ABSTRACT
Recently approved therapies have contributed to a significant progress in the management of ovarian cancer; yet, more options are needed to further improve outcomes in patients with advanced disease. Here we review the rationale and ongoing clinical trials of novel combination strategies involving chemotherapy, poly ADP ribose polymerase, programmed death 1 (PD-1)/PD-ligand 1 immune checkpoint and/or vascular endothelial growth factor receptor inhibitors. Further, we discuss novel agents aimed at targets associated with ovarian cancer growth or progression that are emerging as potential new treatment approaches. Among them, agents targeted to folate receptor α, tissue factor, and protein kinase-mediated pathways (WEE1 kinase, phosphatidylinositol-3 kinase α, cell cycle checkpoint kinase 1/2, ATR kinase) are currently in clinical development as mono- or combination therapies. If successful, findings from these extensive development efforts may further transform treatment of patients with advanced ovarian cancer.
I. Introduction
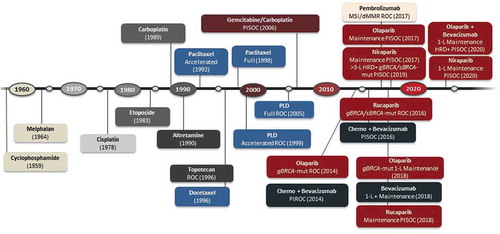
Although incidence rates of ovarian cancer have been decreasing by about 1–2% each year, ~21,750 new cases are expected in the United States in 2020, with a diagnosis of epithelial ovarian cancer in ~90% of these patients.Citation1,Citation2 Primary peritoneal and fallopian tube cancers may be referred to as part of this group of malignancies. The adoption of more effective treatment options with novel chemotherapeutic regimens and targeted agents has significantly contributed to the improvements in patient outcomes observed in the past decade ().Citation1–4
Figure 1. FDA-approved drugs in advanced ovarian cancer
Nonetheless, a substantial proportion of patients with ovarian cancer are primarily resistant to treatment with available agents or develop secondary resistance over time, with disease progression and a poorer prognosis. In 2020, ~13,940 deaths are estimated to occur in the United States due to ovarian cancer. The current, overall 5-year survival rate is 48% and only 29% in the most advanced disease stages.Citation2 This limited survival underscores the need of identifying additional safe and effective treatments to improve outcomes in patients with ovarian cancer, particularly in advanced stage disease. The development of novel agents or regimens with improved tolerability may also contribute to provide treatment options associated with a better health-related quality of life for this patient population.
In this review, we discuss new strategies that are emerging for the treatment of patients with advanced ovarian cancer across a broad range of mechanisms of action, outlining the rationale underlying the selection of new targets in ovarian cancer and the novel combination approaches currently being evaluated in clinical trials.
II. Angiogenesis, genomic instability, and the immune microenvironment in ovarian cancer
Angiogenesis and vascular endothelial growth factor receptor (VEGFR) inhibition
Multiple lines of evidence have demonstrated that angiogenesis may play a key role in the survival and progression of various tumor types, including advanced ovarian cancers.Citation5 Once released within the tumor microenvironment, proangiogenic growth factors such as VEGF can induce activation and proliferation of vascular endothelial cells, promote tumor-associated angiogenesis and contribute to the survival of endothelial cells in the newly formed vessels. In addition, proangiogenic factors modify the vascular tone and increase vascular permeability thereby contributing to further support the growth and survival of tumor cells.Citation5
In ovarian cancer, overexpression of VEGF leads to an increase in tumor microvessel density, which has been found to be associated with disease progression and worse prognosis.Citation5 Consistently, VEGF inhibition by bevacizumab in combination with chemotherapy, followed by maintenance therapy, has proven effective in delaying disease progression in ovarian cancer when administered after initial surgical resection or in recurrent platinum-sensitive disease, and as monotherapy or combined with chemotherapy in patients with platinum-resistant disease.Citation6–8
However, various mechanisms may allow escape of tumor cells from therapeutic control, including selection of tumor clones with increased expression of compensatory signaling pathways or clones with an increased capacity to grow and invade normal tissues in conditions of limited angiogenic support.Citation9,Citation10 Multiple approaches are thus being investigated to further improve clinical outcomes, through the development and characterization of novel antiangiogenic agents, and their use in combination regimens with agents targeting other key pathways in tumor cells (i.e. DNA repair) or immune checkpoints (i.e. programmed death receptor 1 [PD1]/PD-ligand 1 [PD-L1]).Citation11 Both VEGF receptor (VEGFR) 1 and 2 are expressed by microvascular endothelial cells in malignant ovarian tumors and borderline lesions suggesting their potential usefulness as targets for new therapeutic approaches.Citation5,Citation12
Nintedanib is a triple angiokinase inhibitor of the VEGFR, platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor receptor (FGFR).Citation13 Combined treatment with this small-molecule inhibitor and chemotherapy (carboplatin/paclitaxel) in the AGO-OVAR12 phase III trial showed activity in patients with newly diagnosed, advanced ovarian cancer, with an improvement in median progression-free survival (mPFS) versus chemotherapy. However, treatment was associated with limited tolerability and a higher incidence of gastrointestinal adverse events (AEs) compared with the control group.Citation13
Targeted inhibition of VEGFR2 with the small-molecule, tyrosine kinase inhibitor apatinib has demonstrated activity in advanced gastric cancer and other malignancies. Single-agent administration in an initial phase I study induced responses, although of limited duration, in patients with recurrent, platinum-resistant ovarian cancer.Citation14 Treatment with apatinib was most frequently associated with hand-foot syndrome, hypertension, nausea, and vomiting. Further evaluation is ongoing in combination with the DNA topoisomerase II inhibitor etoposide or the anti-PD-1 antibody camrelizumab in patients with advanced, platinum-resistant disease.Citation14
In addition to VEGFR targeting, selective inhibition of angiopoietin binding to the Tie2 tyrosine kinase receptor may result in impairment of tumor-associated angiogenesis.Citation15 Investigation of combination treatment with the angiopoietin inhibitor trebananib (AMG386) plus paclitaxel showed a significant prolongation in mPFS versus paclitaxel alone in patients with recurrent disease (platinum-free interval <12 months), in the randomized phase III TRINOVA-1 trial. Edema (of any grade) was reported in more than half of patients receiving combination treatment, but most of the AEs usually related to VEGF-targeted therapy (i.e. hypertension, proteinuria, thrombotic events, wound-healing complications, gastrointestinal perforation) were infrequently observed.Citation15 At final analysis of this study, OS was significantly prolonged only in the patient subset with baseline ascites.Citation16 Combination treatment with trebananib plus pegylated liposomal doxorubicin in patients with recurrent, partially platinum-sensitive or resistant ovarian cancer (platinum-free interval ≤12 months, ENGOT-ov6/TRINOVA-2 phase III trial) demonstrated an improvement in objective response rate (ORR), but not in mPFS, versus chemotherapy alone.Citation17 Recent findings from the randomized ENGOT-ov2/TRINOVA-3/GOG-3001 phase III trial of trebananib plus carboplatin/paclitaxel as first-line treatment for advanced disease followed by maintenance with trebananib or placebo, showed no significant improvement in mPFS, the primary endpoint, compared with chemotherapy.Citation18 A phase Ib study is currently evaluating trebananib in combination with the PD-1 immune checkpoint inhibitor pembrolizumab in patients with advanced, platinum-resistant ovarian cancer.
Genomic instability and poly ADP ribose polymerase (PARP) inhibition
As in other tumor types, genomic instability may arise in ovarian cancer from the genetic abnormalities associated with malignant transformation, which often affects genes encoding homologous recombination (HR) DNA repair factors such as BRCA1 and BRCA2.Citation19–21 The PARP1 and PARP2 nuclear enzymes mediate repair of single-stranded (ss) DNA breaks, induced by ultraviolet light, radiation, chemotherapy, and other DNA-damaging agents, by base-excision repair.Citation22,Citation23 In patients with germline or somatic defects in genes involved in HR repair (i.e. BRCA1, BRCA2), who cannot repair (ds) DNA breaks, inhibition of PARP activity blocks DNA repair and induces cell death.Citation22,Citation23 Inhibition of PARP becomes synthetically lethal in the context of an inactivating BRCA mutation, because HR defects make BRCA-mutated tumors ‘addicted to’ (dependent on) other DNA repair pathways.Citation24 Although PARP inhibitors were first shown to be effective in BRCA-mutated tumors, responses and clinical benefit have been observed in the presence of wild-type BRCA1 or 2 genes, suggesting that other factors and genetic mutations, involved in HR DNA repair, may confer sensitivity to PARP inhibitors.Citation19,Citation25,Citation26 Beyond BRCA, genes encoding proteins involved in HR repair that may be mutated and associated with HR deficiency (HRD) in ovarian cancer include the Fanconi anemia genes RAD51C, RAD51D, RAD50, BRIP1, BARD1, MRE11A, and PALB2 as well as the DNA mismatch repair genes MLH1, MSH2, MSH6, and PMS2.Citation27 In patients with ovarian cancer, germline mutations in HR genes have been identified more frequently than somatic tumor mutations (<10% of cases).Citation28
Findings from multiple trials have demonstrated that PARP inhibition is an effective therapeutic strategy in ovarian cancer (). Treatment with single-agent rucaparib or olaparib is standard of care for patients with recurrent, platinum-sensitive or platinum-resistant BRCA-mutated disease, after 2 or 3 prior lines of chemotherapy, respectively.Citation4,Citation25,Citation26,Citation29 In addition, the QUADRA study has shown efficacy of niraparib monotherapy in patients with HRD, including BRCA-mutated and non-mutated, recurrent ovarian cancer after four or more lines of chemotherapy (ORR, 28%).Citation30 These results supported the approval of niraparib by the Food and Drug Administration (FDA) for heavily pretreated patients with HRD+ disease.
