ABSTRACT
Immune checkpoint inhibitors have demonstrated promising efficacy and tolerable safety for advanced malignancies. However, a proportion of patients who had received immunotherapy may experience hyperprogressive disease and a resultant poor prognosis. Here, we report a patient with advanced esophageal squamous carcinoma who developed hyperprogressive disease shortly after immunotherapy. This patient received nivolumab after multiple lines of treatment, including chemotherapy, radiotherapy, and antiangiogenic therapy. Through the comprehensive analysis of NGS results, we concluded that the PI3K/AKT signaling pathway might be associated with hyperprogressive disease after immunotherapy. Additionally, potential mechanisms underlying hyperprogressive disease after immunotherapy reported in other malignant tumors were also summarized.
Background
Immune checkpoint inhibitors (ICIs) targeting programmed death 1 (PD1) or programmed death ligand 1 (PD-L1) have become a standard therapy in the clinic and have displayed promising efficacy and acceptable safety among patients with advanced malignancies.Citation1–4 Unfortunately, not all patients treated with ICIs respond to the treatment, while only 20–30% of patients could benefit from ICIs.Citation3–5 In particular, a proportion of patients might develop hyperprogressive disease (HPD) with accelerated tumor growth after immunotherapy. There are still no consistent definitions of HPD. Champiat et al.Citation6 defined HPD as a progressive disease (PD) status at the first evaluation of cancer immunotherapy or a 2-fold or higher increase in the tumor growth rate (TGR). Kato et al.Citation7 defined HPD by a short time to treatment failure (TTF) which is less than 2 months or a more than 50% increase in tumor size. According to the latest reports, HPD rates have been reported in some types of cancers to range from 7% to 29%. HPD is associated with a poor prognosis and, in particular, the overall survival (OS) duration among older patients.Citation6–8 Although multiple molecules or mutated genomic signatures have been proven to be associated with HPD, no conclusive review has been performed to elucidate HPD. Here, we report a case of advanced esophageal squamous carcinoma in a patient who developed HPD after nivolumab treatment and summarize the possible mechanisms and factors underlying HPD.
Case report
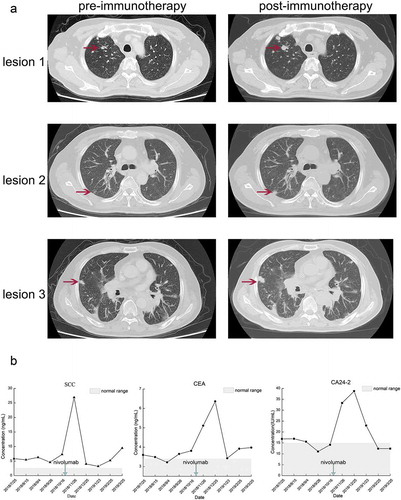
A 52-year-old Asian male was diagnosed as locally advanced esophageal squamous cell carcinoma (medium differentiation, pT3N2M0, IIIB) in February 2017 in China through post-operative pathology. Multiple metastatic lymph nodes were detected, including para-esophageal lymph nodes (3/5), pericardial lymph nodes (2/9), tracheal carinal lymph nodes (4/4) and mid-thoracic paratracheal lymph nodes (5/9). The time-line of the treatment process for this patient is shown in ). After the operation, he received 2 cycles of TP regimen (liposomal paclitaxel [300 mg on day 1] plus nedaplatin [40 mg on days 1 to 4]) on March 20, 2017 and April 14, 2017, respectively. From May 15, 2017 to June 29, 2017, he received concurrent chemoradiotherapy (CCRT) including 2 cycles of chemotherapy (liposomal paclitaxel [300 mg on day 1, every 3 weeks]) and 30 doses of radiotherapy (60 Gy/30 f/2 Gy). After CCRT, the patient started undergoing regular follow-up. His stable disease (SD) status lasted until December 12th 2017. The last CT scan for the patient revealed a PD status, and then he commenced a 2-week regimen of first-line chemotherapy, which consisted of irinotecan (280 mg on day 1) and S-1 (60 mg, twice a day on days 1 to 10) for 3 cycles on December 12, 2017, January 5, 2018 and January 20, 2018, respectively. After 3 cycles of chemotherapy, response assessment indicated SD, and the patient experienced severe grade IV bone marrow suppression. Therefore, the patient received another 3 cycles of this regimen in which the dose of irinotecan was changed to 260 mg. However, disease assessment showed PD after the sixth round of chemotherapy with this regimen. After that, 2 cycles of chemotherapy with the NP regimen (vinorelbine [40 mg on day 1] and cisplatin [50 mg on days 1 to 