ABSTRACT
Signal transducer and activator of transcription 1 (STAT1) is related to the immune microenvironment of tumorigenesis. The programmed cell death 1 (PD-1) and its ligand (PD-L1) have been reported to be important in immunotherapy by mediating tumor immune evasion. Blocking the PD-1/PD-L1 pathway can restore the endogenous anti-tumor immune response. This study aimed to examine the expression of STAT1, PD-1, and PD-L1 and the correlation between selected markers in human epithelial ovarian cancer (EOC). The results showed that malignant tumors contained more STAT1, PD-1, and PD-L1 positive cells. The expression of STAT1 and PD-L1 was associated with age, whereas PD-1 and PD-L1 associated with histopathological type, in patients with ovarian tumors. Moreover, the expression of STAT1 was found to be associated with disease stages and the grade of serous carcinoma. STAT1 expression was higher in OC cells than normal ovarian surface epithelial cells and was positively correlated with PD-L1 expression. The knockdown of STAT1 decreased PD-L1 expression, whereas overexpression of STAT1 increased PD-L1 expression. Furthermore, the expression of STAT1, PD-1, and PD-L1 was lower in paclitaxel-resistant cells than sensitive cells. Finally, STAT1 affected the overall survival and progression-free survival of patients with EOC. These findings suggest that STAT1, PD-1, and PD-L1 are the tissue markers of EOC and imply the possibility that the high level of STAT1, PD-1, and PD-L1 may favor the checkpoint immunotherapy in patients with EOC, but may have a limit in paclitaxel-resistant patients because of the low expression of STAT1, PD-1, and PD-L1 in paclitaxel-resistant cells.
Introduction
Immunotherapy is an important anti-tumor treatment in addition to surgery, chemotherapy, and radiotherapy. It can stimulate the immune system of the body to improve the effectiveness of anti-tumor immune. The association between tumor cells and the immune system is regulated by a large number of immune activators and suppressor factors.Citation1 The programmed cell death 1 (PDCD1, PD-1) and its ligand (PD-L1) play an important role of immunotherapy in cancer patients. The downstream T cell receptor (TCR) signaling can be inhibited by the interaction of PD-1 with PD-L1.Citation2 The immune checkpoint blockade (ICB) therapy, including the anti-PD-1 and anti-PD-L1 antibodies such as nivolumab or pembrolizumab, suppresses PD-1/PD-L1, thereby releasing the endogenous anti-tumor immune response, which has been used in some patients with cancer, including melanoma, renal cell cancer, urothelial carcinoma, non-small cell lung cancer, Hodgkin’s lymphoma, and Merkel cell carcinoma.Citation3,Citation4,Citation5,Citation6,Citation7Citation8 However, only a proportion of patients using this therapy have a durable response. The mechanisms of responsiveness or resistance to ICB in these patients are very complex. A variety of factors may positively or negatively contribute to the anti-tumor response to immune checkpoint inhibitors.Citation9 Therefore, it is reasonable and critical to further investigate the details of molecular mechanisms underlying the responsiveness or resistance to ICB.
A recent study shows that tumor immune evasion is related to the eIF4F-STAT1-PD-L1 axis in melanoma.Citation10 Signal transducer and activator of transcription 1 (STAT1) is a signaling transducer protein that is related to tumor immune microenvironment.Citation11,Citation12 The overexpression and activation of STAT1 can induce the expression of classical interferon-stimulated genes that have key immune effector functions.Citation13 Several studies have shown an important role of STAT1 in the pathogenesis of various tumors.Citation14,Citation15 However, whether STAT1 is a therapeutic partner in the combination with the inhibitors of the PD-1/PD-L1 pathway in epithelial ovarian cancer (EOC) remains unknown.
EOC is the most lethal disease of women because of relapse, metastasis, and lack of efficient therapies at the late stage.Citation16,Citation17 Currently, resistance to the first-line platinum and taxane-based drugs of chemotherapy for EOC is a major clinical challenge.Citation18 Over the past years, the combination of chemotherapy with immunotherapy has been applied in numerous cancers clinically.Citation19 Of note, the presence of intra-epithelial tumor-infiltrating lymphocytes (TILs) implies that EOC may be an immunoreactive tumor type.Citation20 In fact that ICB therapy applied, improved clinical outcomes had been demonstrated in ovarian cancer.Citation21 However, the presence of various inhibitory factors such as PD-1/PD-L1 remains a concern and limits their applications in EOC.Citation22,Citation23 Therefore, identifying reliable biomarkers to evaluate the efficiency of immunotherapy in EOC is needed.
In this study, we aimed to examine the presence of extensive and significant correlations between cancer-related markers in normal and malignant tissues of the ovary. Especially, we investigated the expression of STAT1, PD-1, and PD-L1 in EOC tissues and cell lines. Finally, we explored the correlation between STAT1, PD-1, and PD-L1 in paclitaxel (PTX)-resistant EOC cells and the effect of STAT1 on PD-L1 expression in vitro.
