ABSTRACT
Rearranged during transfection (RET) fusion–positive non–small cell lung cancer (NSCLC) accounts for 1% of lung adenocarcinoma. Although small molecule agents with RET kinase inhibitory activity such as alectinib, vandetanib, and cabozantinib have been clinically evaluated in RET-fusion–positive NSCLC, an effective monotherapy regimen has not been established. We explored agents to use in combination with alectinib to enhance the antitumor effect of alectinib against RET-fusion cells.
Cell proliferation under co-treatment with alectinib plus each of six chemotherapeutic agents or six molecularly targeted agents was evaluated in vitro. The combination effect was analyzed by IC50 isobologram and combination index using LC-2/ad and Ba/F3-KIF5B-RET cells. The in vivo combination effect was investigated in a Ba/F3-KIF5B-RET xenograft model. The phosphorylation levels of proteins regulating proliferation were measured by immunoblotting.
Palbociclib, a CDK4/6 inhibitor, showed the greatest synergy against LC-2/ad cells in the isobologram analysis and combination index. This synergistic effect was also observed against Ba/F3-KIF5B-RET cells. Another CDK4/6 inhibitor, abemaciclib, also showed a synergistic effect. In vivo, the combination of alectinib plus palbociclib showed a more enhanced antitumor effect than each single agent in a mouse xenograft model with transplanted Ba/F3-KIF5B-RET cells. This combination suppressed the phosphorylation of S6 and Rb more intensely than did either single agent in both LC-2/ad and Ba/F3-KIF5B-RET cell lines, both in vitro and in vivo.
Combination therapy with alectinib plus the CDK4/6 inhibitor enhanced the antitumor effect against RET-fusion–positive cells in vitro and in vivo.
Introduction
The oncogenic fusion genes anaplastic lymphoma kinase (ALK), ROS1, and rearranged during transfection (RET) are identified in 5%, 1–2%, and 1% of patients with lung adenocarcinoma, respectively.Citation1 Such fusions result in the constitutive dimerization of the kinase domain leading to aberrant activation of downstream signaling, which promotes tumorigenesis, cellular proliferation, and cancer survival, invasion, and metastasis.Citation2–Citation4
Alectinib is an ALK inhibitor,Citation5 and in the phase III ALEX trial, alectinib showed superior efficacy compared with crizotinib as the primary treatment for ALK-positive NSCLC and was well tolerated.Citation6 Alectinib also shows significant in vitro and in vivo antitumor activity in a RET-fusion gene–positive NSCLC model,Citation7 and a phase I/II study of alectinib in recurrent RET-fusion gene–positive NSCLC is ongoing in Japan.Citation8 Although the RET-tyrosine kinase inhibitors vandetanib and cabozantinib are of great clinical benefit to patients with medullary thyroid cancer with RET mutations,Citation9,Citation10 no effective therapy targeting RET-fusion gene positive NSCLC has yet been established.Citation11 According to case reports, LOXO292 and BLU-667, next-generation selective RET inhibitors, are considered promising monotherapeutic approaches for treating patients with RET-fusion–positive NSCLC,Citation12–Citation14 but their clinical utility is still under investigation, and the emerging clinical data should be closely monitored.
Recently, combination therapies, such as erlotinib plus bevacizumab, have been achieving more attractive outcomes than monotherapy as a first-line therapy in NSCLC patients harboring activating EGFR mutations.Citation15 In vitro, ceritinib in combination with trametinib promotes greater responses against ALK-positive NSCLC cells than does either single agent by minimizing the re-activation of MAPK signaling, which cannot be completely inhibited by ceritinib alone.Citation16 Considering these findings, it is possible that a combination therapy may be a superior treatment for RET-fusion NSCLC compared with treatment with a single agent.
In this study, we searched for a compatible agent that could be used in combination with alectinib, a small molecule agent with RET kinase inhibitory activity, to enhance its antitumor effects. We searched from among 12 compounds launched or developed for the treatment of NSCLC, including 6 chemotherapeutic agents and 6 molecularly targeted agents.Citation17–Citation19 The combination effect was investigated using LC-2/ad cells, which are CCDC6-RET fusion gene–positive NSCLC cells,Citation20 and also using Ba/F3-KIF5B-RET cells, which were constructed from Ba/F3 cells transfected with a KIF5B-RET-fusion gene, the most common RET fusion gene in NSCLC, and like LC-2/ad cells were also sensitive to alectinib.Citation7 Finally, we selected a possible drug to combine with alectinib in the targeting of RET-fusion–positive NSCLC.
