ABSTRACT
Background
Breast cancer is the most common cancer in women, and triple-negative breast cancer (TNBC) accounts for about 15–20% of all breast cancer. High mobility group AT-hook 2 (HMGA2) is overexpressed in some tumors and closely associated with patients’ prognosis. However, the mechanisms involved in the regulation of HMGA2 in TNBC still remain unclear.
Methods
In this study, HMGA2 level in TNBC cell lines was analyzed by western blot. After knockdown of HMGA2 expression by RNA interference in TNBC cell lines MDA-MB-231 and SUM149, wound healing and transwell assays were conducted to examine the effects of HMGA2 on migration and invasion. Tumor metastasis was assessed in amouse xenograft model invivo. Furthermore, expression levels of epithelial–mesenchymal transition (EMT) biomarkers and involvement of the Hippo-YAP pathway were detected by western blot.
Results
Compared to normal breast epithelial cells, the expression levels of HMGA2 were significantly increased in TNBC cell lines (all P< .05). Downregulation of HMGA2 dramatically inhibited the migration and invasion of MDA-MB-231 and SUM149 cells (all P< .01) invitro, and suppressed the tumor metastasis of nude mice xenograft model invivo. Western blot analysis revealed alterations in EMT biomarkers: the expression of mesenchymal markers N-cadherin, Vimentin and Snail were decreased, while the expression of epithelial marker E-cadherin was increased. Downregulated expression of HMGA2 attenuated Hippo-YAP related protein expression and the stability of YAP.
Conclusions
HMGA2 is highly expressed in TNBC cells. Downregulation of HMGA2 inhibits the migration and invasion of TNBC and invivo tumor metastasis mediated through inhibition of EMT and Hippo-YAP pathway.
Introduction
Breast cancer is the most commonly diagnosed cancer and thesecond leading cause of cancer death in women.Citation1 Based on the expression of estrogen receptor (ER), progestogen receptor (PR), and HER2neu expression, breast cancer is divided into four different types: (a) Luminal A: ER/PR+HER2-, (b) Luminal B: ER/PR+HER2+, (c) HER2 overexpression: ER/PR-HER+, (d) Triple-Negative Breast Cancer (TNBC): ER-PR-HER-Citation2 TNBC is characterized by ahigh degree of malignancy, high metastatic capacity, and lacks effective means in clinical treatment. Because of its gene expression pattern, the first three types of breast cancer can be treated with endocrine-targeted or HER2-targeted therapy. TNBC is not sensitive to the above treatments, and can only be treated with chemotherapy with low efficacy. Therefore, TNBC treatment is facing the biggest challenge in clinical practice at present, and it is of great significance to find new and effective targeted treatment for TNBC patients.
High mobility group AT-hook 2 (HMGA2) belongs to ahigh mobility family with aDNA binding domain.Citation3 HMG proteins function as architectural factors and are essential components of the enhanceosome. It is reported that HMGA2 does not directly promote or inhibit the transcription of any genes, but regulates gene expression by binding to the AT enrichment region in DNA or directly interacting with other transcription factors to change DNA conformation.Citation4,Citation5
Studies have shown that the expression of HMGA2 is very low in normal adult tissues, while its overexpression or rearrangement is closely related to many cancers and induces multiple carcinogenic effects in cell cycle, apoptosis, and differentiation.Citation6,Citation7 HMGA2 is mainly expressed in mesenchyme before differentiation, but also in epidermal cancers. The ectopic expression of HMGA2 in epithelial cells can induce epithelial–mesenchymal transition (EMT), which can make epithelial cells lose cell polarity, weaken cell-cell adhesion, and increase cell activity, amilestone characteristic of tumor cell metastasis.Citation8,Citation9 The important role of HMGA2 in cancer progression and tumor invasion has also been reported in colorectal cancer, breast cancer, ovarian cancer, and gastric cancer.Citation9, Citation10, Citation11,Citation12 In addition, astrong nuclear expression of HMGA2 was observed only in TNBC, but not in normal breast samples adjacent to TNBC tissues and in triple-positive samples.Citation13 Thus, HMGA2 protein is apromising biomarker for tumor detection and apotential molecular target in tumor therapy.