Results
Differentially expressed cancer-related markers between normal ovarian tissues and epithelial ovarian malignant tumors and their correlation
Several cancer-related proteins were examined by immunohistochemistry. We found that there were more STAT1, PD-1, and PD-L1 positive staining in human ovarian malignant tumors (positive cases/total cases: STAT1 n = 22/40, PD-1 n = 19/40, PD-L1 n = 21/40) compared to normal ovarian tissues (STAT1 n = 2/40, PD-1 n = 8/40, PD-L1 n = 3/40) (Supplementary Figure S1). Other cancer-related proteins were also observed to be increased in malignant tumors, such as Ki-67, p53, CA125, and WT1 (Supplementary Figure S1, ). Furthermore, we found extensively and significantly positive correlations among these cancer-related proteins in both normal and malignant ovarian tissues. The analyzed data showed that the positive correlation was found between these selected markers, such as STAT1 and PD-L1, PD-1 and Ki-67, ER and PR, ER and CA125, ER and WT1, PR and WT1, p53 and Ki-67, p53 and CEA, CK20 and CEA, calretinin and villin, calretinin and α-inhibin, villin and α-inhibin, and the negative correlation was found between ER and villin, ER and α-inhibin, PR and villin, CK20 and CA125, WT1 and villin, vimentin and CEA, in ovarian cancer tissues (). In normal tissues, a positive correlation was found between STAT1 and PD-L1, STAT1 and villain, PD-1 and CEA, PD-L1 and p53, ER and PR, ER and Ki-67, PR and CA125, WT1 and villin, WT1 and calretinin, CEA and villin, CEA and α-inhibin (Supplementary Table S1). There was no correlation between STAT1 and PD-1 in normal tissues (P = .288, rs 0.172) and malignant tumors (P = .337, rs 0.156).
Table 1. Association between selected markers in epithelial ovarian cancer
Table 2. The association of the expression of STAT1, PD-1, and PD-L1 with clinicopathological features of EOC patients
Overexpression of STAT1, PD-1, and PD-L1 in ovarian malignant tissues and cells
Next, we compared the expression of STAT1, PD-1, and PD-L1 in ovarian tissue derived from patients with or without ovarian tumors. Immunohistochemical staining showed that overexpression of STAT1, PD-1, and PD-L1 was present in malignant tumors ()). There were more STAT1, PD-1, and PD-L1-positive cases in patients with ovarian malignant tumors than normal, benign, and borderline tumors ()). To further validate these outcomes, we examined the expression of STAT1, PD1, and PD-L1 in non-tumorous human ovarian surface epithelial cells (HOSEpiC) and ovarian cancer cells (OVCAR-3). We found that STAT1 and PD-L1 were highly expressed at mRNA and protein levels in OVCAR-3 cells compared with HOSEpiC cells (). PD-1 mRNA expression level was significantly lower in OVCAR-3 cells than HOSEpiC cells, but there was no difference in PD-1 protein expression between these two cell lines.
Figure 1. Immunohistochemical staining of STAT1, PD-1, and PD-L1 proteins and case rate of positive staining. (a) Expression of STAT1, PD-1, and PD-L1 proteins in normal ovarian tissues and epithelium-type ovarian tumors. An enlarged image was inserted in the up-right corner of the picture. A brown color in epithelial cells is considered positive staining. Representative images of STAT1, PD-1, and PD-L1 expression are shown. Original magnification × 100. Scale bar 200 µm. (b) The case rate of STAT1, P-D1, and PD-L1 positive and negative staining. The ratio of positive/negative was 2/38, 8/32, and 3/37 for STAT1, PD-1, and PD-L1, respectively, in control without tumor (40 cases); 0/36, 2/34, and 0/36 for STAT1, PD-1, and PD-L1, respectively, in the benign tumor (36 cases); 2/30, 3/29, and 0/32 for STAT1, PD-1, and PD-L1, respectively, in the borderline tumor (32 cases); 22/18, 19/21, and 21/19 for STAT1, PD-1, and PD-L1, respectively, in the malignant tumor (40 cases). For comparison between the two groups, the χ2 test was applied. Normal, normal ovarian tissue; Benign, benign tumor; Borderline, borderline tumor; Malignant, malignant tumor. *, P < .05; ***, P < .001
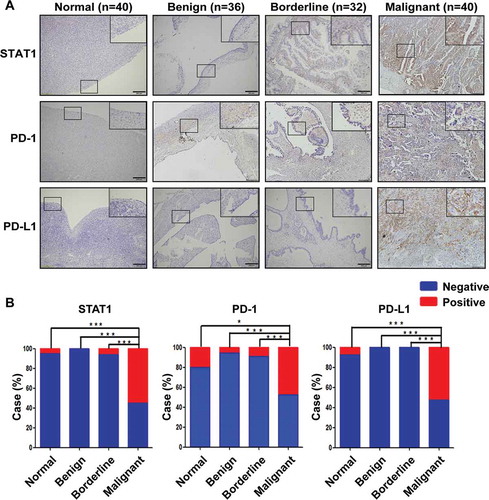
Figure 2. Expression of STAT1, PD-1, and PD-L1 in HOSEpiC and OVCAR-3 cells. (a) The expression of STAT1, PD-1, and PD-L1 mRNA was determined by quantitative RT-PCR. (b) The expression of STAT1, PD-1, and PD-L1 protein was determined by Western blot. (c) Densitometric analysis of the gels in (B). The number above the bar indicates a P-value
Association of STAT1, PD-1, and PD-L1 with clinicopathological features in epithelial-type ovarian tumors
After the analysis of total epithelial-type tumors including benign, borderline, and malignant tumors, we found that the expression of STAT1 and PD-L1 was associated with age (P = .004 and P = .013, respectively), indicating that the positive rates of STAT1 and PD-L1 protein expression were significantly higher in older patients (age >45) than in younger patients (age ≤45) (Supplementary Table S2). Further, the expression of PD-1 and PD-L1 was associated with histopathological types (P = .000 and P = .007 respectively), but STAT1 was not associated with histopathological types (P = .230).. In patients with EOC, STAT1 was associated with stages (P = .044) and grades (P = .048) (). About 83.33% of cases were STAT1-positive at advanced stages (III and IV) and 80% of cases in high-grade serous carcinoma were STAT1-positive.