Table 1. Pivotal trials and FDA approvals of PARP and VEGF inhibitors in advanced ovarian cancer
Based on the results of the SOLO2, study 19, NOVA, and ARIEL 3 studies, olaparib, niraparib, and rucaparib are indicated for the maintenance treatment of patients with recurrent ovarian cancer who are in complete or partial response following prior platinum-based therapy, independent of their biomarker status.Citation4,Citation25,Citation26,Citation29 Results from the randomized phase III SOLO1 trial have demonstrated that maintenance treatment with a PARP inhibitor is effective in newly diagnosed patients following first-line platinum-based chemotherapy.Citation31 Based on the extent of benefit observed, olaparib was approved by the FDA for clinical use in this indication for patients with BRCA-mutated tumors. Furthermore, the phase III studies ENGOT-OV26/PRIMA, VELIA, and PAOLA-1 have shown that both niraparib and veliparib, and the combination of olaparib plus bevacizumab are effective as first-line maintenance therapy in patients with advanced ovarian cancer, following response to prior platinum-based chemotherapy. Detailed study results are presented in for the intent-to-treat populations and the subgroups analyzed (i.e. HRD+, BRCA-mutation+ patients). Although all three trials were positive in the intention-to-treat population, which included ‘all-comers’ despite specific genetic abnormalities, the patients that derived most benefit were HRD+, either due to a BRCA mutation or other HR defect.Citation32–35
Table 2. Pivotal trial results for front-line maintenance therapy in patients with newly diagnosed, advanced ovarian cancer after response to platinum-based therapy
Nonetheless, ovarian tumors may display primary or secondary resistance to treatment with PARP inhibitors, prompting an extensive evaluation of biomarkers that may help to determine the underlying resistance mechanisms and contribute to the identification and selection of therapies/combination regimens, timing, and sequencing of treatments suitable for each patient.Citation36–41 Secondary mutations have been detected in patients with acquired resistance to PARP inhibitor therapy, including somatic mutations that restore BRCA1/2 gene functions, through elimination of the open reading frame shift or by reverse mutation within the coding region.Citation37,Citation41–43 Analysis of baseline and on treatment samples showed that the presence of heterogeneous BRCA2 reversion mutations was associated with resistance to PARP inhibition in prostate and ovarian cancers.Citation42,Citation43 In one of these studies, BRCA reversion mutations were detected in pretreatment samples in 18% of platinum-refractory v 2% of platinum-sensitive, high-grade ovarian carcinomas (p = .049). Patients without BRCA reversion mutations at baseline had significantly longer PFS following treatment with rucaparib compared with patients who had BRCA reversion mutations (median PFS, 9.0 v 1.8 months; HR, 0.12; p < .0001).Citation43 Additionally, heterogeneous mutations were identified in some patients after PARP inhibitor therapy.Citation43
Primary and acquired resistance to PARP inhibition in patients with high-grade ovarian cancers were also found associated with secondary, somatic mutations, including a truncation mutation, in the genes RAD51C and RAD51D, which encode proteins involved in (ds) DNA break repair by HR.Citation44 Complementation assays confirmed the association between the presence of these mutations and resistance to PARP inhibition therapy.Citation44 Combination with other targeted inhibitors as well as earlier administration of a PARP inhibitor in the course of the disease may help to decrease or delay the development of resistance in ovarian cancer, and thus improve treatment outcomes for patients with advanced disease.Citation25,Citation44
The immune microenvironment, activity of PD-1/PD-L1-targeted immune checkpoint inhibitors and other immunotherapeutic approaches
The tumor immune microenvironment in ovarian cancers is quite complex with infiltration by helper or effector T cells and antigen-presenting cells (i.e. dendritic cells), production of interferon (INF) γ, interleukin (IL) 2 and IL-16 (a chemoattractant), which may result in antitumor immune responses. Conversely, the activity of VEGF and other ‘pro-tumor’ immune factors such as transforming growth factor (TGF) β, PD-L1, tumor necrosis (TNF) α, IL-6, and IL-10 often contribute to tumor survival and escape from immune control.Citation45–48
Overexpression of VEGF may induce downregulation of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion-molecule-1 (VCAM-1) and thus reduce the ability of lymphocytes to adhere to the tumor vascular endothelial cells and migrate into the tumor tissues. TGF-β can suppress the activation and proliferation of tumor-infiltrating lymphocytes.Citation45
Engagement of the PD-1 receptor on the immune cells by PD-L1 expressed by tumor cells may lead to a decrease in T cell activation, inhibition of T cell proliferation, and suppression of antitumor CD8 + T cell responses. In ovarian cancer, PD-L1 is detectable in about a third of advanced tumors, but most of the tumor-infiltrating lymphocytes express PD-1.Citation46,Citation47 High-grade tumors have been reported to express PD-L1 to a greater extent than low-grade ovarian tumors (i.e. 42% v 8%). A higher expression of PD-L1 may be associated with a worse prognosis compared with patients with lower PD-L1 levels, although further investigations are warranted to define the prognostic value of PD-L1 expression in ovarian cancer.Citation46,Citation47 Nonetheless, infiltrating CD3+, CD8+, and CD4 + T cells detected in the tumor tissues of approximately half of the ovarian cancers investigated (‘hot tumors’) were associated with better outcomes in patients with advanced ovarian cancer, with prolonged PFS and OS after treatment compared with patients with no infiltrates, suggesting that therapeutic approaches aimed at restoring active antitumor responses may provide benefit in this setting.Citation45–47 However, the complexity of the immune microenvironment associated with ovarian cancer, with the occurrence of immunosuppressive mediators and immunoregulatory cells (i.e. T regulatory cells [Treg] and myeloid-derived suppressor cells),Citation45–47,Citation49 the potential for T cell exhaustionCitation49,Citation50 and the effects of chemotherapy or surgery on antitumor immune responses, are all factors that need to be considered in the selection/sequencing of novel immunotherapeutic approaches and the design of combination regimens. Assessment of the pharmacodynamic effects of novel immunomodulatory approaches, currently ongoing in clinical trials, may also contribute to a better understanding of their impact on antitumor immune responses in patients with advanced ovarian cancer.