YAP (yes-associated protein), also known as YAP1 or YAP65, was identified based on its ability to associate with the SH3 domain of Yes.Citation14 It also binds to other SH3 domain-containing proteins such as Nck, Crk, Src, and Abl. In addition to the SH3 binding motif, YAP contains aPDZ interaction motif, acoiled-coil domain, and WW domains.Citation15,Citation16 YAP and its proximal homologous TAZ (WWTR1) are the main effectors of Hippo tumor suppressor pathway, which plays afundamental and widely conserved role in regulating tissue growth and organ size.Citation17 In many cancers, YAP is avital oncogene whose recurrent amplification can be observed. Moreover, YAP is involved in EMT and the metastatic potential of breast epithelial cells.Citation18,Citation19
However, the biological roles of HMGA2 in TNBC and the underlying mechanisms remain unknown. In this study, we aimed to investigate the biological effects of HMGA2 on TNBC cells and the Hippo-YAP signaling pathway’s mechanisms.
Methods
Cell culture
Human breast cancer cell line MDA-MB-231 (TNBC) was purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), SUM149 (TNBC) was purchased from BioIVT. The above cells were cultured in D/F12 or DMEM medium (Gibco, Grand Island, CA) supplemented with 10% fetal bovine serum (Lonsera, Uruguay) and 100 IU/ml penicillin and 100 µg/ml streptomycin (Solarbio, Beijing, China) in ahumidified 37°C incubator supplemented with 5% CO2.
RNA interference
HMGA2 was interfered with pLKO lentiviral vectors. The short hairpin RNA sequences targeting HMGA2 and empty vector were synthesized by Genewiz (Jiangsu, China). The sequences of short hairpin HMGA2 (shHMGA2) were 5′-AGTCCCTCTAAAGCAGCTCAA-3′, 5′-TTGAGCTGCTTTAGAGGGACT-3′; 5′CAAAAAAGT CCCTCTAAAGCA-3′, 5′-CTCGAGTTGAGCTGCTTTAG A-3′. Knockdown was confirmed by western blotting.
Wound healing assay
Cells were seeded in 12-well plates (MDA-MB-231: 2.5 × 105, SUM149: 2 × 105). When grown to 80%-90% confluence, the cells in each well were scratched with wound lines vertically to the bottom of the well with a200 μl pipette tip. After being washed with PBS 2 times, cells were incubated in agrowth medium containing 4% FBS. The wound width was determined 24 h or 48 h later under amicroscope (Nikon, Tokyo, Japan). The percentage of wound closure was calculated as follows: percentage of wound closure = 1-(widtht/width0)×100%. Wound Healing Assay was performed as previously published.Citation20
Migration assay
Cell migration assay was performed using 24-well transwell chambers with 8 µm pore polycarbonate membrane inserts (Corning, USA). Cells (MDA-MB-231: 2 × 104, SUM149: 1 × 104) in 200 µl serum-free DMEM were seeded in the upper compartment of the transwell units, while 600 µl DMEM with 20% FBS were placed into the lower chambers as achemoattractant. After 24 h, the chamber was fixed with 4% paraformaldehyde and stained with 0.5% crystal violet for 10 min, respectively. After washing the chambers by PBS, cells at atop of the membrane were removed by several Q-tips. Cells that penetrated to the lower surface of the filter membrane were counted in five randomly chosen fields using aNikon Eclipse TS100 microscope at ×200 magnifications.
Invasion assay
Cell invasion assay was performed by atranswell system identical to the cell migration assay except that transwell filters in 24-well plates were coated with diluted matrigel (5 µl in 45 µl serum-free medium) (BD Biosciences, Bedford, MA) for 12 h, hydrated with 200 µl serum-free medium at 37°C for 30 min. The rest of the procedures were the same as the cell migration assay as described above. Transwell Assay was performed as previously published.Citation21
Actinomycin (CHX) assay
CHX, aprotein translation inhibitor, was used to detect the stability of the protein. Cells were seeded in 6-well plates with equivalent quantities for 24 hours and treated with 20 μM concentration of CHX for 0 h, 1 h, 2 h, 4 h, 6 h, 8 h (0 h represents the cells treated with DMSO for 1 h); then, proteins were extracted and quantified at different times.
Proteasome inhibition assay
Cells were seeded in 6-well plates with equivalent quantities and treated with Proteasome inhibitor (MG132) (20 μM) or DMSO for 4 hours. The half-life of protein was obtained according to the protein expression after CHX treatment. In this experiment, the half-life of the YAP protein was 4 hours. Cells were collected with RIPA lysis buffer, and the protein expression was detected by Western blot.