Results
Exploration of agents compatible with alectinib against RET-fusion cell lines in vitro
Firstly, we verified the dependency of LC-2/ad and Ba/F3-KIF5B-RET cells on RET-signaling. Both LC-2/ad and Ba/F3-KIF5B-RET cells were sensitive to alectinib in a dose-dependent manner (Supplementary Figure 1a), and treatment with alectinib suppressed the phosphorylation of RET and also suppressed the phosphorylation of Akt, ERK, STAT3, and S6 which are downstream molecules of RET signaling (Supplementary Figure 1b).
Next, we used IC50 isobologram analysis and a combination index to evaluate the effects of 12 compounds in combination with alectinib on LC-2/ad cells: pemetrexed, paclitaxel, carboplatin, vinorelbine, gemcitabine, irinotecan, palbociclib, SAHA, BKM120, gedatolisib, everolimus, and luminespib. Additive or antagonistic combination effects were observed with 10 of 12 compounds (, ). A weak synergistic effect was observed with BKM120, a class I PI3K inhibitor, and the highest synergistic effect was observed with palbociclib, a CDK4/6 inhibitor (, ). The synergistic effect of alectinib and palbociclib was also observed in Ba/F3-KIF5B-RET cells (, ). Moreover, another CDK4/6 inhibitor, abemaciclib, also had a synergistic effect against Ba/F3-KIF5B-RET cells when combined with alectinib (, ). Furthermore, the combination of palbociclib and each of the other RET inhibitors, BLU-667 and vandetanib, also had a synergistic effect against LC-2/ad cells and Ba/F3-KIF5B-RET cells (Supplementary Figure 2).
Table 1. Combination index for combination of alectinib and each agent in LC-2/ad cells and Ba/F3-KIF5B-RET cells.
Figure 1. The in vitro combination effect of alectinib plus palbociclib in RET-fusion cells (a, b, c) IC50 isobologram analysis of combination of alectinib plus each drug in (a) LC-2/ad cells and (b, c) Ba/F3-KIF5B-RET cells. The diagonal line indicates additivity. The data points located below, on, or above the line indicate synergism, additivity, and antagonism, respectively. The horizontal and vertical axis indicates concentration of alectinib and each combined agent (nM), respectively. (d) Cell growth inhibition of LC-2/ad, Ba/F3-KIF5B-RET, and NCI-H1944 cells treated with either 100 nM of alectinib (ALC), 100 nM of palbociclib (PAL), or the combination (ALC+PAL) for 4 days (n = 6). (e) The fold change of annexin V binding in LC-2/ad and Ba/F3-KIF5B-RET cells treated with either 100 nM of alectinib (ALC), 100 nM of palbociclib (PAL), or the combination (ALC+PAL) in 3-day treatment (n = 6). Each bar represents the mean + SD. a: p < .05 versus control, b: p < .05 versus alectinib, c: p < .05 versus palbociclib by Tukey-Kramer’s HSD test.
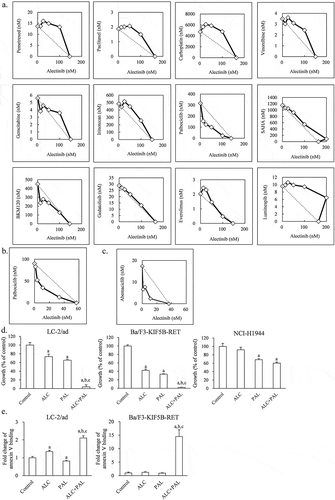
Effect of alectinib plus palbociclib on growth inhibition and induction of apoptosis
We confirmed the combination effect of alectinib plus palbociclib using a method different from that of the isobologram and combination index, both of which are dose–effect-based analyses for finding out which concentrations are needed with each agent to produce the same quantitative effect. In this further evaluation of the combination effect, we used an effect-based analysis to directly compare effects resulting from the whole combination with the effects of the individual components.Citation29 Combination treatment with alectinib plus palbociclib at a concentration at which each agent partially inhibited the cell growth of both LC-2/ad and Ba/F3-KIF5B-RET cells showed a growth inhibition effect significantly greater than that with each single agent in RET-fusion cells (). However, in NCI-H1944 cells, which harbor neither RET-fusion nor ALK-fusion genes, alectinib alone did not inhibit growth and the combination with palbociclib did not improve the effectiveness of the constituent agents (). Furthermore, we investigated the effect of the combination of alectinib plus palbociclib on induction of apoptosis. Regardless of the effect of single agent on the binding of annexin V to cell membranes, the combination of alectinib plus palbociclib significantly increased this binding compared with each single agent in LC-2/ad and Ba/F3-KIF5B-RET cells ().