Low expression of STAT1 and PD-L1 in paclitaxel-resistant ovarian cancer cells
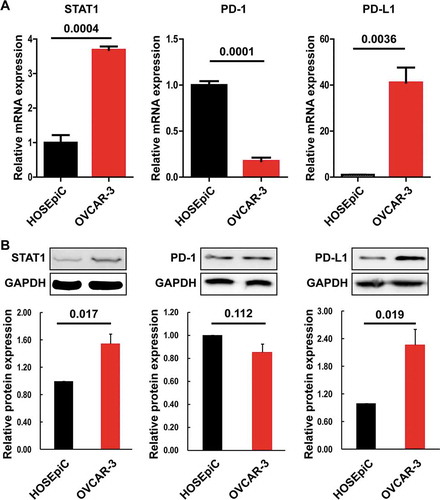
The expression of STAT1, PD-1, and PD-L1 at the transcriptional and translational levels was examined in OVCAR-3 and OV3R-PTX cells. We found that down-regulation of STAT1, PD-1, and PD-L1 at the mRNA level ()) and down-regulation of STAT1 and PD-L1 at the protein level ()) were observed in OV3R-PTX cells compared to OVCAR-3 cells. These results imply that the treatment of PTX leading to the down-regulation of STAT1 and PD-L1 may be linked to the negative response to anti–PD-1/PD-L1 immunotherapy in PTX-resistant patients.
Figure 3. Expression of STAT1, PD-1, and PD-L1 in OVCAR-3 and OV3R-PTX cells. (a) The expression of STAT1, PD-1, and PD-L1 mRNA was determined by quantitative RT-PCR. (b) The expression of STAT1, PD-1, and PD-L1 protein was determined by Western blot. (c) Densitometric analysis of the gels in (B). The number above the bar indicates a P-value
Correlation of STAT1 with PD-L1 in OVACAR-3 and OV3R-PTX cells
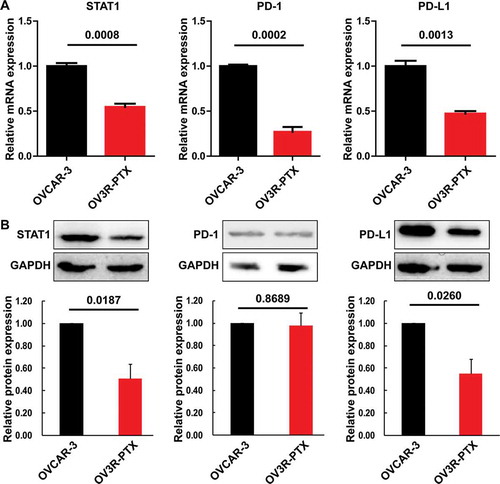
To further examine the effect of STAT1 on PD-L1 expression and validated the correlation between STAT1 and PD-L1, we knocked down STAT1 expression by RNA interference. The decrease of STAT1 mediated by siRNA significantly down-regulated the expression of PD-L1 in OVACAR-3 and OV3R-PTX cells (,)). Next, we increased STAT1 by transient transfection with STAT1-overexpressing plasmid. We found that the expression of PD-L1 was significantly up-regulated after overexpression of STAT1 (,)). Taken together, these results suggested that the expression of PD-L1 was positively correlated with the expression of STAT1.
Figure 4. Expression of STAT1 and PD-L1 protein after transfection with STAT1-siRNA or STAT1 overexpressing plasmid in OVCAR-3 and OV3R-PTX cells. The expression of STAT1 and PD-L1 protein (top panel, Western blot image; middle and low panels, densitometric analysis of the gels) was decreased after STAT1-siRNA transfection in OVCAR-3 cells (a) and OV3R-PTX cells (b). The expression of STAT1 and PD-L1 protein (top panel, Western blot image; middle and low panels, densitometric analysis of the gels) was increased after STAT1 overexpressing plasmid transfection in OVCAR-3 cells (c) and OV3R-PTX cells (d). The number above the bar indicates a P-value
Association of PD1, PD-L1 and STAT1 expression with the overall survival and progress-free survival of patients with ovarian cancer
To assess the prognostic value of STAT1, PD-1, and PD-L1 expression in ovarian cancer, we performed bioinformatics analysis. A high level of STAT1 expression was associated with better overall survival (OS) in 2 studies (n = 248 cases) and progress-free survival (PFS) in 3 studies (n = 982 cases) (Supplementary Figure S2). However, the expression of PD-1 and PD-L1 was not associated with OS and PFS of patients with OC.