Immunofluorescence and confocal imaging
MDA-MB-231 cells were grown on glass coverslips until they reached 60–80% confluence and then fixed and permeabilized with 0.5% Triton-X-100. Rabbit anti-E-cadherin (Abcam, USA) and mouse anti-vimentin (Abcam, USA) antibodies were added to the cells and incubated at 4°C overnight. After washing, the cells were incubated with FITC-conjugated goat anti-rabbit and donkey anti-mouse secondary antibody (1:300, Beyotime) for 1 h.Then, 4ʹ,6-diamidino-2-phenylindole (DAPI, Sigma) was added for 10 min to stain the nuclei of cells, and the cells were observed under afluorescence microscope. The images were analyzed with ImageJ.
Western blotting analysis
Protein expression levels were determined by western blot analysis. After indicated treatments, cells were harvested, an aliquot of 20 µg denatured protein was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred on nitrocellulose membranes, blocked in 5% nonfat milk, and incubated with respective primary antibodies (1:2,000 dilution; Cell Signaling Technology, USA): anti-β-actin antibody, anti-HMGA2 antibody, anti-YAP antibody, anti-p-YAP antibody, anti-N-cadherin antibody, anti-E-cadherin antibody, anti-Vimentin antibody, anti-Snail antibody at 4°C overnight. The membranes were then washed 8 min four times with Tris-buffered saline/0.05% Tween-20 solution (TBST) and incubated with horseradish peroxidase-conjugated secondary antibody (1: 2,000 dilution; Beyotime, Hangzhou, China) for 1 hat room temperature. After washing with TBST for 1 h, the signals were detected and quantified by densitometry using Image Lab Software (Bio-Rad, Hercules, CA).
Tumor xenografts in mice
Sixteen female BALB/C-nu mice (4-week-old) were purchased from Laboratory Animal Company (Nanjing, China) and raised in Atlas Lab. All animal procedures and experimental protocols were approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University (wydw2018-187, 2 November 2018). The mice were randomly divided into two different groups (shControl, shHMGA2). To establish xenografts tumor, MDA-MB-231 cells were harvested from culture and injected into the tail vein (2 × 106 cells each mouse). After 45 days of injection, the mice were sacrificed, and tumors were dissected for further analysis. All animal procedures and experimental protocols were approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University.
Hematoxylin and eosin (H&E) staining
Lung tissues were fixed in Bouin’s fixative solution overnight and transferred to 70% ethanol before paraffin embedding. The sections were de-paraffinization, re-hydration, and counterstained with hematoxylin. Hematoxylin and Eosin staining was performed using standard methods.
Statistical analyses
Statistical analysis was performed with SPSS17.0 software (Chicago, Illinois, USA). Each experiment was repeated at least three times independently. The differences among the control and treatment groups were analyzed by analysis of variance (ANOVA) and two-tailed Student t-test. P-value <0.05 was considered statistically significant.
Results
HMGA2 expression of breast cancer cell lines and successful construction of shHMGA2 cell lines
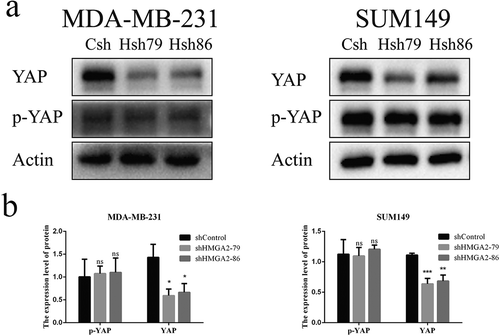
HMGA2 expression was significantly increased in TNBC cells: MDA-MB-231 (p < .001), SUM149 (p < .001), and BT549 (p < .05) than that in normal breast epithelial cells MCF10A and other breast cancer cells (T47D, SKBR3, BT474), respectively ()). MDA-MB-231 and SUM149 cells with high expression of HMGA2 were selected for the following experiments.
Figure 1. Increased expression of HMGA2 in TNBC cell lines and successful construction of shHMGA2 cell lines
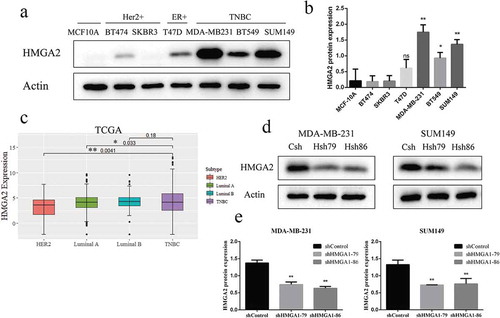
HMGA2 protein levels were significantly decreased in MDA-MB-231 and SUM149 TNBC cells transfected with shHMGA2 (Hsh79, Hsh86) compared with those transfected with shControl (Csh) ()). The results showed that HMGA2-depleted cells were successfully constructed.