3]) and 2 cycles of anlotinib (12 mg on day 1 to 14, oral) were administered, leading to PD. Then, he received another 4 cycles of chemotherapy with irinotecan plus raltitrexed, consisting of irinotecan (280 mg on day 1) and raltitrexed (4 mg on day 1) q3w. During treatment with this regimen, the 2-cycle assessment revealed SD, but the 4-cycle assessment revealed PD. Next-generation sequencing (NGS) was then performed in this patient using both tissue and blood samples, which revealed EGFR amplification, TP53 missense mutation, and PIK3CA activating mutation (). The detailed sequencing procedure has been described in our previous study.Citation9 Immunohistochemistry (IHC) showed positive PD-L1 expression in 5% of tumor cells (TPS: 5%; CPS: 5), as shown in ). We explained in detail to the patient with the effects and risks of immunotherapy and clarified that the genomic signature of the patient might not be benefited from immunotherapy. Given that nivolumab has established the efficacy regardless of PD-L1 status, systemic therapy with nivolumab (200 mg on day 1, every 2 weeks) was administered on October 18, 2018. Unfortunately, less than 2 weeks after the first cycle of nivolumab treatment, the patient experienced apparent cough and chest tightness, and the CT scan revealed a significant progression of lung metastases. Multiple lung metastases have increased by more than 50% of baseline (the percentage of the tumor size enlargement compared with the status when immunotherapy initialed: lesion 1: 102%; lesion 2: 93%; and lesion 3: 215%). Additionally, new metastatic lesions were observed in the lungs, and significant thickening of the esophageal wall was detected. At the same time, the patient experienced immune-related pneumonia (Grade 2) but finally recovered after treatment with glucocorticoids and antibiotics. The comparison of the CT scan is shown in ). In addition, tumor markers, including carcino-embryonic antigen (CEA), squamous cell carcinoma antigen (SCC), and carbohydrate antigen 24–2 (CA24-2), were all drastically increased ()). Nivolumab was withheld for the patient, and he received 4 cycles of treatment consisting of paclitaxel-albumin (200 mg on day 1, day 8, and day 15, every 4 weeks) and nimotuzumab (200 mg every week). The patient achieved partial response (PR) at the 2-cycle assessment but PD at the 4-cycle assessment.
Table 1. Results of NGS examination
Figure 1. Clinical and histological data of the patient from our center. (a) The timeline of treatments for a patient with advanced esophageal squamous carcinoma. (b) The PD-L1 expression in tissue sample by IHC using 22C3 (X200)
Figure 2. CT scan and tumor markers of the patient experienced HPD after nivolumab treatment. (a) The CT scan before and after nivolumab treatment. In this case, HPD was defined as a disease condition after anti-PD1/PD-L1 treatment which has a short TTF (less than 1 month) and a more than 50% increase than baseline in the size of the lesion. (b) The alterations in tumor markers during nivolumab treatment
Discussion
HPD is a new pattern of progression recently described in patients with malignant tumors treated with ICIs, and the rate of HPD ranges from 7% to 29% according to previous reports (summarized in ). However, HPD in patients with advanced esophageal squamous carcinoma treated with PD-1/PD-L1 inhibitors has rarely been reported. In this study, we reported on a patient with advanced esophageal squamous carcinoma after multiple lines of treatment experiencing HPD and immune-related pneumonia during one cycle of nivolumab therapy. Multiple genomic alterations have been proven to be associated with HPD, but no clear mechanism of HPD in esophageal squamous carcinoma has been verified. Genome-wide sequencing of cell-free DNA has been reported to identify copy-number alterations that can be used for monitoring response to immunotherapy and identifying HPD in cancer patients.Citation10 Using the NGS results of the patient in this study and another two patients reported by Xiong et al., Citation11 we revealed that an underlying PI3K/AKT signaling pathway might be associated with HPD induced by nivolumab in advanced esophageal squamous carcinoma.