Discussion
The present study examined the expression of STAT1 and immune checkpoint molecules in ovarian cancer patients. We explore the potential correlations among STAT1, PD-1, and PD-L1 with common cancer-related markers by immunohistochemical staining and the association of these proteins with clinicopathologic features in patients with ovarian tumors.
The current data demonstrated positive correlations of immunohistochemical outcomes extensively and significantly, which imply the role of STAT1 and PD-1/PD-L1 signaling in ovarian tumorigenesis. It has shown that IFN-γ can activate its receptor-associated JAK1/2 pathway, which results in the phosphorylation of STAT1 and its nucleus localization.Citation24 Consequently, STAT1 binds TET2 and recruits it to hydroxymethylate PD-L1 at the promoter region, activating the expression of PD-L1 in solid tumors such as murine melanoma and colon cancer.Citation25 Indeed, our results further confirmed the positive correlation between STAT1 and PD-L1 in human ovarian cancer. Interestingly, we also found that the expression of STAT1 and PD-L1 was associated with the age of patients with ovarian tumors. The expression of STAT1 and PD-L1 was found to be higher in patients aged >45 compared with patients aged ≤45, suggesting that immune checkpoint inhibitors may have a potential to be applied in older patients.
Checkpoint blockade using drugs blocking PD-1 and PD-L1 had shown a promise of treatment in patients with some types of cancer. However, a low response rate to the monotherapy of immune checkpoint inhibitors remains to be addressed. On one hand, practical and consistent biomarkers to identify patients responding to the immunotherapy are needed and the combination of multi-biomarker may be helpful to enrich for responders. On the other hand, improving our understanding of resistance mechanisms of checkpoint inhibitors is a prerequisite for rational combinations of approved drugs and for developing novel agents. STAT1 is a member of the STAT family,Citation26 which transduces external signals into the nucleus and regulates gene expression,Citation27 thereby modulating diverse cellular processes such as cell differentiation, proliferation, and cell death.Citation28,Citation29 Recently, the prognostic value of the STAT family in many cancer has emerged.Citation30,Citation31 However, there are two faces of STAT1 that existed in solid tumors.Citation32,Citation33 Our data analyses from the TCGA datasets showed that though neither PD-1nor PD-L1 was correlated with the OS and PFS of patients with OC,Citation34,Citation35,Citation36,Citation37,Citation38,Citation39,Citation40,Citation41,Citation42,Citation43,Citation44,Citation45,Citation46 STAT1 expression was associated with the OS and PFS of patients with OC.Citation47,Citation48,Citation49 The presence of positive correlation between STAT1 and PD-L1 in ovarian tumors and the overexpression of STAT1 in patients with OC may favor the immune checkpoint inhibitors and thus, provide the novel strategy of therapy against human OC.
Over the past decades, the combined chemotherapy of PTX and carboplatin is the standard treatment in newly diagnosed EOC.Citation50 Here, we intend to evaluate the feasibility of the combination of PTX with anti-PD-1/PD-L1 therapy. The present study showed that the expression of STAT1 and PD-L1 was decreased in PTX-resistant cell (OV3R-PTX) and the levels were significantly lower in OV3R-PTX than PTX-sensitive parental cells (OVCAR-3), suggesting that PTX-induced decrease of STAT1 and PD-L1 expression may limit the therapeutic efficacy of anti-PD-1/PD-L1 therapy. It has been expected that the up-regulation of these two molecules may restore the sensitivity of OC cells to PTX, which is one of the most effective chemotherapeutic drugs for the treatment of OC currently. Further, the PTX-sensitized OC cells may also respond to anti-PD-1/PD-L1 therapy. Giving STAT1 as a regulator of PD-L1 provides new insight into targeting STAT1 and PD-1/PD-L1 in patients with OC.
Although we discovered the correlation between STAT1 and PD-L1 in EOC, a few limitations still exist in the current study. First of all, the sample size is small so that the analysis of clinical data might be influenced due to heterogeneity. We believe that more tumor samples along with the detailed clinical data should be included and conducted in the future to evaluate the importance of STAT1 and PD-L1 in EOC as well as in patients with PTX-resistance. Secondly, the lack of animal study may decrease the applicability of targeting these molecules. Finally, the mechanism of how STAT1 affects PD-L1 expression needs to be explored. Further studies should be focused on the determination of the impact of STAT1 on anti-PD-1/PD-L1 therapy. Taken together, our data demonstrated that STAT1 and PD-L1 are overexpressed and positively correlated in ovarian malignant tumors, which indicates that STAT1 and PD-L1 are two potential biomarkers to the diagnosis and prognosis of patients with EOC. High expression of STAT1 and PD-L1 provides the feasibility of chemotherapy with PD-1/PD-L1 inhibitors. The low expression of STAT1 and PD-L1 in PTX-resistant cells implies to be impracticable to combine taxane drugs with anti-PD-1/PD-L1 therapy.