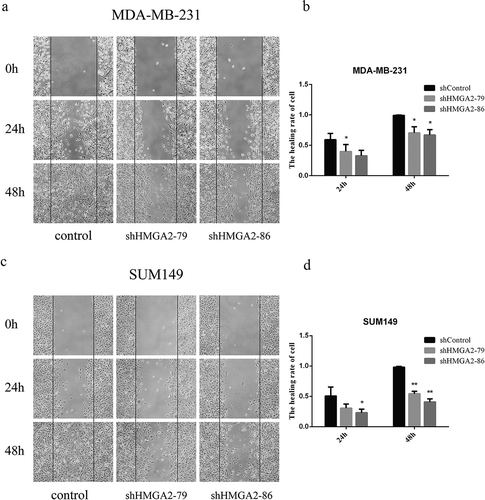
Downregulation of HMGA2 inhibited the wound healing of TNBC cells
Downregulation of HMGA2 inhibited the wound closure rate of MDA-MB-231 to agreat degree with a23% reduction at 24 h and 31% reduction at 48 h, compared with the controls (p < .05, p < .01, respectively) ()). Similar findings were also observed in the SUM149 TNBC cell line. The wound closure rate for SUM149 was 28% reduction at 24 h (p < .05), and 51% reduction at 48 h (p < .01), respectively, compared with the controls ()).
Figure 2. The effects of shHMGA2 on wound healing of TNBC cells
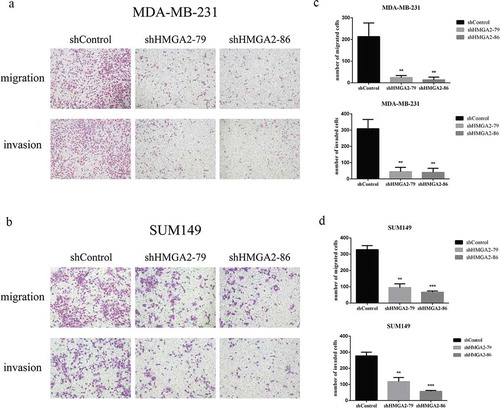
Downregulation of HMGA2 resulted in decreased migration and invasion ability of TNBC cells
The numbers of HMGA2-depleted TNBC cells that migrated through atranswell insert membrane were markedly reduced compared with those of shControl-transfected cells (). Similar results were also observed in the transwell invasion assay. The numbers of HMGA2-depleted TNBC cells penetrating the membrane in the matrigel invasion transwell assay were dramatically lower than that of shControl-transfected cells ().
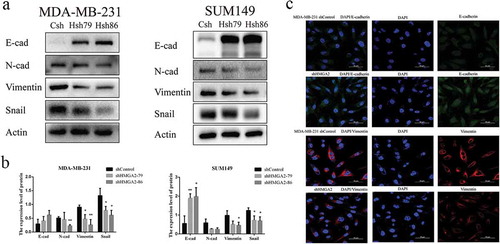
Downregulation of HMGA2 inhibited the expression of EMT-related proteins
Western blot analysis revealed that the protein expression levels of mesenchymal markers N-cadherin, Vimentin, and Snail were decreased, while the expression of epithelial marker E-cadherin was increased in MDA-MB-231, SUM149 TNBC cells after interfering with HMGA2 expression (). Western blot analysis was supported by immunofluorescence imaging in MDA-MB-231 cells ())
Figure 4. Effect of HMGA2 on the expression of EMT-related proteins
Downregulation of HMGA2 decreased the expression and stability of YAP protein
As demonstrated by Western blot, the expression level of YAP protein decreased significantly after the downregulation of HMGA2, while there was no noticeable change in p-YAP protein level ().
Figure 5. Interference with HMGA2 affects the expression of YAP protein
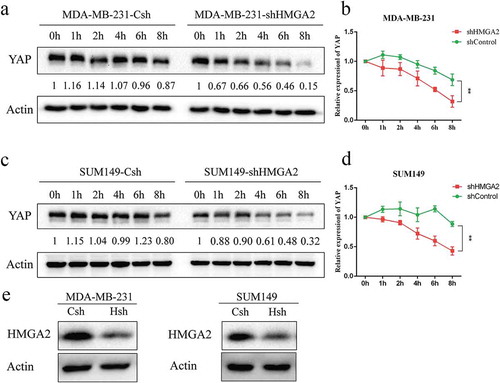
After the MDA-MB-231 shControl and shHMGA2 cells were treated with 20 μM concentration of CHX for different time periods, the protein expression level of YAP decreased gradually with time, and dropped to 50% of its baseline level for 4 h, while there was no significant change of YAP protein levels in shControl cells ()). Similar results were also observed in the SUM149 TNBC cell line ().
