ABSTRACT
The prognosis of hepatocellular carcinoma (HCC), a malignant tumor, is poor. Tumor recurrence and metastasis are the major challenges for the treatment of HCC. Various studies have demonstrated that exosomes, which are loaded with various biomolecules including nucleic acids, lipids, and proteins are involved in the recurrence and metastasis of HCC. Additionally, exosomes mediate various biological processes, such as immune response, cell apoptosis, angiogenesis, thrombosis, autophagy, and intercellular signal transduction. In cancer, exosomes regulate cancer cell differentiation, development, and drug resistance. Circular RNAs, microRNAs, and proteins in the exosomes can serve as early diagnostic and prognostic markers for HCC. As exosomes are characterized by low immunogenicity and high stability in the tissues and circulation, they can be used to deliver the drugs in cancer therapies.
Introduction
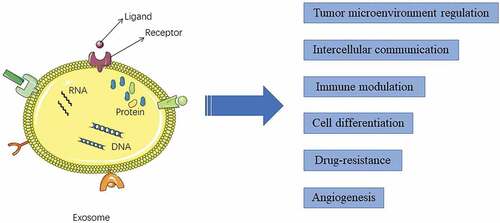
Globally, hepatocellular carcinoma (HCC) is the sixth most common type of cancer and the third leading cause of cancer-related deaths.Citation1 The morbidity rate of HCC is high (more than 20 cases/100,000 individuals) in the East Asia population. Each year, approximately 782,000 new HCC cases and 600,000 HCC-related deaths are reported worldwide.Citation2 Approximately 40% of patients are clinically diagnosed with early-stage liver cancer. Most patients exhibit intrahepatic or distant organ metastasis at diagnosis and are not eligible for radical operation.Citation3 Currently,the primary therapeutic strategies for HCC include surgery, local ablation therapy, and radiation therapy, which have improved the clinical outcomes of patients with the 10-year survival rate reaching approximately 25%.Citation4 The rapid advances in traditional therapy have not resulted in decreased incidences of recurrence and metastasis of HCC. The prevention of HCC metastasis is a major objective in cancer treatment. Cellular signaling is involved in cancer progression, including drug resistance, metastasis, and recurrence.Citation5 Therefore, there is a need to identify the correlation between intercellular communication and cancer progression and to identify the underlying mechanisms and potential therapeutic targets for metastasis in HCC. Exosomes, which are produced and secreted by various cells (including tumor cells), are involved in several physiological and pathological processes in vivo, including intercellular communication, immune system function, cell differentiation, drug resistance and angiogenesis (). Additionally, exosomes are involved in tumor development.Citation6 This review summarizes the recent recent studies on the role of exosomes in cancer and discusses the correlation between HCC and exosomes.
Figure 1. Functions of exosomes in hepatocellular carcinoma development. Exosomes, which harbor proteins, mRNAs, microRNAs, long non-coding RNAs, circular RNAs, and DNAs, are involved in tumor microenvironment regulation, intercellular communication, immune modulation, cell differentiation, drug-resistance, and angiogenesis
Overview of the exosomes
Johnstone et al.Citation7 first reported that vesicles with lipid bilayers were released during the culture of sheep reticulocytes in vitro. Exosomes are detected in the body fluids, such as the serum, milk, semen, and malignant fluid.Citation8 Recently, the International Society for Extracellular Vesicles defined extracellular vesicles (EVs) as the generic term for particles naturally released from the cells that are delimited by lipid bilayers and cannot replicate. There has been a marked increase in the number of studies on the physiological and pathological functions of EVs, a collective term encompassing various subtypes of membranous structures released from the cells, such as microparticles, microvesicles, ectosomes, apoptotic bodies, oncosomes, and exosomes.Citation9 Currently, there is no consensus on the classification of EVs. Some studies have suggested that EV subtypes must be classified based on the physical characteristics(such as size or density), biochemical composition(such as CD63+ EVs), descriptions of conditions, or cell of origin.Citation9 Traditionally, EVs can be divided into the following three subgroups based on their diameter: exosomes (30–100 nm), apoptotic bodies (50–200 nm), and microbubbles (100–1000 nm).Citation10 EVs exhibit a particulate structure and contain biomolecules, such as nucleic acids, proteins, lipids.Citation11 The secreted EVs can be taken up by the recipient cells through endocytosis, phagocytosis, macropinocytosis, or membrane fusion. The contents of EVs are then released into the cells where they exhibit various functions.Citation12 Exosomes, which are a subtype of EVs, are derived from multivesicular bodys(MVBs) of the endosomal bodies and special areas of the cell membrane called endosomal domains.Citation12–14 MVBs can fuse with the plasma membrane and the exosomes are released into the extracellular space.Citation14 The secretion of exosomes is reported to be dependent on the the calcium concentration and pH value.Citation10 In tumor cells (such as HCC cells), the secretion of exosomes is dependent on the GTPase (Rab) family proteins, such as Rab35.Citation15 Zou et al.Citation16 demonstrated that the secretion of exosome was regulated by mechanistic target of rapamycin complex 1(mTORC1) and was dependent on the amino acid changes and growth factor conditions. These findings indicate that exosomes are secreted through multiple pathways. Proteomic analysis has revealed that CD63, TSG101, and flotillin are exosome markers.Citation10 Exosomes function as an intercellular messenger during cell differentiation and cancer development. Additionally, exosomes contain various biomolecules, such as nucleic acids and proteins,Citation17 and are involved in complex biological functions (). Some studies have reported that exosomes are involved in various biological processes (such as inflammatory immune response, apoptosis, angiogenesis, thrombosis, and autophagy), as well as in the occurrence, development, and metastasis of tumors.Citation18–20 These findings suggest that exosomes may serve a pivotal role in intercellular signaling. Recent studies have highlighted the role of exosomes in the tumor microenvironment (TME), which is critical for the occurrence, development, invasion, and metastasis of HCC.Citation21 Previous studies have confirmed that tumor cells release a large number of exosomes during tumorigenesis and that exosome-mediated intercellular signaling can potentially regulate the TME, which affects the progression of tumor.Citation22 Additionally, exosomes are associated with various pathological conditions, such as neurological disorders, drug addiction, and collagen diseases.Citation20,Citation23 Thus, exosomes may exert therapeutic effects. However, the underlying mechanisms involved in the therapeutic effects of exosomes have not been elucidated.