Materials and methods
Ovarian tissue sample preparation
A total of 148 ovarian samples were obtained with informed consent from patients and approved by the Ethics Committee of Jinshan Hospital, Fudan University. Among these ovarian samples, 40 normal samples were from patients without ovarian tumors and 108 were epithelia-type tumor samples (36 benign, 32 borderline, and 40 malignant tumors) with age ranged from 17 to 81 years old between years of 2005 and 2019. The final diagnosis was confirmed by pathological examination. All patients had not received chemotherapy or radiotherapy before surgery.
Immunohistochemical staining and analysis
The 10% formalin-fixed paraffin-embedded ovarian tissue specimens were prepared. Four-micrometer thick tissue was sectioned for the immunohistochemical staining (IHC). Briefly, after blocking with 10% normal goat serum (Maixin Bio, Fuzhou, Fujian, China) for 10 min at room temperature, the sections were incubated with a primary antibody at 4°C overnight. The following antibodies were purchased from ZSBIO (Beijing, China): mouse anti-PD-1, rabbit anti-PD-L1, mouse anti-P53, rabbit anti-ER, rabbit anti-PR, mouse anti-Ki-67, rabbit anti-CK20, mouse anti-CA125, rabbit anti-WT1, mouse anti-vimentin, mouse anti-CEA, rabbit anti-calretinin, mouse anti-villin, mouse anti-α-inhibin. A rabbit anti-STAT1 antibody was obtained from CST (Cell Signaling Technology, Inc., Danvers, MA, USA). The secondary antibody (ZSBIO) was incubated for 1 h at room temperature. Finally, the signal was detected using a DAB Kit (Maixin Bio) and detected under a light microscope (BX43, OLYMPUS, Tokyo, Japan).
Cell culture and transfection of siRNA and plasmid
Human OVCAR-3 (OC cell line), OV3R-PTX (PTX-resistant cell line), and HOSEpiC (non-tumorous cell line) cells were plated into 6-well plates, respectively, at a density of 2 × 105 cells/well and cultured in RPMI-1640 medium supplemented with 10% FBS plus antibiotics for 24 h. For knockdown of STAT1, cells were transfected with 2 μg/well of human STAT1-small interfering RNA (STAT1-siRNA) or nonspecifically scrambled control siRNA (NC-siRNA). The siRNA sequences were 5′-GCGUAAUCUUCAGGAUAAUTT-3′ (sense) and 5′-AUUAUCCUGAAGAUUACGCTT-3′ (anti-sense) for STAT1-siRNA and 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′ (anti-sense) for NC-siRNA synthesized by GenePharma (Shanghai, China). The siRNA was transfected according to the manufacturer’s instruction. STAT1 plasmid was generated as described in our previous publication.Citation51 Cells were plated into a 6-well plate at a density of 2 × 105 cells/well for 24 h and then transfected with 2 μg/well of STAT1 plasmid or nonspecific control plasmid. After transfection for 6 h, the medium was removed and replaced with fresh RPMI-1640 supplemented with 10% FBS without antibiotics. Cells were further incubated as indicated.
Protein extraction and Western blotting
After culture, cells were lysed with SDS lysis buffer (Beyotime, Haimen, Jiangsu, China) with 1% PMSF (Beyotime) and 1% phosphatase inhibitor (KeyGEN, Nanjing, Jiangsu, China), equal amount proteins were separated on 15% SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). After blocking with 5% nonfat milk in Tris-buffered saline with Tween 20 for 1 h, the membrane was incubated with a primary antibody at 4°C overnight and subsequently incubated with goat anti-rabbit IgG (1:5000 dilution, Proteintech, Wuhan, China) or goat anti-mouse IgG (1:5000 dilution, Proteintech) for 1 h at room temperature. The following primary monoclonal antibodies were used: mouse anti-PD1 (1:1000 dilution, Sigma), mouse anti-PD-L1 (1:1000 dilution, Cusabio, China), rabbit anti-STAT1 (1:1000 dilution, CST), and mouse anti-GADPH (1:5000 dilution, Proteintech). Signals were detected using Immobilon™ Western Chemiluminescent Substrate (Millipore) and quantified using the Tanon-4500 Gel Imaging System (Tanon Science and Technology Co., Ltd., Shanghai, China).