At initial clinical evaluation of PD-1/PD-L1 immune checkpoint inhibition therapies, both anti-PD-1 (i.e. nivolumab, pembrolizumab, PF-06801591) and anti-PD-L1 antibodies (i.e. atezolizumab, avelumab), have shown some single-agent, immunomodulatory and anti-tumor activity in patients with advanced ovarian cancer (7%-22%), albeit to a lower extent compared with other anti-PD-1/PD-L1 responsive tumor types (i.e. melanoma, non-small-cell lung cancer) ().Citation51–59 For immune checkpoint inhibition to work in any cancer, T cells must be present in the tumor microenvironment. A substantial proportion of patients with ovarian cancer are resistant to treatment with anti-PD-1/PD-L1 antibodies due to limited infiltration by antitumor immune cells, the activity of immunosuppressive cells and cytokines in the tumor microenvironment, and/or a low expression of PD-1/PD-L1, suggesting that multi-targeted combination approaches including PARP inhibitors, chemotherapeutic agents, angiogenesis inhibitors, radiation, other immunomodulatory agents, vaccines, dendritic cell therapy, adoptive T cell therapy, or other targeted therapy may be more effective to achieve disease control.Citation51–60 Inhibition of DNA damage repair by a PARP inhibitor may induce an increase in the overall tumor mutational burden (TMB) and release of immunogenic, tumor-associated neoantigens. In addition, it promotes type-I interferon signaling, which in turn induces recruitment of T cells. The cell death induced by exposure to chemotherapeutic agents also generates new, tumor-related, immunogenic determinants that can facilitate the antitumor immune responses restored by PD-1/PD-L1 inhibition.Citation60
Table 3. Single-agent treatment with PD-1/PD-L1 immune checkpoint inhibitors in patients with advanced ovarian cancer
VEGFR inhibition may result in tumor vessel normalization and facilitate migration of immune cells into the tumor microenvironment.Citation11,Citation45 Consistently, VEGF inhibition by bevacizumab in combination with atezolizumab was shown to attenuate progression of platinum-resistant ovarian cancer through synergic anti-tumor activity, based on in vitro and in vivo analyses.Citation61 Clinical activity has also been observed with nivolumab plus bevacizumab combination treatment in a phase II trial conducted in patients with recurrent ovarian cancer.Citation62 Furthermore, preclinical investigations have shown that treatment with niraparib increased the activity of IFN-γ and IFN-γ signaling pathways as well as intra-tumor infiltration by CD8+ and CD4 + T cells. Combined administration of niraparib and an anti-PD-1 antibody in breast and ovarian cancer mouse models demonstrated synergic antitumor activity against both mutated BRCA+ and wild-type BRCA tumors, indicating a potential applicability of this combination.Citation63 In addition, a study demonstrated that PARP inhibition by olaparib or talazoparib can induce upregulation of PD-L1 expression in breast cancer cell lines in vitro and in xenograft tumor models in vivo.Citation64 Block of PD-L1 by a targeted antibody was found to restore sensitivity of PARP inhibitor-treated cancer cells to T-cell-mediated cytotoxicity. Combined administration of a PARP inhibitor and an anti-PD-L1 antibody produced greater antitumor activity in vivo versus either agent alone in this animal model, suggesting potential benefit from combined treatment with these agents in humans.Citation64
In addition to PD-1/PD-L1 immune checkpoint inhibitors, other immunotherapeutic approaches such as dendritic cell vaccines, tumor-infiltrating lymphocytes (TILs), and chimeric antigen receptor (CAR)-T cells are being pursued for the treatment of advanced ovarian cancer.Citation60,Citation65–69 Investigational, autologous ovarian cancer vaccines generated by pulsing dendritic cells with oxidized, whole-tumor-cell lysates obtained from individual patients have been evaluated in a pilot study, following intranodal injection in immune-naïve patients with recurrent, platinum-pretreated advanced ovarian cancer.Citation65 A subgroup of patients also received IV bevacizumab to enhance tumor infiltration by immune cells and low-dose cyclophosphamide to decrease infiltration by Treg cells. Vaccination was associated with a tolerable safety profile, high-affinity T-cell responses to autologous tumor antigens, and prolonged survival. Anti-tumor antigen responses were higher in vaccinated patients who had received concomitant treatment with cyclophosphamide. The OS at 2 years was greater in vaccinated patients (78%) who had received bevacizumab plus cyclophosphamide compared with an historical, institutional group of matched patients treated only with bevacizumab plus cyclophosphamide (44%). Although different factors (i.e. tumor sample availability, low lysate immunogenicity, complex production process) may limit the clinical applicability of autologous, dendritic cell vaccines for each patient, these findings indicate the feasibility of inducing beneficial, tumor-specific immune responses in advanced ovarian cancer.Citation65
In a pilot study, adoptive cell therapy with TILs and progressively decreasing IV doses of IL-2 in patients with metastatic, platinum-resistant ovarian cancer, was associated with a manageable toxicity and early signals of clinical activity, although all treated patients ultimately progressed, mostly due to the development of new lesions. Infused TILs were found to express biomarkers potentially associated with T-cell exhaustion (i.e. LAG3 and PD-1), while tumor tissues demonstrated expression of major histocompatibility complex class II antigens and PD-L1, suggesting activation of inhibitory immune checkpoint pathways.Citation66
Results from an adoptive immunotherapy study of gene-modified CAR T cells redirected to the tumor-associated antigen folate receptor alpha (FRα) plus high-dose IL-2 showed large numbers of transferred T cells in the peripheral blood of treated patients within the first 2 days after infusion, followed, however, by a decline to very low levels within 1 month and mostly no localization in tumors.Citation67 A lack of responsiveness to growth factors in vivo, T-cell exhaustion after prolonged in vitro culture, and/or the IV route of administration with intra-organ sequestration may have contributed to the limited in vivo survival and tumor homing of the transferred, gene-modified T cells observed in this study. Optimization of in vitro culture conditions and costimulatory signals as well as selection of an IP delivery route may contribute to improved survival and in vivo antitumor activity of CAR T cells against ovarian cancers.Citation67 In other studies, CAR T cells targeted to the tumor-associated antigens mesothelin or mucin 16 (MUC16), known to be overexpressed on ovarian cancer cells, demonstrated cytotoxic activity in vitro and antitumor activity in vivo, resulting in growth inhibition of ovarian tumors in preclinical animal models.Citation68,Citation69 Combinations with immunomodulatory signals, such as costimulation through CD28 or PD-1/PD-L1 immune checkpoint inhibition, or with growth factors (i.e. IL-12), can contribute to enhance the in vivo efficacy of CAR T-cell-based approaches.Citation68,Citation69
Combination studies of chemotherapy, PARP inhibitors, anti-PD-1/PD-L1 antibodies, and/or VEGFR inhibitors in patients with advanced ovarian cancer
Based on the findings outlined above, multiple combination strategies including PARP inhibition and/or an anti-PD-1/PD-L1 antibody and/or a VEGFR inhibitor are being evaluated in patients with a) newly diagnosed, b) relapsed, platinum-sensitive or c) platinum-resistant disease ().
Table 4. Investigational combination regimens in patients with advanced ovarian cancer
Chemotherapy-based combination regimens (± PARP inhibitor ± anti-PD-1/PD-L1 antibody ± VEGFR inhibitor)
PD-1/PD-L1 targeted immune checkpoint inhibitors are being administered in combination with standard of care platinum/taxane-based chemotherapy in various phase III trials for the treatment of patients with stage III–IV, newly diagnosed ovarian cancer with or without bevacizumab, followed by maintenance therapy with a PARP inhibitor with or without an anti-PD-1/PD-L1 antibody and/or bevacizumab. Details of these trials are presented in .
JAVELIN ovarian PARP 100, which was a phase III study in newly diagnosed ovarian cancer patients, evaluating avelumab in combination with platinum-based chemotherapy followed by maintenance with avelumab and talazoparib versus chemotherapy plus talazoparib maintenance or chemotherapy plus bevacizumab followed by bevacizumab maintenance, was terminated early as the extent of benefit observed with avelumab did not support continuation of this study in an unselected patient population.
Results from the randomized, phase III trial IMagyn050/GOG 3015/ENGOT-ov39 conducted in newly diagnosed patients with stage III–IV ovarian cancer have shown that addition of a PD-L1 inhibitor, atezolizumab, to standard chemotherapy (carboplatin/paclitaxel) plus bevacizumab followed by maintenance with bevacizumab did not significantly improve mPFS (primary endpoint) in the intent-to-treat population (19.5 v 18.4 months; hazard ratio, 0.92) or in PD-L1-positive patients with ≥1% expression (20.8 v 18.5 months; hazard ratio, 0.80). However, an exploratory analysis suggested a trend toward improved mPFS with addition of atezolizumab in the subgroup of patients with PD-L1 expression ≥5%.Citation70
summarizes phase III trials being conducted in patients with recurrent, platinum-sensitive disease, which are evaluating chemotherapy in combination with a PD-1/PD-L1 inhibitor with or without bevacizumab and maintenance treatment with an anti-PD-1/PD-L1 antibody plus/minus a PARP inhibitor and/or bevacizumab.
PARP inhibitor + VEGFR inhibitor combinations
VEGFR inhibition can produce impairment of homologous recombination DNA repair with downregulation of BRCA1/2 and RAD51 in addition to inhibition of tumor-associated angiogenesis, potentially sensitizing target cells to the antitumor activity of PARP inhibitors.Citation71 Results from randomized trials have demonstrated this combination as superior to PARP inhibition alone.Citation72,Citation73
Cediranib is a small-molecule, VEGFR 1–3 tyrosine kinase inhibitor, reported to induce sensitivity to PARP inhibitors (i.e. olaparib) by inducing tumor hypoxia and inhibiting PDGFR, which result in decreased expression of BRCA1/2 and RAD51 and reduced activity of HR DNA repair in target cells.Citation71 In a randomized phase II study, combination treatment with cediranib and olaparib demonstrated a significant improvement in mPFS and overall survival versus olaparib alone in patients with germline BRCA wild-type/unknown, relapsed, platinum-sensitive ovarian cancer.Citation72 A significant improvement in mPFS with VEGF plus PARP inhibition has also been reported with bevacizumab plus niraparib in patients with recurrent, platinum-sensitive disease, in the randomized phase II trial NSGO-AVANOVA2/ENGOT-ov24.Citation73
Thus, phase III trials have been initiated to evaluate combinations of VEGFR and PARP inhibition for potential additive/synergistic effects in relapsed platinum-sensitive or platinum-resistant/refractory, advanced ovarian cancer (). Combination of cediranib with olaparib in the randomized, phase III NRG-GY004 trial has recently demonstrated an mPFS (primary endpoint) comparable rather than significantly improved versus standard platinum-based chemotherapy with carboplatin/paclitaxel, carboplatin/gemcitabine, or carboplatin/pegylated liposomal doxorubicin (10.4 v 10.3 months, hazard ratio 0.86), in the intent-to-treat population of patients with relapsed, platinum-sensitive OvCa.Citation74 However, in a planned subset analysis of patients with germline BRCA mutations, the hazard ratio for improvement in mPFS versus chemotherapy was 0.55 for cediranib plus olaparib and 0.63 for olaparib alone.Citation74 Further phase II or III clinical trials are in progress evaluating cediranib in various combinations with PARP inhibitors, chemotherapy, or anti-PD-1/PD-L1 antibodies.
PARP inhibitor + anti-PD-1/PD-L1 antibody ± VEGFR inhibitor combinations
As previously discussed, preclinical studies have demonstrated synergy between PARP inhibitors and immune checkpoint inhibitors in mediating antitumor activity.Citation63,Citation64 Thus, a number of clinical trials are currently evaluating combination treatment with these two classes of agents in patients with recurrent ovarian cancer, including niraparib plus pembrolizumab, talazoparib plus avelumab, BGB-290 (pamiparib) plus BGB-A317 (tislelizumab), rucaparib plus nivolumab, and olaparib plus durvalumab. In addition, multiple phase II or III studies are assessing triple combinations of a PARP inhibitor with an anti-PD-1/PD-L1 antibody and a VEGFR inhibitor in platinum-sensitive or resistant disease ().