Table 2. The HPD incidences among patients received ICIs
According to the NGS results for the patient in this report, 16 altered genes were detected for genomic analysis. We submitted the 16 genes into DAVID, an online tool for functional enrichment. There were seven related signaling pathways with a kappa value larger than 0.5 (similarity score: very high [0.75–1]; high [0.5–0.75]; moderate [0.25–0.5]; and low [<0.25]), including Phosphoinositide-3-kinase (PI3K)/Akt signaling pathway, Rap signaling pathway, Ras signaling pathway, MAPK signaling pathway, HIF-1 signaling pathway and FoxO signaling pathway, of which the PI3K/Akt signaling pathway was strongly activated in this patient (kappa = 1.00). Then, we took the intersection of the seven signaling pathways and the HPD-related signaling pathways reported in the latest literature and found that the PI3K/Akt signaling pathway was activated in all three patients who experienced HPD. To the best of our knowledge, this is the first report of an advanced esophageal cancer patient who experienced HPD after nivolumab treatment, which might be associated with the PI3K/AKT signaling pathway, but the signaling pathway requires further study. Several predictive factors, including pathological, genomic, and immune characteristics, have been revealed to be associated with HPD. The potential factors contributing to HPD are summarized in .
Figure 3. Potential mechanisms associated with HPD induced by immunotherapy. In addition to PI3K/AKT pathway, several genetic alterations and clinical factors have been stated to be involved in HPD, which including mouse double minute 2 (MDM2) or MDM4 amplification, alteration of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK). The potential cell signaling pathways contributed to HPD were displayed
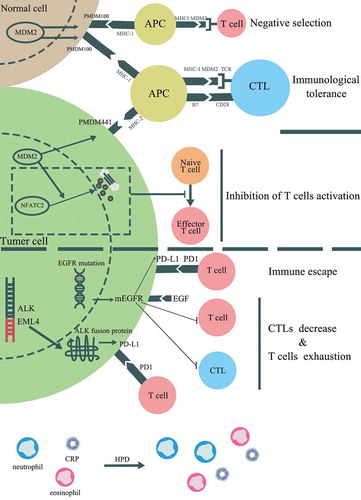
The results from a recent study indicated a close association between MDM2/MDM4 amplification and HPD among stage IV cancer patients after anti-PD1 therapy.Citation12 There is no clear mechanism of MDM2/MDM4 amplification resulting in HPD, but previous studies may offer a potential idea for further experiments. MDM2 is recognized as a tumor-associated antigen (TAA) and can be identified by antigen-specific CD8+ autologous T lymphocytes.Citation13 The epitopes of MDM2 can act as a TAA to activate cytotoxic T lymphocytes (CTLs), which in turn kill tumor cells expressing MDM2. However, after long-term stimulation of the MDM2 epitope, the effector CTLs disappear and result in the limitation of the antigen-specific antitumor response.Citation14 Meanwhile, according to The Human Protein Atlas, MDM2 is also expressed in multiple normal tissues, which can induce the central and/or peripheral tolerance of T lymphocytes and prevent effective control of tumor growth. In normal tissues expressing MDM2, an epitope derived from MDM2 called pMDM100, can bind to the H2-Kb major histocompatibility complex (MHC) I molecules with high affinity and generate antigen-specific CTLs with low avidity to the target tissue. Although pMDM100-specific CTLs can target this epitope and exhibit the ability of protein-specific killing, they are unable to induce the lysis of MDM2-expressing tumor cells. High-avidity CTLs can be generated by pMDM441, an epitope derived from MDM2 expressed in tumor tissues, which have the ability to induce the lysis of tumor cells but bind to H2-Db MHC I molecules with low affinity.Citation15 According to these studies, the immunological tolerance of MDM2 may play an important role in HPD after anti-PD1/PD-L1 treatment. Despite this, MDM2 can directly inhibit the activation of T cells, which could be a mechanism of HPD. Zou et al.Citation16 demonstrated that MDM2 participates in the negative regulation of T cell activation by inducing the ubiquitination and degradation of the transcription factor NFATc2. The results from a study by Gasparini et al.Citation17 revealed that the nutlin-3, a small molecular inhibitor of the MDM2/P53 interaction, can promote the ability of dendritic cells to stimulate T cell activation. In summary, MDM2 amplification is likely to induce HPD among patients treated with anti-PD1/PD-L1 therapy by inducing immunological tolerance and direct inhibition of T cell activation. MDM2 inhibitors can serve as an auxiliary therapeutic agent that may benefit patients with a genomic signature displaying MDM2 family amplification.