RNA extraction and quantitative real-time PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer’s instruction. Five-hundred nanogram of total RNA was reversely transcribed using a reverse transcription kit (TaKaRa Biotechnology Co., Ltd., Dalian, Liaoning, China). The primer sequences were 5′-TCCGTTTTCATGACCTCCTG-3′ (forward) and 5′-TGAATATTCCCCGACTGAGC-3′ (reverse) for human STAT1, 5′-CCAAGGCGCAGATCAAAGAGA-3′ (forward) and 5′-AGGACCCAGACTAGCAGCA-3′ (reverse) for human PD-1, 5′-ACCTACTGGCATTTGCTGAAC-3′ (forward) and 5′-AGTGCAGCCAGGTCTAATTGTT-3′ (reverse) for human PD-L1. PCR amplification was performed at 95°C for 5 sec and 60°C for 30 sec for 40 cycles using an SYBR Premix TaqTM II (Tli RNaseH Plus) kit (TaKaRa) with an initial step of denaturing RNA at 95°C for 30 sec. PCR was conducted in triplicate and repeated at least three times. The targets (STAT1, PD-1, and PD-L1) were normalized by an endogenous control (GADPH) given by 2ΔΔCt, in which threshold cycle (Ct) was obtained using Sequence Detection Software v1.4 (7300 Real-Time PCR System, Applied Biosystems, Foster City, CA, USA).
Bioinformatics analysis
The public data were searched from PubMed, Web of Science, and EMBASE databases thoroughly between January 2000 and February 2019 using the following terms (ovarian cancer or tumor or tumor or carcinoma or malignant or malignancy or neoplasm) AND (STAT1 or PD-1 or PDL1) AND (prognostic or prognosis or survival) AND (prognostic or prognosis or survival) AND (mortality or outcome). The comprehensive database search was performed independently by two individuals. The inclusion criteria were the following: (a) Evaluation of STAT1, PD-1, and PD-L1 expression in ovarian tumor tissues for predicting patient prognosis; (b) Studies with survival data; (c) Articles published in English. The exclusion criteria were the following: (a) Meta-analysis, review paper, conference abstract, case reports, letters to the editor, and experimental studies without patient data; (b) Duplicate publications or overlapping database; (c) Articles in non-English. Two outcome endpoints of overall survival (OS) and progress-free survival (PFS) were analyzed. The pooled hazard ratios (HRs) and 95% confidence intervals (CIs) for OS and PFS were calculated using Stata version 12.0 (Stata Corporation, College Station, TX, USA).
Statistical analyses
The data were analyzed using SPSS Statistics 24 for Windows (SPSS, Chicago, IL, USA). For comparison between two groups of positivity and the association of STAT1, PD-1, and PD-L1 protein expression with histological types or the clinicopathological characteristics, the Chi-square test with or without continuity correction was applied. A P value <.05 was considered to be significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download Zip (9 MB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi:10.1016/j.ccell.2015.03.001.
- Wilky BA. Immune checkpoint inhibitors: the linchpins of modern immunotherapy. Immunol Rev. 2019;290:6–23. doi:10.1111/imr.12766.
- Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., Gutzmer R, Linette G, Chmielowski B, Lao CD, et al. Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J Clin Oncol. 2018;36(4):383–390. doi:10.1200/JCO.2016.71.8023.
- Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi:10.1056/NEJMoa1510665.
- Ning YM, Suzman D, Maher VE, Zhang L, Tang S, Ricks T, Palmby T, Fu W, Liu Q, Goldberg KB, et al. FDA Approval Summary: atezolizumab for the Treatment of Patients with Progressive Advanced Urothelial Carcinoma after Platinum-Containing Chemotherapy. Oncologist. 2017;22:743–749. doi:10.1634/theoncologist.2017-0087.
- Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA Approval Summary: pembrolizumab for the Treatment of Patients With Metastatic Non-Small Cell Lung Cancer Whose Tumors Express Programmed Death-Ligand 1. Oncologist. 2016;21:643–650. doi:10.1634/theoncologist.2015-0498.
- Ramchandren R, Domingo-Domenech E, Rueda A, Trneny M, Feldman TA, Lee HJ, Provencio M, Sillaber C, Cohen JB, Savage KJ, et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: safety and Efficacy in the Phase II CheckMate 205 Study. J Clin Oncol. 2019;37(23):1997–2007. doi:10.1200/JCO.19.00315.
- Singh P, Visger Von J, Prosek J, Rovin B, Pesavento TE, Olencki T, Pandey D. Preserved Renal Allograft Function and Successful Treatment of Metastatic Merkel Cell Cancer Post Nivolumab Therapy. Transplantation. 2019;103(2):e52–e3. doi:10.1097/TP.0000000000002502.
- Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30(2):219–235. doi:10.1093/annonc/mdy551.
- Cerezo M, Guemiri R, Druillennec S, Girault I, Malka-Mahieu H, Shen S, Allard D, Martineau S, Welsch C, Agoussi S, et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat Med. 2018;24:1877–1886. doi:10.1038/s41591-018-0217-1.
- Wang Y, Song X, Zheng Y, Liu Z, Li Y, Qian X, Pang X, Zhang Y, Yin Y. Cancer/testis Antigen MAGEA3 Interacts with STAT1 and Remodels the Tumor Microenvironment. Int J Med Sci. 2018;15:1702–1712. doi:10.7150/ijms.27643.