Figure 6. HMGA2 mediates the stability of YAP protein in TNBC
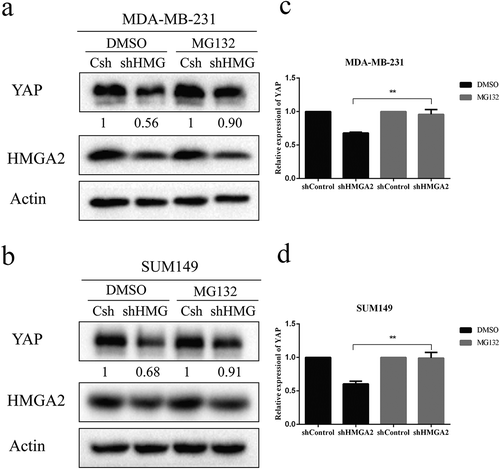
The proteasome is involved in downregulation of YAP in HMGA2-depleted cells
In order to verify whether HMGA2 regulates the protein level of YAP through the proteasome system, MDA-MB-231 and SUM149 cells were treated with DMSO and proteasome inhibitor MG132 for 4 h. The expression of YAP protein in the HMGA2-depleted cells was decreased significantly after DMSO treatment. In contrast, when cells were treated with MG132, the decrease of YAP protein in HMGA2-depleted group was not as low as that observed in the DMSO-treated group ().
Figure 7. The decrease of the YAP protein level caused by proteasome activity
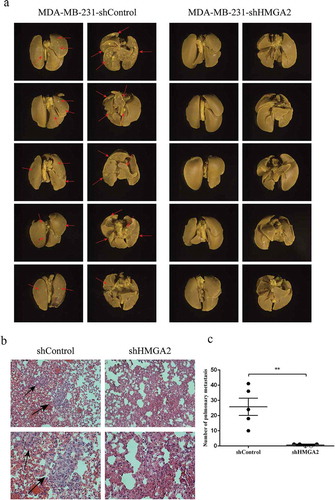
HMGA2 promotes the metastasis of MDA-MB-231 cells in BALB/c nude mice
MDA-MB-231 shControl and shHMGA2 group of TNBC cells were injected via the tail vein to construct the metastatic tumor model invivo. Forty-five days later, the nude mice were sacrificed, and lung tissues were taken out to observe the metastases. After the fixation of lung tissues, HE staining was performed to observe the metastatic tissues. There were obvious metastatic lesions in the shControl group (red arrows in )), and the number of metastases was significantly higher than that in the shHMGA2 group ()). However, no lesion was found in the lung tissue of the shHMGA2 group. Furthermore, obvious heteromorphic cells were observed in the tissue after HE staining in the shControl group compared with the shHMGA2 group (black arrow in )).
Figure 8. HMGA2 depletion suppresses metastasis in amouse xenograft model
Discussion
In the present study, we have demonstrated that HMGA2 is highly expressed in TNBC cells. Downregulation of HMGA2 inhibits the migration and invasion of TNBC and invivo tumor metastasis but had no effect on cell proliferation (Figure S1). Our results suggested that HMGA2 plays acritical role in regulating malignant phenotypes of TNBC.
HMGA2 was significantly upregulated in cancerous tissues.Citation13,Citation22–24 HMGA2 overexpression is involved in invasive growth, early metastasis, and poor prognosis in Papillary thyroid carcinoma,Citation25 Pancreatic cancer,Citation26 and Breast cancer.Citation27 The association between HMGA2 and tumor transformation has been reported previously.Citation28,Citation29 In general, HMGA2 knockdown could inhibit cell metastasis in several types of cancer,Citation30–32 which was similar to our findings in TNBC.