Gainor et al.Citation18 demonstrated that non-small cell lung cancer (NSCLC) patients harboring EGFR mutations or ALK rearrangements displayed low objective response rate (ORR) to anti-PD1/PD-L1 therapy. In addition, the results from another study revealed a correlation between EGFR mutations and the low TTF (less than 2 months), and patients harboring EGFR mutations were more likely to experience HPD.Citation19 While EGFR mutations and ALK rearrangements are associated with the activation of corresponding signaling pathways, whether there exists a correlation between the mutation-driven signaling pathway and HPD remains to be identified. Recently, studies have clarified the role of the EGFR signaling pathway in remodeling the tumor immune microenvironment (TIME).Citation20 The EGFR signaling pathway can positively regulate the expression of PD1, which might contribute to the immune escape mediated by the PD1/PD-L1 pathway. Meanwhile, Akbay et al.Citation21 confirmed a correlation between the EGFR signaling pathway and multiple immunosuppressive factors, including PD-L1, cytotoxic T lymphocyte antigen-4 (CTLA-4). In addition, CTLs reduction and T cells exhaustion can be detected in the xenografted mouse models of lung cancer driven by the EGFR signaling pathway. Coincidentally, the overexpression of ALK fusion protein can up-regulate the expression of PD-L1 and result in the apoptosis of T cells.Citation22 In addition, a series of microRNAs (miRNAs) that are associated with both innate and adaptive immune responses have been shown to be regulated by ALK. In particular, the miRNA-181 family, which regulates the phenotype, development, and modulation of T cell receptor (TCR) signaling strength, is down-regulated in anaplastic large cell lymphoma (ALCL) harboring ALK rearrangement.Citation23,Citation24 However, the relationship between the miRNA-181 family and ALK rearrangements in solid tumors requires further research. Given the correlation between driven mutations and HPD, it is interesting whether patients with cancer driven by EGFR or ALK will benefit from treatment combined with anti-PD1/PD-L1 therapy and corresponding tyrosine kinase inhibitors (TKIs). Several studies have already focused on this issue, and multiple clinical trials are currently undergoing.Citation25,Citation26
Although baseline features of cancer patients at first diagnosis play important roles in the prognosis of the disease, the importance of these features in the prediction of HPD remains unclear. Sasaki et al.Citation8 noted that a larger tumor size is significantly associated with HPD in patients with gastric cancer, while the study by Saâda-Bouzid E et al.Citation27 provided a different perspective that the tumor size at baseline had no correlation with the incidence of HPD during the treatment. Meanwhile, Saâda-Bouzid E et al. also provided us with a clue for the prediction of HPD that HPD occurs in patients with metastatic cervical lymph nodes at initial diagnosis more frequently. Hematological changes were detected after the diagnosis of the HPD. Early hypereosinophilia concurrent with HPD was reported to occur in an elderly woman treated with anti-PD1 therapy.Citation28 Other studies have shown that neutrophil counts and C-reactive protein (CRP) levels increased significantly in a lung cancer patientCitation29 and a gastric cancer patientCitation8 who experience HPD. Interestingly, the results from a recent study based on Asian NSCLC patients revealed that HPD is closely associated with the phenotypes of peripheral blood CD8 + T lymphocytes. Researchers found that a lower frequency of effector/memory subsets and a higher frequency of severely exhausted populations were associated with HPD.Citation27 Nevertheless, hematological changes in patients with HPD after anti-PD1/PD-L1 therapy still require larger-scale research.
In this review, the TIME is emphasized to be associated with HPD of cancer immunotherapy. According to the latest researches, the regulatory T cells (Treg) are blamed to be tightly associated with the HPD. The immune suppression induced by Treg was detected augmented after the blockage of PD-1 signaling pathway and the significant proliferation of Treg was discovered in HPD tissues.Citation30 EGFR and ALK pathways were all confirmed to be positively associated with the proliferation of TregCitation31,Citation32 and might participate in the resultant immune suppression. EGFR and ALK pathways share the same downstream PI3K/AKT pathwayCitation33–35 which is proved to be over-activated after the HPD in this report. Interestingly, the activation of PI3K/AKT pathway plays an important role in the generation of TregCitation36 and changes the ratio of Th17/Treg.Citation37 Therefore, PI3K/AKT pathway could mingle the tyrosine kinase-associated pathway with the regulation of TIME which might serve as the underlying mechanism explaining the development of HPD during cancer immunotherapy.