Results from the phase I–II study (TOPACIO/Keynote-162) of niraparib in combination with pembrolizumab showed an ORR of 18% in patients with recurrent, advanced ovarian cancer, with no new safety signals from the combination treatment. The majority of patients had platinum-resistant/refractory disease.Citation75 This compares with historical ORRs of ≤10% with PD-1 checkpoint inhibitor treatment (irrespective of PD-1 levels) or PARPi monotherapy in similar patient populations without BRCA-mutations.Citation75
Initial findings from the phase II study (NCT02484404) of olaparib and durvalumab in patients with mostly platinum-resistant, recurrent disease, showed an overall ORR of ~15%, with responses in both BRCA-mutated and BRCA-wild type patients. Grade 3–4 anemia and lymphopenia were reported in 26% and 14% of patients, respectively. Dose reductions of olaparib were required in <1% of patients.Citation76 Preliminary results from the same phase II study of a triple combination of olaparib, durvalumab, and cediranib in a small number of patients with recurrent ovarian cancer have shown tolerability, antitumor activity with partial responses, and correlation of clinical benefit with PD-L1 expression levels in tumors.Citation77 Furthermore, a triple combination of olaparib, durvalumab, and bevacizumab investigated in the phase II, non-randomized MEDIOLA trial, in patients with relapsed, platinum-sensitive ovarian cancer (non-germline BRCA-mutated) demonstrated a high 24-week disease control rate (77.4%) and confirmed ORR (77.4%), with a mPFS of 14.7 months. In the olaparib plus durvalumab arm, the ORR was 31.3% and the mPFS was 5.5 months.Citation78,Citation79 Anemia, hypertension, fatigue, increased lipase levels, and neutropenia were the grade ≥3 AEs most frequently observed with the triple combination; 16% of patients discontinued one or more study drugs in this combination regimen.Citation79
III. Targeting tumor-associated antigens and signaling pathways in ovarian cancer
A number of novel agents to tumor targets associated with ovarian cancer growth and progression are currently in clinical development, to identify new options for single-agent or combination treatment in patients with advanced ovarian cancer (). Details of the clinical studies in progress with these agents are presented in .
Table 5. Targeted agents in development for the treatment of patients with advanced ovarian cancer
Table 6. Clinical trials evaluating emerging agents for the treatment of patients with advanced ovarian cancer
Folate receptor alpha (FRα)
FRα is a transmembrane glycoprotein mediating transport of folate into cells, which is overexpressed in the majority of epithelial ovarian cancers but absent in normal epithelial cells of the ovary.Citation80 Such selective expression in tumor cells and the ability to internalize following ligand binding make FRα a suitable target for antibody-drug conjugates (ADCs) designed to deliver cytotoxic payloads to tumor cells.Citation80
Mirvetuximab soravtansine is an FRα-targeted ADC composed of the anti-FRα M9346A antibody and the microtubule-disrupting agent maytansinoid DM4. The presence of a cleavable linker also allows release of active DM4 molecules and killing of proximal tumor cells.Citation80,Citation81 Combination treatment with mirvetuximab soravtansine plus carboplatin followed by maintenance with mirvetuximab soravtansine had an acceptable tolerability profile in a phase I study of patients with recurrent, platinum-sensitive ovarian cancer, with an ORR of 71% and median PFS of 15 months. The most frequent AEs were nausea, diarrhea, thrombocytopenia, blurred vision, and fatigue (mostly ≤ grade 2).Citation80
The randomized phase III FORWARD I trial evaluated safety and efficacy of mirvetuximab soravtansine versus chemotherapy of choice in patients with FRα-positive, platinum-resistant ovarian cancer, who had received up to three lines of prior treatment.Citation81 Although the response rate was higher in the experimental arm, the study did not meet the primary endpoint of a significant improvement in mPFS. Nonetheless, patients with high levels of FRα expression treated with mirvetuximab soravtansine had longer mPFS (4.8 v 3.3 months) and a higher ORR (24% v 10%), with less grade ≥3 AEs and treatment discontinuations due to AEs compared with chemotherapy, thus suggesting a potentially favorable risk/benefit profile in this patient population.Citation82 The most frequent AEs reported with mirvetuximab soravtansine in this study were nausea (54%), diarrhea (44%), and blurred vision (43%).Citation82 Two phase III trials, MIRASOL and SORAYA, are further evaluating mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer and high FRα expression. In MIRASOL, safety and efficacy of mirvetuximab soravtansine are assessed versus chemotherapy of choice with paclitaxel, pegylated liposomal doxorubicin, or topotecan (primary endpoint, mPFS) ().
Furthermore, mirvetuximab soravtansine is being evaluated in combination with chemotherapy, bevacizumab, the anti-PD-1 antibody pembrolizumab, or the PARP inhibitor rucaparib in phase I–II studies, to identify potential synergies with these inhibitors (). Results from cohorts of patients with platinum-resistant, ovarian cancer treated with mirvetuximab soravtansine and bevacizumab in the FORWARD II study have shown an ORR of 39% in all patients (mPFS, 6.9 months) and 56% in bevacizumab-naïve patients with medium/high FRα expression (≥50% positive cells) (mPFS, 9.9 months). AEs were generally mild or moderate in severity; 9% of patients developed grade 1–2 pneumonitis.Citation83
In addition, a triple combination of mirvetuximab soravtansine with carboplatin and bevacizumab (followed by mirvetuximab soravtansine/bevacizumab maintenance), in a phase Ib/II study of patients with recurrent, platinum-sensitive ovarian cancer and medium/high FRα expression, was associated with a manageable safety profile, confirmed responses in 81% of patients (median duration, 10.7 months) and a mPFS of 12.0 months. The most frequently reported treatment-related AEs were diarrhea, nausea, fatigue, and blurred vision, consistent with the known safety profile of mirvetuximab soravtansine. Grade 2 peripheral neuropathy was observed in 22% of patients.Citation84
The ADC MORAb-202 consists of the humanized anti-FRα antibody farletuzumab conjugated to the microtubule-targeted agent eribulin (maleimido-PEG2-valine-citrulline-p-aminobenzylcarbamyl-eribulin) through reduced inter-chain disulfide bonds. Preclinical studies demonstrated durable antitumor responses in human cell lines and patient-derived xenograft tumor models, supporting evaluation of this novel ADC for the treatment of patients with FRα-positive tumors.Citation85 Administration of MORAb-202 was associated with complete and partial responses in patients with advanced ovarian cancer enrolled in a first-in-human, phase I study. Leukopenia and neutropenia, observed in approximately half of the patients, were the most frequent treatment-related AEs.Citation86 Further evaluation of MORAb-202 is ongoing in patients with FRα-positive, platinum-resistant ovarian cancer and other selected tumor types in advanced stage (endometrial, non-small-cell lung, and triple-negative breast cancer) ().