- Zemek RM, De Jong E, Chin WL, Schuster IS, Fear VS, Casey TH, Forbes C, Dart SJ, Leslie C, Zaitouny A, et al. Sensitization to immune checkpoint blockade through activation of a STAT1/NK axis in the tumor microenvironment. Sci Transl Med. 2019;11(501):eaav7816. doi:10.1126/scitranslmed.aav7816.
- Regis G, Pensa S, Boselli D, Novelli F, Poli V. Ups and downs: the STAT1: STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19:351–359. doi:10.1016/j.semcdb.2008.06.004.
- Timofeeva OA, Plisov S, Evseev AA, Peng S, Jose-Kampfner M, Lovvorn HN, Dome JS, Perantoni AO. Serine-phosphorylated STAT1 is a prosurvival factor in Wilms’ tumor pathogenesis. Oncogene. 2006;25:7555–7564. doi:10.1038/sj.onc.1209742.
- Goodman ML, Trinca GM, Walter KR, Papachristou EK, D’Santos CS, Li T, Liu Q, Lai Z, Chalise P, Madan R, et al. Progesterone Receptor Attenuates STAT1-Mediated IFN Signaling in Breast Cancer. J Immunol. 2019;202:3076–3086. doi:10.4049/jimmunol.1801152.
- Glasgow MA, Argenta P, Abrahante JE, Shetty M, Talukdar S, Croonquist PA, Khalifa MA, Starr TK. Biological Insights into Chemotherapy Resistance in Ovarian Cancer. Int J Mol Sci. 2019;20(9):2131. doi:10.3390/ijms20092131.
- Savant SS, Sriramkumar S, O’Hagan HM. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers. 2018;10(8):251. doi:10.3390/cancers10080251.
- Levy A, Alhazzani K, Dondapati P, Alaseem A, Cheema K, Thallapureddy K, Kaur P, Alobid S, Rathinavelu A. Focal Adhesion Kinase in Ovarian Cancer: A Potential Therapeutic Target for Platinum and Taxane-Resistant Tumors. Curr Cancer Drug Targets. 2019;19:179–188. doi:10.2174/1568009618666180706165222.
- Luo Q, Zhang L, Luo C, Jiang M. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019;454:191–203. doi:10.1016/j.canlet.2019.04.017.
- Kandalaft LE, Odunsi K, Coukos G. Immunotherapy in Ovarian Cancer: are We There Yet? J Clin Oncol. 2019;37:2460–2471. doi:10.1200/JCO.19.00508.
- Odunsi K. Immunotherapy in ovarian cancer. Ann Oncol. 2017;28:viii1–viii7. doi:10.1093/annonc/mdx444.
- Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, George AJT, Ghaem-Maghami S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63(3):215–224. doi:10.1007/s00262-013-1503-x.
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi:10.1073/pnas.1003345107.
- Hu X, Ivashkiv LB. Cross-regulation of Signaling Pathways by Interferon-γ: implications for Immune Responses and Autoimmune Diseases. Immunity. 2009;31(4):539–550. doi:10.1016/j.immuni.2009.09.002.
- Xu YP, Lv L, Liu Y, Smith MD, Li WC, Tan XM, Cheng M, Li Z, Bovino M, Aubé J, et al. Tumor suppressor TET2 promotes cancer immunity and immunotherapy efficacy. J Clin Invest. 2019;130:4316–4331. doi:10.1172/JCI129317.
- Copeland NG, Gilbert DJ, Schindler C, Zhong Z, Wen Z, Darnell JE Jr., Mui ALF, Miyajima A, Quelle FW, Ihle JN, et al. Distribution of the mammalian Stat gene family in mouse chromosomes. Genomics. 1995;29(1):225–228. doi:10.1006/geno.1995.1235.
- Adamkova L, Souckova K, Kovarik J. Transcription protein STAT1: biology and relation to cancer. Folia Biologica. 2007;53:1–6.
- Kim HS, Lee MS. STAT1 as a key modulator of cell death. Cell Signal. 2007;19:454–465. doi:10.1016/j.cellsig.2006.09.003.
- Wang H, Yang Y, Sharma N, Tarasova NI, Timofeeva OA, Winkler-Pickett RT, Tanigawa S, Perantoni AO. STAT1 activation regulates proliferation and differentiation of renal progenitors. Cell Signal. 2010;22:1717–1726. doi:10.1016/j.cellsig.2010.06.012.
- Gordziel C, Bratsch J, Moriggl R, Knoesel T, Friedrich K. Both STAT1 and STAT3 are favourable prognostic determinants in colorectal carcinoma. Br J Cancer. 2013;109:138–146. doi:10.1038/bjc.2013.274.
- Tymoszuk P, Charoentong P, Hackl H, Spilka R, Muller-Holzner E, Trajanoski Z, Obrist P, Revillion F, Peyrat J-P, Fiegl H, et al. High STAT1 mRNA levels but not its tyrosine phosphorylation are associated with macrophage infiltration and bad prognosis in breast cancer. BMC Cancer. 2014;14(1):257. doi:10.1186/1471-2407-14-257.
- Meissl K, Macho-Maschler S, Muller M, Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine. 2017;89:12–20.