EMT is acomplex process involving multiple extracellular signaling pathways. It plays akey role in embryonic development, so as in the pathogenesis of cancer, and cancer cells might become more invasive and drug-resistant via EMT.Citation33–35 Shell etal. found that HMGA2 was one of the few genetic markers that can distinguish most of the type I(mesenchymal gene marker) and type II (epithelial gene marker) cancer cell lines.Citation36 Accumulating evidence have revealed that the migration and invasion of cancer cells can be modulated by EMT.Citation6,Citation37 We observed the effect of HMGA2 on EMT-related proteins that the expression of epithelial marker E-cadherin increased after interfering HMGA2, while the expression of mesenchymal markers Vimentin, Snail, and N-cadherin decreased. These findings demonstrated that inhibition of HMGA2 could retard migration and invasion by suppressing EMT in TNBC.
Hippo-YAP signaling is one of the major pathways involved in the development and progression of many kinds of malignancies.Citation19,Citation38 To further explore the mechanism by which HMGA2 promotes the migration and invasion of TNBC, we quantified the expression level of YAP protein, which is akey factor in the Hippo signaling pathway. Our results showed that the expression level of YAP protein was decreased after interfering HMGA2, while phosphorylated YAP ser127 showed no obvious change. Therefore, we speculate that the effect of HMGA2 on TNBC may be related to YAP. So how does HMGA2 regulate the expression of YAP protein?
CHX inhibits ribosomal function and prevents cells from synthesizing proteins.Citation39 If the translated protein is not stable after treatment with CHX, it will be degraded gradually with time. On the contrary, if the protein is stable, its expression does not change with time. We found that the expression level of YAP protein in HMGA2-deleted cells decreased significantly with time. This indicates that HMGA2 promotes the stabilization of YAP protein. At what level does HMGA2 regulate YAP? The proteasome system often degrades the ubiquitinated proteins. Ubiquitin-proteasome-dependent degradation system, which is involved in various physiological processes of the cell, and its disorder can cause various diseases, including tumors.Citation40 Originally, the YAP protein level would be decreased after interfering with HMGA2, but we treated cells with proteasome inhibitor MG132,Citation41,Citation42 TAZ protein level was increased. Our results have demonstrated that HMGA2 regulates the protein level of YAP through the proteasome system. Therefore, we concluded that HMGA2 may inhibit the ubiquitination of YAP, promoting the stabilization of YAP protein (Figure S2).
However, how does HMGA2 regulate the stability of YAP is still unclear. We looked into the expression level and phosphorylation level of LATS, and the results showed that YAP was not associated with LATS expression and activity in TNBC cell lines. Jia etal. further confirmed that HMGA2 activated Src promoted the metastasis of HCC cells through cortactin.Citation43 The relationship between HMGA2 and Src needs to be proved in future studies. In tumors, YAP is involved in the EMT, cancer stem cell properties and acquisition of metastatic potential.Citation44 We found that inhibition of HMGA2 suppressed YAP’s expression. Thus, we propose that these two proteins might interact with each other in tumorigenesis. But whether YAP can rescue invasion and metastasis of HMGA2-knockdown cells is unclear. Further study is needed to demonstrate the role of YAP1 in invasion and metastasis and the specific relationship with HMGA2 in TNBC cells.
In summary, we have demonstrated that downregulation of HMGA2 inhibited the migration and invasion of TNBC and the expression of interstitial markers, but up-regulated the expression of epithelial markers. Meanwhile, we found that HMGA2 may inhibit the ubiquitination of YAP, regulate its stability, and thereby regulate the EMT progression of TNBC. Therefore, we came to the conclusion that this biological effect of HMGA2 in tumorigenesis and metastasis is associated with increased EMT and Hippo-YAP pathway. However, the findings of the biological effects of HMGA2 and its role in EMT are preliminary. Further studies to illustrate the role of HMGA2 in the clinical course of TNBC are needed.
Disclosure of interest
The authors confirm that there are no conflicts of interest.
Ethics approval
All animal procedures and experimental protocols were approved by the Laboratory Animal Ethics Committee of Wenzhou Medical University.
Supplemental Material
Download Zip (199.8 KB)Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer JClin. 2019;69:7–34.
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proce National Acad Sci USA. 2003;100:8418–8423.
- Ashar HR, Cherath L, Przybysz KM, Chada K. Genomic characterization of human HMGIC, amember of the accessory transcription factor family found at translocation breakpoints in lipomas. Genomics. 1996;31:207–214. doi:10.1006/geno.1996.0033.
- Cleynen I. Van de Ven WJ. The HMGA proteins: Amyriad of functions (Review). IntJOncol. 2008;32:289–305.
- Pfannkuche K, Summer H, Li O, Hescheler J, Droge P. The high mobility group protein HMGA2: a co-regulator of chromatin structure and pluripotency in stem cells? Stem Cell Rev Reports. 2009;5:224–230. doi:10.1007/s12015-009-9078-9.