Antitumor effect of the combination of alectinib plus palbociclib
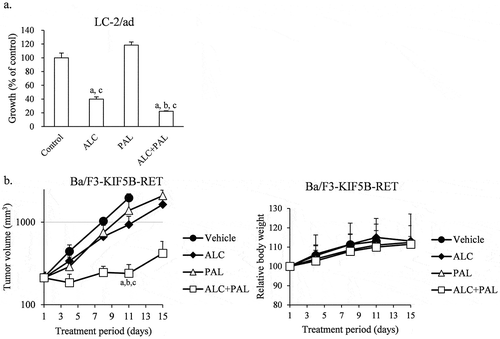
We evaluated the antitumor efficacy of the combination of alectinib plus palbociclib in 3 dimension (3D) assay and in a mouse xenograft model with transplanted Ba/F3-KIF5B-RET cells. While palbociclib alone did not decrease the growth of 3D cultured LC-2/ad cells, palbociclib significantly enhanced the antitumor effect of alectinib in combination compared with each single agent (). Furthermore, these combination significantly enhanced the antitumor effect compared with each single agent on Day 11 without reducing body weight (). In accordance with tumor volumes measured in vivo, tumors resected on Day 11 showed that this combination also significantly decreased tumor weights compared with single agents (Supplementary Figure 3a), and the tumors were visually confirmed to be smaller (Supplementary Figure 3b).
Figure 2. The antitumor effect of the combination of alectinib plus palbociclib in 3D cultured LC-2/ad cells and in Ba/F3-KIF5B-RET cells transplanted mouse xenograft model (a) The antitumor effect of combination of alectinib (ALC) plus palbociclib (PAL) in 3D cultured-LC-2/ad cells. Cells were treated with 200 nM of ALC, 200 nM of PAL, or the combination (ALC+PAL) for 7 days (n = 6). Each bar represents the mean + SD. a: significant versus control; b: significant versus alectinib; c: significant versus palbociclib by Wilcoxon test and the Holm method. (b) In vivo antitumor effect and relative body weight change of combination of alectinib (ALC) plus palbociclib (PAL) in mice bearing Ba/F3-KIF5B-RET cells (n = 6). Vehicle and each agent were orally administered daily from Day 1. a: significant versus vehicle group; b: significant versus alectinib group; c: significant versus palbociclib group by Wilcoxon test and the Holm method.
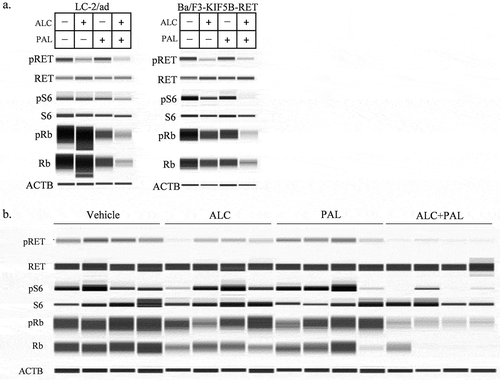
Effect of alectinib plus palbociclib on downstream signaling inhibition
Next, we investigated whether the combination of alectinib plus palbociclib suppressed the downstream signaling of RET or CDK4/6 in RET-fusion cells. The combination suppressed the phosphorylation of S6 in LC-2/ad and Ba/F3-KIF5B-RET cells compared with each single agent (). Especially in LC-2/ad cells, with alectinib alone, although the phosphorylation of S6 was completely diminished at 6 hours (Supplementary Figure 1b), it was not suppressed at 24 hours. However, the combination treatment suppressed the phosphorylation of S6 at 24 hours (). Whereas palbociclib alone suppressed the phosphorylation of Rb, which is downstream of CDK4/6, the combination treatment suppressed this phosphorylation more intensely than did either single agent in LC/2-ad and Ba/F3-KIF5B-RET cells in vitro (). Moreover, this was also the case in Ba/F3-KIF5B-RET tumors in vivo ().
Figure 3. The effect of alectinib plus palbociclib on downstream signaling inhibition (a, b) Immunoblots of (a) cell lysates of LC-2/ad and Ba/F3-KIF5B-RET cells after 24-hour treatment with either 100 nM of alectinib (ALC), 100 nM of palbociclib (PAL), or the combination, and (b) tumor lysates from a mouse xenograft model with transplanted Ba/F3-KIF5B-RET cells after 2-day treatment with vehicle, alectinib 20 mg/kg (ALC), palbociclib 75 mg/kg (PAL), or the combination (ALC+PAL) (n = 4).