Tissue factor (TF)
TF, usually involved as a cofactor in the coagulation process, can be abnormally expressed on the surface of cancer cells in various tumor types and thus provide a potential new target for anticancer therapy.Citation87,Citation88 Tisotumab vedotin is a TF-targeted ADC consisting of a human IgG1 antibody conjugated to monomethyl auristatin E (MMAE) through a protease-cleavable valine citrulline linker.Citation87 Durable tumor responses were achieved with tisotumab vedotin in TF-positive xenograft solid tumor models, including patient-derived xenografts.Citation87
Preliminary findings from a phase I–II study of tisotumab vedotin in patients with advanced solid malignancies, unselected for predefined TF expression levels, showed an ORR of ~16%. The most common AEs were epistaxis, fatigue, nausea, alopecia, and conjunctivitis; grade ≥3 AEs (fatigue, anemia, abdominal pain, and hypokalemia) were reported in ≤10% of patients.Citation87 A randomized, open-label phase II study (innovaTV 208) is evaluating a standard and a dose-dense regimen of tisotumab vedotin in patients with advanced, platinum-resistant ovarian cancer.Citation89
Protein tyrosine kinase 7 (PTK7)
PTK7 is a catalytically inactive receptor tyrosine kinase, involved in the Wnt signaling pathway, which was shown to be enriched in tumor-initiating cells (TICs) in patient tumor xenografts.Citation90 Thus, PTK7 represents a tumor target that can lead to elimination of cancer cells responsible for tumor recurrence and dissemination. The anti-PTK7 ADC PF-06647020 consists of a humanized, monoclonal antibody joined to the auristatin microtubule inhibitor Aur0101 by a cleavable valine-citrulline-based linker. Following internalization and cleavage, auristatin-0101 inhibits tubulin polymerization leading to apoptotic cell death in target cells.Citation90 In preclinical, patient-derived tumor xenograft models, PF-06647020 induced durable responses and showed greater efficacy compared with chemotherapy.Citation90
Results from a phase I study of PF-06647020 demonstrated a manageable safety profile, with a disease control rate of 73% (ORR, 27%) in patients with advanced ovarian cancer.Citation91 The majority of the treatment-related AEs were grade 1–2, including most frequently nausea, alopecia, fatigue, headache, neutropenia, and vomiting.Citation91 Biomarker analysis showed that clinical responses to PF-06647020 correlated with higher baseline PTK7 tumor expression levels.Citation92
Protein kinase-mediated pathways
The WEE1 kinase is a key intracellular checkpoint at the G₂-M transition that mediates arrest of the cell cycle to allow for pre-mitotic DNA repair in the G₂ phase. Differently from normal cells, DNA repair in cancer cells occurs more frequently in G₂ than in G1 arrest, thus conferring tumor selectivity to WEE1 targeting. Inhibition of WEE1 in the presence of DNA damaging agents can result in mitotic catastrophe due to initiation of mitosis with unrepaired lethal DNA damage.Citation93 Consistently, preclinical studies have demonstrated increased cell death, reduced tumor burden, and prolonged survival in experimental animal models following WEE1 inhibition.Citation93
Adavosertib (AZD1775) is a WEE1 tyrosine kinase inhibitor which has demonstrated antitumor activity in a small number of treatment-refractory patients with BRCA-mutated tumors including ovarian cancer.Citation94 Supraventricular tachyarrhythmia and myelosuppression were reported as dose-limiting toxicities, while myelosuppression and diarrhea were common treatment-related AEs.Citation94 Evaluation of adavosertib plus carboplatin in patients with TP53-mutated, platinum-resistant/refractory ovarian cancer showed an ORR of 43% and a median PFS of 5.3 months, with responses lasting more than 31 months in 2 of 24 patients. The most frequent, grade 3–4, treatment-related AEs were thrombocytopenia and neutropenia.Citation95 Further trials are in progress investigating combination treatment with adavosertib and olaparib in a phase II study of patients with ovarian cancer progressing after prior PARP inhibitor therapy, and adavosertib monotherapy in the phase II NCI MATCH screening trial/subprotocol Z1I in patients with BRCA-mutated tumors.Citation96
Alpelisib (BYL719) is a small-molecule, selective inhibitor of the phosphatidylinositol 3-kinase (PI3K) α subunit, approved by the FDA for the treatment of hormone receptor positive, HER2-negative, PIK3CA mutation-positive breast cancer.Citation97 Preclinical studies have shown that PI3K inhibitors may impair homologous recombination repair and sensitize ovarian cancer cells to PARP inhibitors.Citation98 Results from a phase Ib combination study of alpelisib with olaparib, in patients with recurrent ovarian cancer after prior platinum-based therapy, indicated a partial response rate of 36% and stable disease in 50% of patients. Treatment-related grade 3–4 AEs included hyperglycemia, nausea, and increased alanine aminotransferase levels. Hyperglycemia and neutropenic fever occurred as dose-limiting toxicities.Citation98
Ralimetinib mesylate (LY2228820 dimesylate) is a small-molecule inhibitor of p38α and β mitogen-activated protein kinase 1 (MAPK1), a kinase that may facilitate cell survival and resistance to standard treatment by modulation of cytokine production in the tumor microenvironment. Pharmacodynamic studies showed inhibition of p38 MAPK-induced phosphorylation of MAP kinase-activated protein kinase 2 (MAPKAP-K2) by ralimetinib in patient peripheral blood mononuclear cells.Citation99 Preliminary evaluation of ralimetinib in a randomized phase Ib-II study in combination with gemcitabine and carboplatin chemotherapy demonstrated a significant, although limited prolongation in median PFS (10.3 v 7.9 months), but no significant difference in ORR and median OS, in patients with recurrent, platinum-sensitive ovarian cancer. Neutropenia, thrombocytopenia, and anemia were the most frequent grade 3–4 AEs in both treatment arms; grade 3–4 elevations in alanine aminotransferase levels were observed more frequently in patients receiving the triple combination.Citation100
A further, novel kinase inhibitor that has shown activity against ovarian cancer in preclinical and early clinical studies is prexasertib (LY2606368), a selective, ATP-competitive inhibitor of the cell cycle checkpoint kinase 1 and 2 (CHK 1/2), which are expressed at higher levels in cancer cells compared with normal tissues.Citation101,Citation102 Inhibition of CHK 1/2 activity leads to replication catastrophe, thereby inducing cell death and sensitizing cancer cells to the antitumor activity of PARP inhibitors. Prexasertib in combination with olaparib has shown antitumor activity in patient-derived, ovarian cancer xenograft models with acquired resistance to olaparib and in olaparib-sensitive models with an increase in the extent and durability of the antitumor responses observed.Citation101 Initial findings from a phase II study of prexasertib in patients with recurrent, mostly platinum-resistant/refractory, BRCA-non mutated, high-grade ovarian cancer demonstrated a 33% response rate in evaluable patients.Citation102 Neutropenia, leukopenia, and thrombocytopenia were the most frequent grade 3–4 AEs associated with treatment (≥25% of patients). However, grade 4 neutropenia observed in ~79% of cases after first dose administration appeared transient and improved without growth factor therapy.Citation102 Further evaluation of prexasertib is in progress in patients with advanced, BRCA-mutated ovarian cancer.Citation103
Acting in concert with CHKs (i.e. CHK1), the ataxia telangiectasia mutated and Rad3-related (ATR) serine threonine protein kinase contributes to genomic stability by regulating initiation of DNA replication and DNA repair.Citation104 The ATR kinase inhibitor AZD6738 is an ATP-competitive, small-molecule inhibitor of ATR which inhibits phosphorylation of CHK1 Ser345, leading to an impairment in cell cycle progression and cell proliferation.Citation105 Synergistic activity was observed against tumor cells with AZD6738 and DNA-damaging agents (i.e. cisplatin, carboplatin, gemcitabine), ionizing radiation, or PARP inhibitors.Citation106 A phase II study is assessing AZD6738 in combination with olaparib in patients with recurrent platinum-sensitive or platinum-resistant ovarian cancer.
Consistent with its mechanism of action, the selective ATR kinase inhibitor berzosertib (M6620/VX-970/VE-822) has shown enhanced induction of double-strand DNA breaks and antitumor activity in combination with the topoisomerase I inhibitor topotecan in patients with platinum-refractory, solid tumors.Citation107,Citation108 The most frequent treatment-related AEs were hematologic, with grade 3–4 anemia, leukopenia, and neutropenia observed in 19% and thrombocytopenia in 10% of treated patients.Citation108 Preliminary results from a randomized phase II study in platinum-resistant ovarian cancer have recently demonstrated a prolongation in mPFS with berzosertib in combination with gemcitabine versus gemcitabine alone in the overall intent-to-treat population (22.9 v 14.7 weeks, p = .047), which was mainly due to the benefit observed in a subgroup analysis of the patients stratified for platinum-free interval ≤3 months.Citation109 Berzosertib is currently being evaluated in a further, phase I study in triple combination with carboplatin and gemcitabine in recurrent, platinum-sensitive disease ().
Conclusions
The identification of new, effective combination regimens including chemotherapeutic agents, PARP inhibitors, angiogenesis inhibitors and/or other novel, targeted agents may provide therapeutic options that could prove beneficial for a substantial proportion of patients, particularly in the early lines of treatment for advanced disease. Accordingly, based on the findings reported from multiple phase III trials and recent FDA approvals, PARP inhibitors may become a new standard of care for maintenance treatment of newly diagnosed patients with advanced ovarian cancer. Validation of key biomarkers able to predict response to agents in each drug class may further result in improved drug selection at treatment initiation, maintenance phase, or switch to a new therapeutic option in case of tumor resistance and disease progression.
Chemotherapy-free regimens of potentially comparable or greater efficacy, based on various combinations of PARP inhibitors, PD-1/PD-L1 antibodies, and/or VEGFR inhibitors are being actively pursued to reduce the burden of toxicity associated with treatment. The eagerly awaited results from the ongoing phase II–III trials outlined in this review will provide evidence on the feasibility of these new therapeutic approaches and their potential to change the current standards of care for advanced disease. Investigational agents with novel mechanisms of action (i.e. inhibition of key factors in the cell cycle, DNA repair and protein kinase pathways), designed to overcome the limitations imposed by primary and secondary tumor resistance to available therapies, also appear to be opening new, promising avenues for selective targeting of ovarian tumors in concert with other DNA-damaging or targeted agents and PD-1/PD-L1 immune-checkpoint inhibitors.
Acknowledgments
Medical writing was provided by S. Mariani, MD, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Disclosure statement
R. C. Arend disclosed advisory board honoraria from AstraZeneca, Clovis, Pfizer, and Tesaro. A. Jackson-Fisher, I. A. Jacobs, and J. Chou were employees of Pfizer and held stock/stock options in Pfizer at the time of this study. B. J. Monk disclosed consulting honoraria from Aravive, Asymmetric Therapeutics, Boston Biomedical, ChemoCare, ChemoID, Circulogene, Conjupro Biotherapeutics, Eisai, Geistlich, Genmab/Seattle Genetics, Gynecologic Oncology Group Foundation, ImmunoGen, Immunomedics, Incyte, Laekna Health Care, Mateon/Oxigene, Merck, Mersana, Myriad, Nucana, Oncomed, Oncoquest, Oncosec, Perthera, Pfizer, Precision Oncology, Puma, Regeneron, Samumed, Takeda, VBL, and Vigeo; and consulting/speaker honoraria from AstraZeneca, Clovis, Janssen/Johnson & Johnson, Roche/Genentech, and Tesaro/GSK.