Conclusion
Nivolumab induced HPD in a patient with advanced esophageal squamous carcinoma, and the mechanism might be associated with the PI3K/AKT pathway. NGS examination may facilitate the exploration mechanisms underlying HPD after treatment with ICIs. The predictive factors for HPD, including clinicopathological, genomic, and immune characteristics, require further study.
List of abbreviations
| PD1 | = | programmed death 1 |
| PD-L1 | = | programmed death legend 1 |
| HPD | = | hyperprogressive disease |
| TTF | = | time to treatment failure |
| TGR | = | tumor growth rate |
| OS | = | overall survival |
| PI3K | = | phosphoinositide-3-kinase |
| EGFR | = | epidermal growth factor receptor |
| MDM2 | = | mouse double minute 2 |
| NGS | = | next generation sequencing |
| TAA | = | tumor associated antigen |
| MAPK | = | mitogen-activated protein kinase |
| ORR | = | obstetric response rate |
| CTLA-4 | = | cytotoxic T lymphocyte antigen-4 |
| TIME | = | tumor immunomicroenviroment |
| miRNA | = | micro RNA |
| TKI | = | tyrosine kinase inhibitor |
| CRP | = | c-reactive protein |
| ICI | = | immune checkpoint inhibitor |
Availability of data and material
The data used during the current study are available from the corresponding author on a reasonable request.
Consent for publication
The article was approved by all authors for publication.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. Written informed consent was obtained from the patient included in the study.
Acknowledgments
We thanked Lu Tian (Ocean University of China), Tao Wang (Huaiyin Normal University), and Xue Zhang (Qingdao University) for the technical assistance in diagramming.
Additional information
Funding
Notes on contributors
Helei Hou
Helei Hou: Conceptualization and writing the original draft;
Dantong Sun: Collecting data and writing the original draft;
Dong Liu: NGS examination and IHC test;
Qiaoling Liu: Data analysis.
All the authors reviewed and approved the final version of the manuscript.
References
- Kanjanapan Y, Day D, Wang L, Al-Sawaihey H, Abbas E, Namini A, Siu LL, Hansen A, Razak AA, Spreafico A, et al. 2019. Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer. 125(8):1341–1349.
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi:10.1093/annonc/mdz011.
- Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903.
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6.
- Harrington KJ, Ferris RL, Blumenschein G Jr, Colevas AD, Fayette J, Licitra L, Kasper S, Even C, Vokes EE, Worden F, et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (Checkmate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017;18(8):1104–1115. doi:10.1016/S1470-2045(17)30421-7.
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–1928. doi:10.1158/1078-0432.CCR-16-1741.
- Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–4250. doi:10.1158/1078-0432.CCR-16-3133.
- Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, et al. 2019. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 22(4):793–802.
- Hou H, Zhu H, Zhao H, Yan W, Wang Y, Jiang M, Liu B, Liu D, Zhou N, Zhang C, et al. Comprehensive molecular characterization of young chinese patients with lung adenocarcinoma identified a distinctive genetic profile. Oncol. 2018;23(9):1008–1015. doi:10.1634/theoncologist.2017-0629.
- Jensen TJ, Goodman AM, Kato S, Ellison CK, Daniels GA, Kim L, Nakashe P, McCarthy E, Mazloom AR, McLennan G, et al. Genome-wide sequencing of cell-free DNA identifies copy-number alterations that can be used for monitoring response to immunotherapy in cancer patients. Mol Cancer Ther. 2019;18(2):448–458. doi:10.1158/1535-7163.MCT-18-0535.
- Xiong D, Wang Y, Singavi AK, Mackinnon AC, George B, You M. Immunogenomic landscape contributes to hyperprogressive disease after anti-PD-1 immunotherapy for cancer. iSci. 2018;9:258–277. doi:10.1016/j.isci.2018.10.021.
- Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi:10.1001/jamaoncol.2018.3676.
- Mayr C, Bund D, Schlee M, Bamberger M, Kofler DM, Hallek M, Wendtner CM. MDM2 is recognized as a tumor-associated antigen in chronic lymphocytic leukemia by CD8+ autologous T lymphocytes. Exp Hematol. 2006;34(1):44–53. doi:10.1016/j.exphem.2005.09.016.
- Ramírez F, Ghani Y, Stauss H. Incomplete tolerance to the tumor-associated antigen MDM2. Int Immunol. 2004;16(2):327–334. doi:10.1093/intimm/dxh040.