Discussion
Published reports have shown that combinations of tyrosine kinase inhibitors (TKIs) plus CDK4/6 inhibitors, including palbociclib, exert a superior combination effect in TKI-naïve and TKI-resistant cell lines, both in vitro and in vivo.Citation30–Citation34 We demonstrated that the combination of alectinib plus palbociclib enhanced the growth inhibition effect while inducing apoptosis of RET-fusion cells. Kodama T. et al. reported that alectinib showed significant in vitro and in vivo antitumor effects against RET-fusion gene–positive NSCLC cells.Citation7 In that paper, alectinib activated caspase 3/7 as a single agent, indicating that alectinib induces apoptosis. In fact, in our experiment as well, a high concentration of alectinib alone induced apoptosis in both LC-2/ad and Ba/F3-KIF5B-RET cells (data not shown). In contrast, palbociclib by itself didn’t increase the binding of annexin V in RET-fusion cells (). Thus, it is likely that in combination, palbociclib promotes the apoptosis induced by alectinib. The synergistic effect was also shown in combination with another CDK4/6 inhibitor, abemaciclib, suggesting that the combination of alectinib and a CDK4/6 inhibitor can show a superior combination effect in RET-fusion cells. Furthermore, the synergistic effect of the combination of palbociclib with each of the other two RET inhibitors suggests that the observed synergistic effect is caused by a RET signaling inhibition-dependent mechanism. We showed that the phosphorylation of Rb and S6 were suppressed further under co-treatment with alectinib plus palbociclib in RET-fusion cells than under each single agent.
In HER2-positive breast cancer cells, CDK4/6 and HER2 trigger TSC2 phosphorylation. The combination of CDK4/6 inhibitors plus HER2 inhibitors induces a more marked suppression of TSC2 phosphorylation, and this suppression in turn induces suppression of S6 and Rb which is more potent than is achieved by either inhibitor alone. Moreover, the CDK4/6 inhibitor induces suppression of mTORC1 activity and activates HER2 kinase by relieving feedback inhibition, which renders cells more sensitive to the HER2 inhibitor. These mechanisms induce a synergistic growth inhibition effect.Citation31 In RET-fusion cells, we checked the dependency of the phosphorylation of the S6 on mTOR using a combination of mTOR inhibitor, rapamycin or everolimus, with alectinib plus palbociclib (Supprementary Figure 4). As single drugs, both mTOR inhibitors suppressed phospho-S6, and the combination of alectinib, palbociclib plus either mTOR inhibitor further suppressed it (Supprementary Figure 4). This result suggests that the repression of phospho-S6 is mediated via not only mTOR but also other molecules. While the mechanisms underlying the inhibition of the phosphorylation of S6 and Rb have not yet been resolved, it is likely that the inhibition of these through the combination of alectinib plus a CDK4/6 inhibitor also contributes to enhancing the growth inhibition effect.
Because alectinib has been approved for use in the treatment of ALK-fusion gene–positive NSCLC, we preliminarily investigated the combination of alectinib plus palbociclib against SNU-2535 cells (Supplementary Figure 5), which are ALK-fusion gene–positive NSCLC cells.Citation35 As with in RET-fusion cells, this combination had a synergistic effect according to isobologram analysis. This combination also significantly enhanced growth inhibition, increased apoptosis, and suppressed the phosphorylation of Rb and S6 compared with each single agent (Supplementary Figure 5). These results indicate that the combination of alectinib plus a CDK4/6 inhibitor could be effective against RET-fusion and ALK-fusion tumors, and could be a promising treatment option for RET-fusion and ALK-fusion NSCLC.
Although a mouse model with transplanted human RET-fusion gene–positive NSCLC cell line LC-2/ad has been used to evaluate small molecule agents with RET kinase inhibitory activity,Citation7,Citation12 we could not establish in vivo experimental conditions for evaluating the combination effect because of the slow tumor growth of LC-2/ad in vivo. Thus, we evaluated the antitumor effect of the combination of alectinib plus palbociclib in a three-dimensional culture assay, which more accurately reflects clinical profiles than those in two-dimensional culture assay.Citation36 In addition to this, we evaluated in vivo antitumor effect of these combination using a RET fusion gene transfected mouse pro-B cell line Ba/F3-KIF5B-RET, a model which has been used to evaluate the antitumor effect of some RET inhibitors.Citation7,Citation13 We considered this mouse model as a suitable for evaluating the antitumor effects of these combinations because it is dependent to RET signaling, forms solid tumor (Supplementary Figure 3), and expands sufficiently in mouse subcutaneous.