- Zhao XP, Zhang H, Jiao JY, Tang DX, Wu YL, Pan CB. Overexpression of HMGA2 promotes tongue cancer metastasis through EMT pathway. JTransl Med. 2016;14:26. doi:10.1186/s12967-016-0777-0.
- Gao X, Dai M, Li Q, Wang Z, Lu Y, Song Z. HMGA2 regulates lung cancer proliferation and metastasis. Thoracic Cancer. 2017;8:501–510. doi:10.1111/1759-7714.12476.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. JClin Invest. 2009;119:1420–1428. doi:10.1172/JCI39104.
- Morishita A, Zaidi MR, Mitoro A, Sankarasharma D, Szabolcs M, Okada Y, D’Armiento J, Chada K. HMGA2 is adriver of tumor metastasis. Cancer Res. 2013;73:4289–4299. doi:10.1158/0008-5472.CAN-12-3848.
- Wang X, Liu X, Li AY, Chen L, Lai L, Lin HH, Hu S, Yao L, PengJ, Loera S, etal. Overexpression of HMGA2 promotes metastasis and impacts survival of colorectal cancers. Clini Cancer Res. 2011;17:2570–2580. doi:10.1158/1078-0432.CCR-10-2542.
- Wu J, Liu Z, Shao C, Gong Y, Hernando E, Lee P, Narita M, Muller W, Liu J, Wei -J-J, etal. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Res. 2011;71(2):349–359. doi:10.1158/0008-5472.CAN-10-2550.
- Motoyama K, Inoue H, NakamuraY, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clini Cancer Res. 2008;14:2334–2340. doi:10.1158/1078-0432.CCR-07-4667.
- Pallante P, Sepe R, Puca F, Fusco A. High mobility group aproteins as tumor markers. Frontiers Med. 2015;2:15. doi:10.3389/fmed.2015.00015.
- Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152.
- Mohler PJ, Kreda SM, Boucher RC, Sudol M, Stutts MJ, Milgram SL. Yes-associated protein 65 localizes p62 c-Yes to the apical compartment of airway epithelia by association with EBP50. JCell Biol. 1999;147:879–890. doi:10.1083/jcb.147.4.879.
- Espanel X, Sudol M. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. JBiol Chem. 2001;276:14514–14523. doi:10.1074/jbc.M008568200.
- Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–828. doi:10.1016/j.cell.2015.10.044.
- Shao DD, Xue W, Krall EB, Bhutkar A, PiccioniF, WangX, SchinzelA, SoodS, RosenbluhJ, KimJ, etal. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi:10.1016/j.cell.2014.06.004.
- Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM, Fu K, Datta K, Palermo N, Chen Y, etal. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015;35(8):1350–1362. doi:10.1128/MCB.00102-15.
- Chen X, Li C, He T, Mao J, Li C, Lyu J, Meng QH. Metformin inhibits prostate cancer cell proliferation, migration, and tumor growth through upregulation of PEDF expression. Cancer Biol Ther. 2016;17:507–514. doi:10.1080/15384047.2016.1156273.
- Yang Y, Meng H, Peng Q, Yang X, Gan R, Zhao L, Chen Z, Lu J, Meng QH. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22(1):23–29. doi:10.1038/cgt.2014.66.
- Sarhadi VK, Wikman H, Salmenkivi K, Kuosma E, SiorisT, Salo J, Karjalainen A, Knuutila S, Anttila S. Increased expression of high mobility group Aproteins in lung cancer. JPathol. 2006;209(2):206–212. doi:10.1002/path.1960.
- Aguirre-Gamboa R, Gomez-Rueda H, Martínez-Ledesma E, Martínez-Torteya A, Chacolla-Huaringa R, Rodriguez-Barrientos A, Tamez-Peña JG, TreviñoV. SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PloS One. 2013;8:e74250. doi:10.1371/journal.pone.0074250.
- MeyerB, LoeschkeS, SchultzeA, WeigelT, SandkampM, GoldmannT, VollmerE, BullerdiekJ. HMGA2 overexpression in non-small cell lung cancer. Mol Carcinog. 2007;46(7):503–511. doi:10.1002/mc.20235.
- Chiappetta G, Ferraro A, Vuttariello E, Monaco M, Galdiero F, De Simone V, Califano D, Pallante P, Botti G, Pezzullo L, et al. HMGA2 mRNA expression correlates with the malignant phenotype in human thyroid neoplasias. Eur J Cancer. 2008;44:1015–1021. doi:10.1016/j.ejca.2008.02.039.