- Bendle GM, Holler A, Downs AM, Xue SA, Stauss HJ. Broadly expressed tumour-associated proteins as targets for cytotoxic T lymphocyte-based cancer immunotherapy. Expert Opin Biol Ther. 2005;5(9):1183–1192. doi:10.1517/14712598.5.9.1183.
- Zou Q, Jin J, Hu H, Li HS, Romano S, Xiao Y, Nakaya M, Zhou X, Cheng X, Yang P, et al. 2014. USP15 stabilizes MDM2 to mediate cancer-cell survival and inhibit antitumor T cell responses. Nat Immunol. 15(6):562–570.
- Gasparini C, Tommasini A, Zauli G. The MDM2 inhibitor Nutlin-3 modulates dendritic cell-induced T cell proliferation. Hum Immunol. 2012;73(4):342–345. doi:10.1016/j.humimm.2012.01.018.
- Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-Small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi:10.1158/1078-0432.CCR-15-3101.
- Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28(7):1532–1539. doi:10.1093/annonc/mdx183.
- De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214(3):559–567. doi:10.1002/jcp.21260.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi:10.1158/2159-8290.CD-13-0310.
- Hong S, Chen N, Fang W, Zhan J, Liu Q, Kang S, He X, Liu L, Zhou T, Huang J, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunol. 2015;5(3):e1094598. doi:10.1080/2162402X.2015.1094598.
- Steinhilber J, Bonin M, Walter M, Fend F, Bonzheim I, Quintanilla-Martinez L. Next-generation sequencing identifies deregulation of microRNAs involved in both innate and adaptive immune response in ALK+ ALCL. PLoS One. 2015;10(2):e0117780. doi:10.1371/journal.pone.0117780.
- Ghorbani S, Talebi F, Chan WF, Masoumi F, Vojgani M, Power C, Noorbakhsh F. MicroRNA-181 variants regulate T cell phenotype in the context of autoimmune neuroinflammation. Front Immunol. 2017;8:758–772. doi:10.3389/fimmu.2017.00758.
- Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):465–469. doi:10.1080/14740338.2017.1300656.
- Tabchi S, Kourie HR, Kattan J. Adding checkpoint inhibitors to tyrosine kinase inhibitors targeting EGFR/ALK in non-small cell lung cancer: a new therapeutic strategy. Invest New Drugs. 2016;34(6):794–796. doi:10.1007/s10637-016-0383-2.
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. doi:10.1093/annonc/mdx178.
- Occhipinti M, Falcone R, Onesti C, Marchetti P. Hyperprogressive disease and early hypereosinophilia after anti-PD-1 treatment: a case report. Drug Saf Case Rep. 2018;5(1):12. doi:10.1007/s40800-018-0078-z.
- Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104–1113. doi:10.1093/annonc/mdz123.
- Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, Sato E, Fukuoka S, Tada Y, Tanaka A, et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116(20):9999–10008. doi:10.1073/pnas.1822001116.
- He H, Qi L, Hou Y. Altered Treg and IL-1A expression in the immune microenvironment of lung squamous-cell cancer after EGFR blockade. Zhongguo Fei Ai Za Zhi. 2017;20(3):143–148.
- Kasprzycka M, Marzec M, Liu X, Zhang Q, Wasik MA. Nucleophosmin/anaplastic lymphoma kinase (NPM/ALK) oncoprotein induces the T regulatory cell phenotype by activating STAT3. Proc Natl Acad Sci U S A. 2006;103(26):9964–9969. doi:10.1073/pnas.0603507103.
- Yoshida A, Tsuta K, Nakamura H, Kohno T, Takahashi F, Asamura H, Sekine I, Fukayama M, Shibata T, Furuta K, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol. 2011;35:1226–1234. doi:10.1097/PAS.0b013e3182233e06.
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi:10.1038/nature05945.
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899.
- Liu Y, Marin A, Ejlerskov P, Rasmussen LM, Prinz M, Issazadeh-Navikas S. Neuronal IFN-beta-induced PI3K/Akt-FoxA1 signalling is essential for generation of FoxA1+Treg cells. Nat Commun. 2017;8:14709. doi:10.1038/ncomms14709.
- Gao L, Dong Y, Lin R, Meng Y, Wu F, Jia L. The imbalance of Treg/Th17 cells induced by perinatal bisphenol A exposure is associated with activation of the PI3K/Akt/mTOR signaling pathway in male offspring mice. Food Chem Toxicol. 2020;137:111177. doi:10.1016/j.fct.2020.111177.