In conclusion, we demonstrated the enhanced antitumor efficacy of alectinib in combination with the CDK4/6 inhibitor against RET-fusion cells, both in vitro and in vivo. This combination suppressed the phosphorylation of S6 and Rb, which are possible molecules contributing to the combination effect. Further study to analyze the detailed mechanisms and to confirm the clinical utility of the combination of RET inhibitor plus CDK4/6 inhibitor against RET-fusion–positive NSCLC is required.
Materials and methods
Compounds and cell lines
Alectinib was synthesized at Chugai Pharmaceutical Co., Ltd. Pemetrexed, paclitaxel, carboplatin, vinorelbine, irinotecan, and gemcitabine were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). BKM120 and everolimus were purchased from AdooQ BioScience (Irvine, CA, USA). Gedatolisib and abemaciclib were purchased from Selleck Chemicals LLC (Houston, TX, USA). Luminespib was purchased from Akt Pharm, Inc (Arlington Heights, IL, USA). Palbociclib was purchased from Sigma-Aldrich (St. Louis, MO, USA). SAHA was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan).
For in vitro studies, all compounds were dissolved in dimethyl sulfoxide (Sigma-Aldrich). For in vivo studies, alectinib was dissolved in a 6% (w/v) solution of Captisol, and palbociclib was suspended in a 6% (w/v) solution of Captisol.
LC-2/ad cells were obtained from RIKEN Bioresorce Research Center (Ibaraki, Japan) and maintained in RPMI-1640 medium (Sigma-Aldrich) and Ham’s F-12 medium (Gibco, Life Technologies Corp., Grand Island, NY, USA) supplemented with 15% fetal bovine serum (FBS) (Bovogen Biologicals Pty Ltd., East Keilor, VIC, Australia) and 25 mM HEPES (Sigma-Aldrich). Ba/F3-KIF5B-RET cells, which are Ba/F3 cells transfected with the KIF5B-RET fusion gene, that had been previously established,Citation7 were maintained in RPMI-1640 medium supplemented with 10% FBS (Sigma-Aldrich). NCI-H1944 cells were purchased from ATCC and maintained in RPMI-1640 medium supplemented with 10% FBS (Sigma-Aldrich), 0.45% D-glucose (Sigma-Aldrich), 10 mM HEPES, and 1 mM sodium pyruvate (Thermo Fisher Scientific, Waltham, MA, USA). All cells were maintained at 37°C under 5% CO2.
Animals
Male 5-week-old BALB/c-nu/nu mice (CAnN Cg-Foxn1< nu>/CrlCrlj nu/nu) were purchased from Charles River Laboratories Japan, Inc (Yokohama, Japan). All animals were allowed to acclimatize and recover from shipping-related stress for more than 5 days prior to the study. Chlorinated water and irradiated food were provided ad libitum, and the animals were kept under a controlled 12-h light/12-h dark cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee at Chugai Pharmaceutical.
In vitro cell proliferation assay
LC-2/ad cells and NCI-H1944 cells were seeded on 96-well plates at 1 × 104 cells per well and pre-cultured overnight. The cells were then treated with compounds at the indicated concentrations for 4 days. Viable cells were measured by crystal violet assay and analyzed as described previously.Citation37 Briefly, the cells were fixed with formalin, stained with 0.5% (w/v) crystal violet in 6% methanol, then washed and dried. Finally, the cells were dissolved with 70% ethanol and their absorbance was measured at 595 nm. Pre-cultured cells before treatment were also subjected to crystal violet viability assay. The percentage of cell growth was calculated as follows: Growth (% of control) = [(absorbance of treatment well − absorbance of pre-cultured well)/(absorbance of non-treatment well − absorbance of pre-cultured well)] × 100.
Ba/F3-KIF5B-RET cells were seeded on 96-well plates at 5 × 103 cells per well. The cells were treated with compounds at the indicated concentrations for 4 days. Viable cells were measured by CellTiter-Glo Luminescent Cell Viability Assay (Promega Corp., Madison, WI, USA). The percentage of cell growth was calculated as follows: Growth (% of control) = [(luminescence of treatment well − luminescence of blank well)/(luminescence of non-treatment well − luminescence of blank well)] × 100.
The absorbance was measured by a Benchmark Plus microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) or a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific), and luminescence was measured by a Varioskan LUX multimode microplate reader.
IC50 isobologram analysis and combination index
Cells were treated with alectinib alone or either combined agent alone to calculate IC50, and their combinations to analyze combination effect. Each agent was diluted serially, and their concentrations are described in .