- Hristov AC, Cope L, Reyes MD, Singh M, Iacobuzio-Donahue C, Maitra A, et al. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Modern Pathol. 2008;22:43–49. doi:10.1038/modpathol.2008.140.
- Fabjani G, Tong D, Wolf A, Roka S, Leodolter S, Hoecker P, Fischer MB, Jakesz R, Zeillinger R, et al. HMGA2 is associated with invasiveness but not asuitable marker for the detection of circulating tumor cells in breast cancer. Oncol Rep. 2005;14:737–741.
- Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi:10.1126/science.1137999.
- Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, SchuldenfreiA, Kowalski J, Bhattacharya R, Ashfaq R, Resar LMS, etal. HMGA2 participates in transformation in human lung cancer. Molecular Cancer Res. 2008;6:743–750. doi:10.1158/1541-7786.MCR-07-0095.
- Watanabe S, Ueda Y, Akaboshi S, HinoY, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells. Am JPathol. 2009;174:854–868. doi:10.2353/ajpath.2009.080523.
- Shi Z, Li X, Wu D, Tang R, Chen R, Xue S, Sun X. Silencing of HMGA2 suppresses cellular proliferation, migration, invasion, and epithelial–mesenchymal transition in bladder cancer. Tumour Biol. 2016;37:7515–7523. doi:10.1007/s13277-015-4625-2.
- Shi Z, Wu D, Tang R, Li X, Chen R, XueS, ZhangC, Sun X. Silencing of HMGA2 promotes apoptosis and inhibits migration and invasion of prostate cancer cells. JBiosci. 2016;41:229–236. doi:10.1007/s12038-016-9603-3.
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi:10.1038/nrc2620.
- Shang Y, Cai X, Fan D. Roles of epithelial-mesenchymal transition in cancer drug resistance. Curr Cancer Drug Targets. 2013;13:915–929. doi:10.2174/15680096113136660097.
- Chen T, You Y, Jiang H, Wang ZZ. Epithelial-mesenchymal transition (EMT): Abiological process in the development, stem cell differentiation, and tumorigenesis. JCell Physiol. 2017;232:3261–3272. doi:10.1002/jcp.25797.
- Shell S, Park SM, Radjabi AR, Schicke lR, Kistner EO, Jewell DA, Feig C , Lengye lE , Peter ME. Let-7 expression defines two differentiation stages of cancer. Proc National Acad Sci USA. 2007;104:11400–11405.
- Zhou X, Guo Y, Ye L, Long L, Wang H, Tan P, Xu M. MicroRNA-588 regulates invasion, migration and epithelial–mesenchymal transition via targeting EIF5A2 pathway in gastric cancer. Cancer Manag Res. 2018;10:5187–5197. doi:10.2147/CMAR.S176954.
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, etal. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi:10.1101/gad.1602907.
- Reich E, Franklin RM, Shatkin AJ, Tatum EL. Action of actinomycin Don animal cells and viruses. Proc Natl Acad Sci USA. 1962;48:1238–1245. doi:10.1073/pnas.48.7.1238.
- Ciechanover A. Intracellular protein degradation: from avague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Best Pract Clin Haematol. 2017;30:341–355. doi:10.1016/j.beha.2017.09.001.
- Yan H, Ma YL, Gui YZ, WangSM, Wang XB, Gao F, Wang YP. MG132, aproteasome inhibitor, enhances LDL uptake in HepG2 cells invitro by regulating LDLR and PCSK9 expression. Acta Pharmacol Sin. 2014;35:994–1004. doi:10.1038/aps.2014.52.
- Shimizu S, Kadowaki M, Yoshioka H, Kambe A, Watanabe T, Kinyamu HK, Eling TE. Proteasome inhibitor MG132 induces NAG-1/GDF15 expression through the p38 MAPK pathway in glioblastoma cells. Biochem Biophys Res Commun. 2013;430:1277–1282. doi:10.1016/j.bbrc.2012.11.137.
- Jia D, Jing Y, Zhang Z, Liu L, Ding J, Zhao F, Ge C, Wang Q, Chen T, Yao M, et al. Amplification of MPZL1/PZR promotes tumor cell migration through Src-mediated phosphorylation of cortactin in hepatocellular carcinoma. Cell Res. 2014;24(2):204–217. doi: 10.1038/cr.2013.158.
- Mo JS, Park HW, Guan KL. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi:10.15252/embr.201438638.