Additional information
Funding
References
- Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28(Suppl 8):viii61–5. doi:10.1093/annonc/mdx443.
- American Cancer Society. Cancer facts and figures 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. [Accessed 2020 Nov 8].
- Monk BJ, Randall LM, Grisham RN. The evolving landscape of chemotherapy in newly diagnosed advanced epithelial ovarian cancer. Am Soc Clin Oncol Educ Book. 2019;39(39):e141–151. doi:10.1200/EDBK_239007.
- NCCN. National Comprehensive Cancer Network Guidelines. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. v1.2020. https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Accessed 2020 Nov 8
- Bamberger ES, Perrett CW. Angiogenesis in epithelial ovarian cancer. Mol Pathol. 2002;55(6):348–359. doi:10.1136/mp.55.6.348.
- Ruan G, Ye L, Liu G, An J, Sehouli J, Sun P. The role of bevacizumab in targeted vascular endothelial growth factor therapy for epithelial ovarian cancer: an updated systematic review and meta-analysis. Onc Targets Ther. 2018;11:521–528. doi:10.2147/OTT.S155581.
- Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi:10.1200/JCO.2013.51.4489.
- Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, Kim BG, Fujiwara K, Tewari KS, O’Malley DM, et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18(6):779–791. doi:10.1016/S1470-2045(17)30279-6.
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi:10.1038/nrc2442.
- Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30(32):4026–4034. doi:10.1200/JCO.2012.41.9242.
- Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi:10.1038/nrclinonc.2018.29.
- Spannuth WA, Nick AM, Jennings NB, Armaiz-Pena GN, Mangala LS, Danes CG, Lin YG, Merritt WM, Thaker PH, Kamat AA, et al. Functional significance of VEGFR-2 on ovarian cancer cells. Int J Cancer. 2009;124(5):1045–1053. doi:10.1002/ijc.24028.
- Du Bois A, Kristensen G, Ray-Coquard I, Reuss A, Pignata S, Colombo N, Denison U, Vergote I, Del CJM, Ottevanger P, et al. AGO Study Group led Gynecologic Cancer Intergroup/European Network of Gynaecologic Oncology Trials Groups Intergroup Consortium. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17(1):78–89. doi:10.1016/S1470-2045(15)00366-6.
- Miao M, Deng G, Luo S, Zhou J, Chen L, Yang J, He J, Li J, Yao J, Tan S, et al. A phase II study of apatinib in patients with recurrent epithelial ovarian cancer. Gynecol Oncol. 2018;148(2):286–290. doi:10.1016/j.ygyno.2017.12.013.
- Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae DS, Oaknin A, Ray-Coquard PDM, Provencher DM, Karlan BY, et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(8):799–808. doi:10.1016/S1470-2045(14)70244-X.
- Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae DS, Oaknin A, Ray-Coquard PDM, Provencher DM, Karlan BY, et al. Final results of a phase 3 study of trebananib plus weekly paclitaxel in recurrent ovarian cancer (TRINOVA-1): long-term survival, impact of ascites, and progression-free survival-2. Gynecol Oncol. 2016;143(1):27–34. doi:10.1016/j.ygyno.2016.07.112.
- Marth C, Vergote I, Scambia G, Oberaigner W, Clamp A, Berger R, Kurzeder C, Colombo N, Vuylsteke P, Lorusso D, et al. ENGOT-ov-6/TRINOVA-2: randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer. Eur J Cancer. 2017;70:111–121. doi:10.1016/j.ejca.2016.09.004.
- Vergote I, Scambia G, O’Malley DM, Van Calster B, Park SY, Del Campo JM, Meier W, Bamias A, Colombo N, Wenham RM, et al. TRINOVA-3/ENGOT-ov2/GOG-3001 investigators. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(6):862–876. doi:10.1016/S1470-2045(19)30178-0.
- Konstantinopoulos PA, Matulonis UA. Targeting DNA damage response and repair as a therapeutic strategy for ovarian cancer. Hematol Oncol Clin North Am. 2018;32(6):997–1010. doi:10.1016/j.hoc.2018.07.006.
- Bertelsen B, Tuxen IV, Yde CW, Gabrielaite M, Torp MH, Kinalis S, Oestrup O, Rohrberg K, Spangaard I, Santoni-Rugiu E, et al. High frequency of pathogenic germline variants within homologous recombination repair in patients with advanced cancer. NPJ Genom Med. 2019;4(1):13. doi:10.1038/s41525-019-0087-6.
- Lopes JL, Chaudhry S, Lopes GS, Levin NK, Tainsky MA. FANCM, RAD1, CHEK1 and TP53I3 act as BRCA-like tumor suppressors and are mutated in hereditary ovarian cancer. Cancer Genet. 2019;235:57–64. doi:10.1016/j.cancergen.2019.04.061.
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi:10.1038/nature03445.
- Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355(6330):1152–1158. doi:10.1126/science.aam7344.
- Hoppe MM, Sundar R, Tan DSP, Jeyasekharan AD. Biomarkers for homologous recombination deficiency in cancer. J Natl Cancer Inst. 2018;110(7):704–713. doi:10.1093/jnci/djy085.
- Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, Cruz C, Oaknin A, Kaye SB, de Bono JS. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30(9):1437–1447. doi:10.1093/annonc/mdz192.
- Taylor KN, Eskander RN. PARP inhibitors in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. 2018;13(2):145–158. doi:10.2174/1574892813666171204094822.
- Randall LM, Pothuri B. The genetic prediction of risk for gynecologic cancers. Gynecol Oncol. 2016;141(1):10–16. doi:10.1016/j.ygyno.2016.03.007.
- Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Rendi MH, Thornton A, Norquist BM, Casadei S, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–775. doi:10.1158/1078-0432.CCR-13-2287.
- LaFargue CJ, Dal Molin GZ, Sood AK, Coleman RL. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15–e28. doi:10.1016/S1470-2045(18)30786-1.
- Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, Wahner Hendrickson AE, Azodi M, DiSilvestro P, Oza AM, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):636–648. doi:10.1016/S1470-2045(19)30029-4.
- Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–2505. doi:10.1056/NEJMoa1810858.
- Monk BJ, Mirza MR, Vergote I, Li Y, Malinowska I, Gupta D, Graybill WA, Pothuri B, Gonzalez-Martin A. A prospective evaluation of tolerability of niraparib dosing based upon baseline body weight and platelet count: blinded pooled interim safety data from the ENGOT-OV26/PRIMA study. Gynecol Oncol. 2019;154(suppl 1):3–4. doi:10.1016/j.ygyno.2019.04.018.
- González Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D, Hoskins P, Freyer G, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–2402. doi:10.1056/NEJMoa1910962.
- Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381(25):2403–2415. doi:10.1056/NEJMoa1909707.
- Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N, Mäenpää J, et al. PAOLA-1 Investigators. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381(25):2416–2428. doi:10.1056/NEJMoa1911361.
- George A, Kaye S, Banerjee S. Delivering widespread BRCA testing and PARP inhibition to patients with ovarian cancer. Nat Rev Clin Oncol. 2017;14(5):284–296. doi:10.1038/nrclinonc.2016.191.
- D’Andrea AD. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair (Amst). 2018;71:172–176. doi:10.1016/j.dnarep.2018.08.021.
- Chartron E, Theillet C, Guiu S, Jacot W. Targeting homologous repair deficiency in breast and ovarian cancers: biological pathways, preclinical and clinical data. Crit Rev Oncol Hematol. 2019;133:58–73. doi:10.1016/j.critrevonc.2018.10.012.
- Hodgson DR, Dougherty BA, Lai Z, Fielding A, Grinsted L, Spencer S, O’Connor MJ, Ho TW, Robertson JD, Lanchbury JS, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018;119(11):1401–1409. doi:10.1038/s41416-018-0274-8.
- Jiang X, Li X, Li W, Bai H, Zhang Z. PARP inhibitors in ovarian cancer: sensitivity prediction and resistance mechanisms. J Cell Mol Med. 2019;23(4):2303–2313. doi:10.1111/jcmm.14133.
- Gourley C, Balmaña J, Ledermann JA, Serra V, Dent R, Loibl S, Pujade-Lauraine E, Boulton SJ. Moving from poly (ADP-ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J Clin Oncol. 2019;37(25):2257–2269. doi:10.1200/JCO.18.02050.
- Quigley D, Alumkal JJ, Wyatt AW, Kothari V, Foye A, Lloyd P, Aggarwal R, Kim W, Lu E, Schwartzman J, et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017;7(9):999–1005. doi:10.1158/2159-8290.CD-17-0146.
- Lin KK, Harrell MI, Oza AM, Oaknin A, Ray-Coquard I, Tinker AV, Helman E, Radke MR, Say C, Vo LT, et al. BRCA reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2019;9(2):210–219. doi:10.1158/2159-8290.CD-18-0715.
- Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, Kuiper MJ, Ho GY, Barker H, Jasin M, et al. AOCS Study Group. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7(9):984–998. doi:10.1158/2159-8290.CD-17-0419.
- Drakes ML, Stiff PJ. Regulation of ovarian cancer prognosis by immune cells in the tumor microenvironment. Cancers (Basel). 2018;10(9):pii:E302. doi:10.3390/cancers10090302.