IC50 isobologram analysis derived from the Loewe additivity model provides a graphical presentation of the interaction of two drugs.Citation38 First, in a two-coordinate plot with one coordinate representing concentration of alectinib and the other representing the concentration of the combined compound, the concentrations of alectinib and the combined compound required to produce a 50% growth inhibition effect, when used as single agents, were placed on the horizontal and vertical axis, respectively. The line representing additivity is constructed by connecting these two points. Second, concentrations of the two drugs used in combination to provide the same effect, denoted by points, are placed in the same plot. The data points located below, on, or above the line indicate synergism, additivity, and antagonism, respectively.
To calculate the combination index, the combination concentrations of drug A and drug B required to produce a 50% inhibition effect, C50,A and C50,B, are normalized by their corresponding concentrations to produce the same effect as single agents, IC50,A and IC50,B, respectively. The sum of C50,A/IC50,A and C50,B/IC50,B is defined as the combination index.Citation38 Combination index <1 means synergism, = 1 means additivity, and >1 means antagonism.
Annexin V assay
LC-2/ad cells were seeded on 96-well plates at 2 × 104 cells per well and Ba/F3-KIF5B-RET cells were seeded at 1 × 104 cells per well, and the cells were pre-cultured overnight. The cells were treated with alectinib and/or palbociclib at 100 nM each. After 3 days, the apoptotic cells were measured by RealTime Glo Annexin V Apoptosis and Necrosis Assay (Promega Corp.) without using Necrosis Detection Reagent. At the same time, live cells were measured by the CellTiter-Glo Luminescent Cell Viability Assay. The luminescence was measured with a Varioskan LUX multimode microplate reader. The luminescence of annexin V was normalized by the luminescence of CellTiter-Glo as follows: (luminescence of annexin V − luminescence of blank of annexin V)/(luminescence of CellTiter-Glo − luminescence of blank of CellTiter-Glo). The fold change of annexin V binding was calculated as follows: (normalized luminescence of annexin V of treatment cells)/(normalized luminescence of annexin V of unstimulated cells).
Three dimension assay
Three dimension assay was carried out by using a CytoSelect 96-Well In Vitro Tumor Sensitivity assay (Cell Biolabs, San Diego, CA, USA). LC-2/ad cells were treated with alectinib and/or palbociclib at 200 nM each for 7 days. Viable cells were measured by Cell counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). The absorbance was measured by a Varioskan LUX multimode microplate reader. Growth (% of control) = [(absorbance of treatment well − absorbance of blank well)/(absorbance of non-treatment well − absorbance of blank well)] × 100.
In vivo tumor growth inhibition study
5 × 106 Ba/F3-KIF5B-RET cells were inoculated subcutaneously into the right flank of each mouse. Mice bearing tumors were randomly allocated to vehicle control or treatment groups (six mice in each group), and treatment was started. These studies consisted of the following four groups: (i) vehicle control (treated with solvent vehicle [6% Captisol]); (ii) alectinib at 20 mg/kg; (iii) palbociclib at 75 mg/kg; and (iv) alectinib at 20 mg/kg plus palbociclib at 75 mg/kg. Mice bearing Ba/F3-KIF5B-RET tumors were treated until the day before the final measurement of tumor volume and body weight. The first day of treatment was defined as Day 1. All treatments were administered orally and daily. Tumor volume (TV) and body weight were measured twice a week. TV was estimated as follows: TV = abCitation2/2, where a and b are tumor length and width.
In vitro immunoblotting
LC-2/ad cells were seeded on 6-well plates at 4 × 105 cells per well and pre-cultured for 2 or 3 days. Ba/F3-KIF5B-RET cells were seeded on 15 mL conical tubes or 6-well plates at 1 × 106 cells per tube or per well. The cells were treated with alectinib and/or palbociclib at 100 nM each for the indicated time. The cells were lysed in cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA) containing protease inhibitor cocktail (Sigma-Aldrich) and phosphatase inhibitor cocktail (NACALAI TESQUE, INC., Kyoto, Japan). RET, phospho-RET, phospho-Rb, S6, phospho-S6, ACTB (Cell Signaling Technology), and Rb (abcam, Cambridge, MA, USA) were detected by a capillary electrophoresis-based protein analysis system (Sally Sue; ProteinSimple, San Jose, CA, USA).
