208–9218. doi:10.1200/JCO.2005.03.2151.
- Amitai I, Gurion R, Vidal L, Dann EJ, Raanani P, Gafter-Gvili A. PET-adapted therapy for advanced Hodgkin lymphoma - systematic review. Acta Oncol. 2018;57(6):765–772. doi:10.1080/0284186X.2018.1426877.
- Shariff SZ, Cuerden MS, Jain AK, Garg AX. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19(5):841–843. doi:10.1681/ASN.2007121354.