- Zhang Y, Liu ZY. STAT1 in Cancer: friend or Foe? Discov Med. 2017;24:19–29.
- Chatterjee J, Dai W, Aziz NHA, Teo PY, Wahba J, Phelps DL, Maine CJ, Whilding LM, Dina R, Trevisan G, et al. Clinical Use of Programmed Cell Death-1 and Its Ligand Expression as Discriminatory and Predictive Markers in Ovarian Cancer. Clin Cancer Res. 2017;23(13):3453–3460. doi:10.1158/1078-0432.CCR-16-2366.
- Drakes ML, Mehrotra S, Aldulescu M, Potkul RK, Liu Y, Grisoli A, Joyce C, O'Brien TE, Stack MS, Stiff PJ. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J Ovarian Res. 2018;11:43. doi:10.1186/s13048-018-0414-z.
- Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2):1486–1499. doi:10.18632/oncotarget.6429.
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi:10.1073/pnas.0611533104.
- Li M, Li H, Liu F, Bi R, Tu X, Chen L, Ye S, Cheng X. Characterization of ovarian clear cell carcinoma using target drug-based molecular biomarkers: implications for personalized cancer therapy. J Ovarian Res. 2017;10(1):9. doi:10.1186/s13048-017-0304-9.
- Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, Gouy S, Morice P, Bentivegna E, Lhomme C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017;28(3):651–657. doi:10.1093/annonc/mdw625.
- Zhu J, Wen H, Ju X, Bi R, Zuo W, Wu X, Shridhar V. Clinical Significance of Programmed Death Ligand‑1 and Intra-Tumoral CD8+ T Lymphocytes in Ovarian Carcinosarcoma. PLoS One. 2017;12(1):e0170879. doi:10.1371/journal.pone.0170879.
- Zhu J, Wen H, Bi R, Wu Y, Wu X. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J Gynecol Oncol. 2017;28:e77. doi:10.3802/jgo.2017.28.e77.
- Wang Q, Lou W, Di W, Wu X. Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol. 2017;52:7–14. doi:10.1016/j.intimp.2017.08.017.
- Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141:293–302. doi:10.1016/j.ygyno.2016.03.008.
- Webb JR, Milne K, Nelson BH. PD-1 and CD103 Are Widely Coexpressed on Prognostically Favorable Intraepithelial CD8 T Cells in Human Ovarian Cancer. Cancer Immunol Res. 2015;3:926–935. doi:10.1158/2326-6066.CIR-14-0239.
- Xu M, Zhang B, Zhang M, Liu Y, Yin FL, Liu X, ZHUO S-C. Clinical relevance of expression of B7-H1 and B7-H4 in ovarian cancer. Oncol Lett. 2016;11:2815–2819. doi:10.3892/ol.2016.4301.
- Mills AM, Peres LC, Meiss A, Ring KL, Modesitt SC, Abbott SE, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy ML, et al. Targetable Immune Regulatory Molecule Expression in High-Grade Serous Ovarian Carcinomas in African American Women: A Study of PD-L1 and IDO in 112 Cases From the African American Cancer Epidemiology Study (AACES). Int J Gynecol Pathol. 2019;38(2):157–170. doi:10.1097/PGP.0000000000000494.
- Au KK, Le Page C, Ren R, Meunier L, Clement I, Tyrishkin K, Peterson N, Kendall-Dupont J, Childs T, Francis JA, et al. STAT1-associated intratumoural TH1 immunity predicts chemotherapy resistance in high-grade serous ovarian cancer. J Pathol Clin Res. 2016;2:259–270. doi:10.1002/cjp2.55.
- Josahkian JA, Saggioro FP, Vidotto T, Ventura HT, Candido Dos Reis FJ, de Sousa CB, Tiezzi DG, de Andrade JM, Koti M, Squire JA, et al. Increased STAT1 Expression in High Grade Serous Ovarian Cancer Is Associated With a Better Outcome. Int J Gynecol Cancer. 2018;28(3):459–465. doi:10.1097/IGC.0000000000001193.
- Koti M, Siu A, Clement I, Bidarimath M, Turashvili G, Edwards A, Rahimi K, Mes-Masson A-M, Squire JA. Erratum: A distinct pre-existing inflammatory tumour microenvironment is associated with chemotherapy resistance in high-grade serous epithelial ovarian cancer. Br J Cancer. 2015;113(12):1746. doi:10.1038/bjc.2015.459.
- Khalifa AM, Elsheikh MA, Khalifa AM, Elnaggar YSR. Current strategies for different paclitaxel-loaded Nano-delivery Systems towards therapeutic applications for ovarian carcinoma: A review article. J Control Release. 2019;311-312:125–137. doi:10.1016/j.jconrel.2019.08.034.
- Tian X, Guan W, Zhang L, Sun W, Zhou D, Lin Q, Ren W, Nadeem L, Xu G. Physical interaction of STAT1 isoforms with TGF-beta receptors leads to functional crosstalk between two signaling pathways in epithelial ovarian cancer. J Exp Clin Cancer Res. 2018;37:103. doi:10.1186/s13046-018-0773-8.