In vivo immunoblotting
Mice were inoculated with Ba/F3-KIF5B-RET cells as in the in vivo tumor growth inhibition study. Tumors were resected 2 days after treatment with vehicle, alectinib (20 mg/kg), palbociclib (75 mg/kg), or alectinib (20 mg/kg) plus palbociclib (75 mg/kg), and were lysed after being homogenized (Multi-Beads Shocker; Yasui Kikai, Osaka, Japan). The lysates were detected as with in vitro immunoblotting.
Statistical analysis
Statistical analyses were performed using JMP version 11.2.1 software (SAS Institute). In in vitro experiments, statistical significance was analyzed by Tukey-Kramer’s HSD test. In in vivo experiments, statistical significance was analyzed by Wilcoxon test. The significant p-values were adjusted for multiple comparisons by the Holm method.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (257.3 KB)Acknowledgments
We thank Kumiko Kondoh and Mirei Kohno (Product Research Department, Chugai Pharmaceutical Co., Ltd.) for technical assistance; Kiyoaki Sakata (Research Division, Chugai Pharmaceutical Co., Ltd.) for instruction on the use of isobolograms; and Dr. Kazushige Mori and Kaori Fujimoto-Ouchi (Product Research Department, Chugai Pharmaceutical Co., Ltd.) for helpful suggestions and comments about this study.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Chan BA, Hughes BG. 2015. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 4:36–54.4. doi:10.3978/j..2218-6751.2014.05.01.
- Sgambato A, Casaluce F, Maione P, Gridelli C. 2018. Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev Anticancer Ther. 18:71–80.5. doi:10.1080/14737140.2018.1412260.
- Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16:441–467.6. doi:10.1016/j.cytogfr.2005.05.010.
- Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J. 2016. Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget. 7:59236–59244.7. doi:10.18632/oncotarget.10985.
- Sakamoto H, Tsukaguchi T, Hiroshima S, Kodama T, Kobayashi T, Fukami TA, Oikawa N, Tsukuda T, Ishii N, Aoki Y. 2011. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 19:679–690.8. doi:10.1016/j.ccr.2011.04.004.
- Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Perol M, Dziadziuszko R, Rosell R, et al. 2017. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 377:829–838.10. doi:10.1056/NEJMoa1704795.
- Kodama T, Tsukaguchi T, Satoh Y, Yoshida M, Watanabe Y, Kondoh O, Sakamoto H. 2014. Alectinib shows potent antitumor activity against RET-rearranged non-small cell lung cancer. Mol Cancer Ther. 13:2910–2918.11. doi:10.1158/1535-7163.MCT-14-0274.
- Takeuchi S, Murayama T, Yoshimura K, Kawakami T, Takahara S, Imai Y, Kuribayashi Y, Nagase K, Goto K, Nishio M, et al. 2017. Phase I/II study of alectinib in lung cancer with RET fusion gene: study protocol. J Med Invest. 64:317–320.13. doi:10.2152/jmi.64.317.
- Wells SA Jr., Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, et al. 2012. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 30:134–141.38. doi:10.1200/jco.2011.35.5040.
- Sherman SI, Clary DO, Elisei R, Schlumberger MJ, Cohen EE, Schoffski P, Wirth LJ, Mangeshkar M, Aftab DT, Brose MS. 2016. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer. 122:3856–3864.39. doi:10.1002/cncr.30252.
- Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, Camidge R, Narayanan V, Doebele RC, Besse B, et al. 2017. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 35:1403–1410.37. doi:10.1200/JCO.2016.70.9352.
- Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, Wirth LJ, Stock S, Smith S, Lauriault V, et al. 2018. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 29:1869–1876.29. doi:10.1093/annonc/mdy137.
- Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, Hu W, Cao Q, Sheets MP, Wilson D, et al. 2018. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 8:836–849.53. doi:10.1158/2159-8290.cd-18-0338.
- Ackermann CJ, Stock G, Tay R, Dawod M, Gomes F, Califano R. 2019. Targeted therapy for RET-rearranged non-small cell lung cancer: clinical development and future directions. Onco Targets Ther. 12:7857–7864.54. doi:10.2147/ott.s171665.
- Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al. 2014. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 15:1236–1244.30. doi:10.1016/s1470-2045(14)70381-x.
- Hrustanovic G, Olivas V, Pazarentzos E, Tulpule A, Asthana S, Blakely CM, Okimoto RA, Lin L, Neel DS, Sabnis A, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med. 2015;21:1038–1047.15. doi:10.1038/nm.3930.
- Valentino F, Borra G, Allione P, Rossi L. 2018. Emerging targets in advanced non-small-cell lung cancer. Future Oncol. 14:61–72.20. doi:10.2217/fon-2018-0099.