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi:10.1073/pnas.0611533104.
- Drakes ML, Mehrotra S, Aldulescu M, Potkul RK, Liu Y, Grisoli A, Joyce C, O’Brien TE, Stack MS, Stiff PJ. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand-1 (PD-L1) in ovarian cancer. J Ovarian Res. 2018;11(1):43. doi:10.1186/s13048-018-0414-z.
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi:10.1056/NEJMoa020177.
- Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment to T cell activity: a case for synergistic therapies. Cancer Cell. 2017;31(3):311–325. doi:10.1016/j.ccell.2017.02.008.
- Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22(8):1856–1864. doi:10.1158/1078-0432.CCR-15-1849.
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi:10.1200/JCO.2015.62.3397.
- Omatsu K, Hamanishi J, Katsumata N, Nishio S, Sawada K, Takeuchi S, Aoki D, Fujiwara K, Sugiyama T, Konishi I. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant (advanced or recurrent) ovarian cancer: open-label, randomized trial in Japan (NINJA trial). Ann Oncol. 2020;31(suppl. 4):S611. doi:10.1016/j.annonc.2020.08.946.
- Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, Yang P, Ruman J, Matei D. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol. 2019;152(2):243–250. doi:10.1016/j.ygyno.2018.11.017.
- Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase 2 KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi:10.1093/annonc/mdz135.
- Johnson ML, Braiteh F, Grilley-Olson JE, Chou J, Davda J, Forgie A, Li R, Jacobs I, Kazazi F, Hu-Lieskovan S. Assessment of subcutaneous vs intravenous administration of anti-PD-1 antibody PF-06801591 in patients with advanced solid tumors: a phase 1 dose-escalation trial. JAMA Oncol. 2019;5(7):999–1007. doi:10.1001/jamaoncol.2019.0836.
- Infante JR, Braiteh F, Emens LA, Balmanoukian AS, Oaknin A, Wang Y, Liu B, Molinero L, Fasso M, O’Hear C, et al. 2016. Safety, clinical activity and biomarkers of atezolizumab (atezo) in advanced ovarian cancer (OC). Ann Oncol. 27(suppl.6):vi296–vi312. doi:10.1093/annonc/mdw374.
- Liu JF, Gordon M, Veneris J, Braiteh F, Balmanoukian A, Eder JP, Oaknin A, Hamilton E, Wang Y, Sarkar I, et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol Oncol. 2019;154(2):314–322. doi:10.1016/j.ygyno.2019.05.021.
- Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393–401. doi:10.1001/jamaoncol.2018.6258.
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481.
- Kandalaft LE, Odunsi K, Coukos G. Immunotherapy in ovarian cancer: are we there yet? J Clin Oncol. 2019;37(27):2460–2471. doi:10.1200/JCO.19.00508.
- Zhang L, Chen Y, Li F, Bao L, Liu W. Atezolizumab and bevacizumab attenuate cisplatin resistant ovarian cancer cells progression synergistically via suppressing epithelial-mesenchymal transition. Front Immunol. 2019;10:867. doi:10.3389/fimmu.2019.00867.
- Liu JF, Herold C, Luo W, Penson R, Horowitz N, Konstantinopoulos P, Castro C, Curtis J, Matulonis UA, Cannistra S, et al. 2018. A phase 2 trial of combination nivolumab and bevacizumab in recurrent ovarian cancer. Ann Oncol. 29(suppl8):viii332–viii358. doi:10.1093/annonc/mdy285.
- Wang Z, Sun K, Xiao Y, Feng B, Mikule K, Ma X, Feng N, Vellano CP, Federico L, Marszalek JR, et al. Niraparib activates interferon signaling and potentiates anti-PD-1 antibody efficacy in tumor models. Sci Rep. 2019;9(1):1853. doi:10.1038/s41598-019-38534-6.
- Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH, et al. PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res. 2017;23(14):3711–3720. doi:10.1158/1078-0432.CCR-16-3215.
- Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, Baumgartner P, Stevenson BJ, Iseli C, Dangaj D, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018;10(436):436. doi:10.1126/scitranslmed.aao5931.
- Pedersen M, Westergaard MCW, Milne K, Nielsen M, Borch TH, Poulsen LG, Hendel HW, Kennedy M, Briggs G, Ledoux S, et al. Adoptive cell therapy with tumor-infiltrating lymphocytes in patients with metastatic ovarian cancer: a pilot study. Oncoimmunology. 2018;7(12):e1502905. doi:10.1080/2162402X.2018.1502905.
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20):6106–6115. doi:10.1158/1078-0432.CCR-06-1183.
- Zhang Z, Jiang D, Yang H, He Z, Liu X, Qin W, Li L, Wang C, Li Y, Li H, et al. Modified CAR T cells targeting membrane-proximal epitope of mesothelin enhances the antitumor function against large solid tumor. Cell Death Dis. 2019;10(7):476. doi:10.1038/s41419-019-1711-1.
- Yeku OO, Rafiq S, Koneru M, Purdon TJ, Song M, Wang P, Hendrickson R, Brentjens RJ, Spriggs D. MUC16-directed immunotherapy for ovarian cancer. Clin Cancer Res. 2020;26(suppl. 13):IA21.
- Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, Myers T, Taskiran C, Robison K, Maenpaa J, et al. Primary results from IMagyn050/GOG 3015/ENGOT-OV39, a double-blind placebo (pbo)-controlled randomised phase III trial of bevacizumab (bev)-containing therapy ± atezolizumab (atezo) for newly diagnosed stage III/IV ovarian cancer (OC). Ann Oncol. 2020;31(suppl 4):S1161–S1162. doi:10.1016/j.annonc.2020.08.2261.
- Kaplan AR, Gueble SE, Liu Y, Oeck S, Kim H, Yun Z, Glazer PM. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med. 2019;11(492):eaav4508. doi:10.1126/scitranslmed.aav4508.
- Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, Rimel BJ, Buss MK, Nattam SR, Hurteau J, et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol. 2019;30(4):551–557. doi:10.1093/annonc/mdz018.
- Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, Anttila M, Werner TL, Lund B, Lindahl G, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20(10):1409–1419. doi:10.1016/S1470-2045(19)30515-7.
- Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, Tew WP, Cloven NG, Muller C, Bender D, et al. A phase III study comparing single-agent olaparib or the combination of cediranib and olaparib to standard platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer. J Clin Oncol. 2020;38(suppl. 15):6003. doi:10.1200/JCO.2020.38.15_suppl.6003.
- Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, Van Le L, Sachdev JC, Chapman-Davis E, Colon-Otero G, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5(8):1141–1149. doi:10.1001/jamaoncol.2019.1048.
- Lee J, Annunziata CM, Houston N, Kohn EC, Lipkowitz S, Minasian L, Nichols E, Trepel J, Trewhitt K, Zia F, et al. 2018. A phase 2 study of durvalumab, a PD-L1 inhibitor and olaparib in recurrent ovarian cancer (OvCa). Ann Oncol. 29(suppl.8):viii332–viii358. doi:10.1093/annonc/mdy285.
- Zimmer AS, Nichols E, Cimino-Mathews A, Peer C, Cao L, Lee MJ, Kohn EC, Annunziata CM, Lipkowitz S, Trepel JB, et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J Immunother Cancer. 2019;7(1):197. doi:10.1186/s40425-019-0680-3.
- Penson RT, Drew Y, de Jonge MJA, Hong S, Park YH, Wolfer A, Brown J, Ferguson M, Gore ME, Alvarez RH, et al. 2018. MEDIOLA: a Phase I/II trial of olaparib (PARP inhibitor) in combination with durvalumab (anti-PD-L1 antibody) in pts with advanced solid tumours. Ann Oncol. 29(suppl 8):viii133–viii148. doi:10.1093/annonc/mdy279.
- Drew Y, Penson RT, O’Malley DM, Kim J, Zimmermann S, Roxburgh P, Sohn S, Stemmer SM, Bastian S, Ferguson M, et al. Phase II study of olaparib (O) plus durvalumab (D) and bevacizumab (B) (MEDIOLA): initial results in patients (pts) with non-germline BRCA-mutated (non-gBRCAm) platinum sensitive relapsed (PSR) ovarian cancer (OC). Ann Oncol. 2020;31(suppl. 4):S615–S616. doi:10.1016/j.annonc.2020.08.953.
- Moore KN, O’Malley DM, Vergote I, Martin LP, Gonzalez-Martin A, Malek K, Birrer MJ. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. Gynecol Oncol. 2018;151(1):46–52. doi:10.1016/j.ygyno.2018.07.017.
- Moore KN, Vergote I, Oaknin A, Colombo N, Banerjee S, Oza A, Pautier P, Malek K, Birrer MJ. FORWARD I: a Phase III study of mirvetuximab soravtansine versus chemotherapy in platinum-resistant ovarian cancer. Future Oncol. 2018;14(17):1669–1678. doi:10.2217/fon-2017-0646.
- Moore KN, Oza AM, Colombo N, Oaknin A, Scambia G, Lorusso D, Farias-Eisner R, Banerjee S, Murphy CG, Tanyi JL, et al. 2019. FORWARD I (GOG 3011): a Phase III study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), versus chemotherapy in patients (pts) with platinum-resistant ovarian cancer (PROC). Ann Oncol. 30(suppl5):v403–v434. doi:10.1093/annonc/mdz250.