- Dolly SO, Collins DC, Sundar R, Popat S, Yap TA. 2017. Advances in the development of molecularly targeted agents in non-small-cell lung cancer. Drugs. 77:813–827.21. doi:10.1007/s40265-017-0732-2.
- Pillai RN, Ramalingam SS. 2014. Heat shock protein 90 inhibitors in non-small-cell lung cancer. Curr Opin Oncol. 26:159–164.41. doi:10.1097/cco.0000000000000047.
- Matsubara D, Kanai Y, Ishikawa S, Ohara S, Yoshimoto T, Sakatani T, Oguni S, Tamura T, Kataoka H, Endo S, et al. 2012. Identification of CCDC6-RET fusion in the human lung adenocarcinoma cell line, LC-2/ad. J Thorac Oncol. 7:1872–1876.42. doi:10.1097/JTO.0b013e3182721ed1.
- National Cancer Institute, NCI drug dictionary, pemetrexed disodium. [accessed 2019 Jun 27]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/pemetrexed-disodium
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392.45. doi:10.1056/NEJMra035536.
- Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438.47.
- U.S. Food and Drug Administration, Label for NDA 021991. [accessed 2019 Jun 27]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021991s001lbl.pdf
- Burger MT, Pecchi S, Wagman A, Ni ZJ, Knapp M, Hendrickson T, Atallah G, Pfister K, Zhang Y, Bartulis S, et al. 2011. Identification of NVP-BKM120 as a potent, selective, orally bioavailable class I PI3 kinase inhibitor for treating cancer. ACS Med Chem Lett. 2:774–779.50. doi:10.1021/ml200156t.
- National Cancer Institute, NCI Drug dictionary, gedatolisib. [accessed 2019 Jun 27]. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/gedatolisib
- U.S. Food and Drug Administration, Labels for NDA 022334. [accessed 2019 Jun 27]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022334lbl.pdf
- U.S. Food and Drug Administration, Labels for NDA 208716. [accessed 2019 Jun 27]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208716Orig1s000lbl.pdf
- Foucquier J, Guedj M. 2015. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 3:e00149.40. doi:10.1002/prp2.149.
- Zhou J, Zhang S, Chen X, Zheng X, Yao Y, Lu G, Zhou J. 2017. Palbociclib, a selective CDK4/6 inhibitor, enhances the effect of selumetinib in RAS-driven non-small cell lung cancer. Cancer Lett. 408:130–137.22. doi:10.1016/j.canlet.2017.08.031.
- Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, Ramm S, Palmer AC, Yuzugullu H, Varadan V, et al. 2016. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell. 29:255–269.25. doi:10.1016/j.ccell.2016.02.006.
- Wood AC, Krytska K, Ryles HT, Infarinato NR, Sano R, Hansel TD, Hart LS, King FJ, Smith TR, Ainscow E, et al. 2017. Dual ALK and CDK4/6 inhibition demonstrates synergy against neuroblastoma. Clin Cancer Res. 23:2856–2868.2. doi:10.1158/1078-0432.CCR-16-1114.
- Zhou J, Wu Z, Wong G, Pectasides E, Nagaraja A, Stachler M, Zhang H, Chen T, Zhang H, Liu JB, et al. 2017. CDK4/6 or MAPK blockade enhances efficacy of EGFR inhibition in oesophageal squamous cell carcinoma. Nat Commun. 8:13897.26. doi:10.1038/ncomms13897.
- Liu M, Xu S, Wang Y, Li Y, Li Y, Zhang H, Liu H, Chen JPD. 0332991, a selective cyclin D kinase 4/6 inhibitor, sensitizes lung cancer cells to treatment with epidermal growth factor receptor tyrosine kinase inhibitors. Oncotarget. 2016;7:84951–84964.33. doi:10.18632/oncotarget.13069.
- Kim S, Kim TM, Kim DW, Go H, Keam B, Lee SH, Ku JL, Chung DH, Heo DS. 2013. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol. 8:415–422.43. doi:10.1097/JTO.0b013e318283dcc0.
- Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. 2010. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 148:3–15.55. doi:10.1016/j.jbiotec.2010.01.012.
- Yamashita-Kashima Y, Iijima S, Yorozu K, Furugaki K, Kurasawa M, Ohta M, Fujimoto-Ouchi K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17:–5060–5070.19. doi:10.1158/1078-0432.ccr-10-2927.
- Zhao L, Au JL, Wientjes MG. Comparison of methods for evaluating drug-drug interaction. Front Biosci (Elite Ed). 2010;2:241–249.36. doi:10.2741/e86.