- O’Malley DM, Matulonis UA, Birrer MJ, Castro CM, Gilbert L, Vergote I, Martin LP, Mantia-Smaldone GM, Martin AG, Bratos R, et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. 2020;157(2):379–385. doi:10.1016/j.ygyno.2020.01.037.
- O’Malley DM, Richardson DL, Vergote IB, Gilbert L, Castro C, Provencher D, Matulonis UA, Mantia-Smaldone G, Martin L, Zweidler-Mckay PA, et al. 2020. Mirvetuximab soravtansine (MIRV), a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin (CARBO) and bevacizumab (BEV): final results from a study in patients (pts) with recurrent platinum sensitive ovarian cancer. Ann Oncol. 31(suppl. 4):S626–S627.
- Cheng X, Li J, Tanaka K, Majumder U, Milinichik AZ, Verdi AC, Maddage CJ, Rybinski KA, Fernando S, Fernando D, et al. MORAb-202, an antibody-drug conjugate utilizing humanized anti-human FRα farletuzumab and the microtubule-targeting agent eribulin, has potent antitumor activity. Mol Cancer Ther. 2018;17(12):2665–2675. doi:10.1158/1535-7163.MCT-17-1215.
- Shimizu T, Fujiwara Y, Yonemori K, Koyama T, Shimomura A, Tamura K, Iwasa S, Jun Sato J, Kitano S, Ikezawa H, et al. First-in-human (FIH) phase 1 (Ph1) study of MORAb-202 in patients (pts) with advanced folate receptor alpha (FRA)-positive solid tumors. J Clin Oncol. 2019;37(suppl 15):5544. doi:10.1200/JCO.2019.37.15_suppl.5544.
- de Bono JS, Concin N, Hong DS, Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH, Nielsen D, Windfeld K, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(3):383–393. doi:10.1016/S1470-2045(18)30859-3.
- Theunissen JW, Cai AG, Bhatti MM, Cooper AB, Avery AD, Dorfman R, Guelman S, Levashova Z, Migone TS. Treating tissue factor-positive cancers with antibody-drug conjugates that do not affect blood clotting. Mol Cancer Ther. 2018;17(11):2412–2426. doi:10.1158/1535-7163.MCT-18-0471.
- Mahdi H, Schuster SR, O’Malley DM, McNamara DM, Rangwala RA, Liang S-Y, Jain S, Nicacio L, Chon HS. Phase 2 trial of tisotumab vedotin in platinum-resistant ovarian cancer (innovaTV 208). J Clin Oncol. 2019;37(suppl15):TPS5602. doi:10.1200/JCO.2019.37.15_suppl.TPS5602.
- Damelin M, Bankovich A, Bernstein J, Lucas J, Chen L, Williams S, Park A, Aguilar J, Ernstoff E, Charati M, et al. A PTK7-targeted antibody-drug conjugate reduces tumor-initiating cells and induces sustained tumor regressions. Sci Transl Med. 2017;9(372):372. doi:10.1126/scitranslmed.aag2611.
- Sachdev JC, Maitland ML, Sharma M, Moreno V, Boni V, Kummar S, Stringer-Reasor EM, Forero-Torres A, Lakhani NJ, Gibson B, et al. PF-06647020 (PF-7020), an antibody-drug conjugate (ADC) targeting protein tyrosine kinase 7 (PTK7), in patients (pts) with advanced solid tumors: results of a phase I dose escalation and expansion study. J Clin Oncol. 2018;36(suppl 15):5565. doi:10.1200/JCO.2018.36.15_suppl.5565.
- Jackson-Fisher A, Mehra N, Gianani R, Whalen P, Vizcarra P, Deng S, McComish S, Xin X, Powell EL. Protein tyrosine kinase 7 (PTK7) biomarker analysis in patients (pts) treated with PF-06647020, a PTK7 antibody-drug conjugate (ADC), in a phase I dose expansion study. Cancer Res. 2019;79(suppl 13):4035.
- De Witt Hamer PC, Mir SE, Noske D, Van Noorden CJ, Wurdinger T. WEE1 kinase targeting combined with DNA-damaging cancer therapy catalyzes mitotic catastrophe. Clin Cancer Res. 2011;17(13):4200–4207. doi:10.1158/1078-0432.CCR-10-2537.
- Do K, Wilsker D, Ji J, Zlott J, Freshwater T, Kinders RJ, Collins J, Chen AP, Doroshow JH, Kummar S. Phase I study of single-agent AZD1775 (MK-1775), a Wee1 kinase inhibitor, in patients with refractory solid tumors. J Clin Oncol. 2015;33(30):3409–3415. doi:10.1200/JCO.2014.60.4009.
- Leijen S, van Geel RM, Sonke GS, de Jong D, Rosenberg EH, Marchetti S, Pluim D, van Werkhoven E, Rose S, Lee MA, et al. Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patients with TP53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol. 2016;34:4354–4361. doi:10.1200/JCO.2016.67.5942.
- Hamilton E, Falchook GS, Wang JS, Fu S, Oza A, Karen S, Imedio ER, Kumar S, Ottesen L, Mugundu GM, et al. 2019. Phase Ib study of adavosertib in combination with olaparib in patients with refractory solid tumors: dose escalation. Cancer Res. 79(suppl 13):CT025.
- André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, et al. SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi:10.1056/NEJMoa1813904.
- Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, Mayer EL, O’Cearbhaill RE, Coleman RL, Kochupurakkal B, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019;20(4):570–580. doi:10.1016/S1470-2045(18)30905-7.
- Patnaik A, Haluska P, Tolcher AW, Erlichman C, Papadopoulos KP, Lensing JL, Beeram M, Molina JR, Rasco DW, Arcos RR, et al. A first-in-human phase i study of the oral p38 MAPK inhibitor, ralimetinib (LY2228820 dimesylate), in patients with advanced cancer. Clin Cancer Res. 2016;22(5):1095–1102. doi:10.1158/1078-0432.CCR-15-1718.
- Vergote I, Heitz F, Buderath P, Powell M, Sehouli J, Lee CM, Hamilton A, Fiorica J, Moore KN, Teneriello M, et al. A randomized, double-blind, placebo-controlled phase 1b/2 study of ralimetinib, a p38 MAPK inhibitor, plus gemcitabine and carboplatin versus gemcitabine and carboplatin for women with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol. 2020;156(1):23–31. doi:10.1016/j.ygyno.2019.11.006.
- Parmar K, Kochupurakkal BS, Lazaro JB, Wang ZC, Palakurthi S, Kirschmeier PT, Yang C, Sambel LA, Färkkilä A, Reznichenko E, et al. The CHK1 inhibitor prexasertib exhibits monotherapy activity in high-grade serous ovarian cancer models and sensitizes to PARP inhibition. Clin Cancer Res. 2019;25(20):6127–6140. doi:10.1158/1078-0432.CCR-19-0448.
- Lee JM, Nair J, Zimmer A, Lipkowitz S, Annunziata CM, Merino MJ, Swisher EM, Harrell MI, Trepel JB, Lee MJ, et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol. 2018;19(2):207–215. doi:10.1016/S1470-2045(18)30009-3.
- Angius G, Tomao S, Stati V, Vici P, Bianco V, Tomao F. Prexasertib, a checkpoint kinase inhibitor: from preclinical data to clinical development. Cancer Chemother Pharmacol. 2020;85(1):9–20. doi:10.1007/s00280-019-03950-y.
- Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14(4):397–402.
- Foote KM, Nissink JWM, McGuire T, Turner P, Guichard S, Yates JWT, Lau A, Blades K, Heathcote D, Odedra R, et al. Discovery and characterization of AZD6738, a potent inhibitor of ataxia telangiectasia mutated and Rad3 related (ATR) kinase with application as an anticancer agent. J Med Chem. 2018;61(22):9889–9907. doi:10.1021/acs.jmedchem.8b01187.
- Bradbury A, Hall S, Curtin N, Drew Y. Targeting ATR as cancer therapy: a new era for synthetic lethality and synergistic combinations? Pharmacol Ther. 2020;207:107450. doi:10.1016/j.pharmthera.2019.107450.
- Gorecki L, Andrs M, Rezacova M, Korabecny J. Discovery of ATR kinase inhibitor berzosertib (VX-970, M6620): clinical candidate for cancer therapy. Pharmacol Ther. 2020 Feb 26;210:107518. doi:10.1016/j.pharmthera.2020.107518.
- Thomas A, Redon CE, Sciuto L, Padiernos E, Ji J, Lee MJ, Yuno A, Lee S, Zhang Y, Tran L, et al. Phase I study of ATR inhibitor M6620 in combination with topotecan in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1594–1602. doi:10.1200/JCO.2017.76.6915.
- Konstantinopoulos PA, Wahner Hendrickson AE, Penson RT, Doyle A, Kohn EC, Duska RL, Crispens MA, Olawaiye AB, Winer IS, Barroilhet LM, et al. Randomized phase 2 (RP2) study of ATR inhibitor M6620 in combination with gemcitabine versus gemcitabine alone in platinum-resistant high grade serous ovarian cancer (HGSOC). Ann Oncol. 2019;30(suppl 5):v897. doi:10.1093/annonc/mdz394.057.