Table 1. Overview of the roles of exosomal contents in HCC.
Exosomal RNAs as a diagnostic marker and a therapeutic target for HCC
As the early diagnosis of HCC is challenging, there are ongoing efforts to develop noninvasive diagnostic methods for HCC. The advances in sequencing technology have enabled the elucidation of the role of exosomes in cancers.Citation24 Previous studies have indicated that exosomes mediate the transportation of proteins, DNAs and various RNAs (such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and messenger RNAs (mRNAs)) in the cells, which promotes the development of HCC.Citation25,Citation26 Thus, the contents of the exosomes can be utilized for the early diagnosis and follow-up of patients with cancer. The exosomal proteins, RNAs and DNAs may be potential diagnostic markers for HCC. Additionally, exosomes can be potential therapeutic targets for HCC.Citation23 This section summarizes the functions of RNAs from HCC-derived exosomes ().
Figure 2. Roles of RNAs from hepatocellular carcinoma (HCC)-derived exosomes. Exosomes mediate the transport of various RNAs. Exosomes-associated miRNAs, such as miR-221, miR-222, and miR-224 can be potential diagnostic biomarkers for HCC. miR-155, lncR-HULC, and circR-DB promote HCC progression and invasion, whereas miR-194 and miR-424-5p suppress HCC growth and invasion. Exosome-associated RNAs, such as miR-1247-3p, which are transferred from the donor cells, can regulate stromal cells, such as cancer-related fibroblasts. The secretion of cytokines, such as chemokine and interleukins from the exosomes promotes HCC growth and invasion, including angiogenesis. miR-32-5p, lncRNA-RoR, and lncRNA-VLDLR are involved in the development of drug-resistance.Citation131Citation132Citation133Citation134Citation135
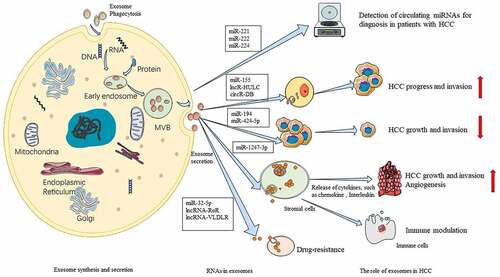
Non-coding RNAs, which are abundant in exosomes, are involved in all stages of tumor development.Citation27 miRNAs can regulate various target genes through their short sequences that are not completely complementary. Therefore, miRNAs may regulate tumor progression, including proliferation, metabolism, and apoptosis.Citation28 For example, miR-194 inhibits the growth and migration of primary liver cancer cells by suppressing the Wnt/β-catenin signaling pathway via the downregulation of polyclonal inhibitory complex 1 (PRC1).Citation29 Sun et al.Citation30 demonstrated that miR-155 was enriched in the exosomes released by HCC cells. These miR-155-containing exosomes were taken up by other HCC cells, which resulted in enhanced proliferation. In addition, exosomal miR-155 can directly bind to the 3ʹ-untranslated region of PTEN (tumor suppressor), which results in the downregulation of target genes in the recipient liver cells. Animal experiments have demonstrated that the exosomes enriched with miR-155 promoted the development of HCC xenotransplants. The expression of miRNAs markedly varied between the benign tumors or non-tumorous cells and the cancer cells. Compared with those in patients with chronic hepatitis B (CHB), the serum levels of miR-18a, miR-221, miR-222, and miR-224 were significantly higher, while those of miR-101, miR-106b, miR-122, and miR-195 were markedly lower in patients with HCC. This suggested that these miRNAs may act as biomarkers for HCC.Citation31 Compared with the biomarkers detected in the conventional specimens, exosomes are highly stable. The plasma levels of exosomal tumor-specific molecules (such as miR-718) in patients with HCC are significantly higher than those in healthy control.Citation32 These studies suggest that the miRNA in the exosomes can be detected using noninvasive methods, which can aid in the early diagnosis of cancer, especially the diagnosis of HCC.Citation136Citation137Citation138Citation139Citation140
LncRNAs are also involved in the pathogenesis of HCC. Yang et al.Citation15 reported that lncRNA HOTAIR regulated the secretion of exosomes from the liver tumor cells. HOTAIR promotes the release of exosomes via inducing the transport of MVBs to the plasma membrane and regulating the expression and localization of RAB35. The expression level of lncRNA HULC in serum exosomes and liver cancer tissues is correlated with the tumor TNM stage. The overexpression of HULC promotes the growth and invasion and inhibits apoptosis of HCC cells. Notably, HULC promotes the secretion of exosomes from the HCC cells.Citation33 Conigliaro et al.Citation34 demonstrated that lncRNA H19 in CD90+ HCC modulates the TME balance via promoting angiogenesis. These findings illustrate the importance of the lncRNAs in HCC and provide novel insights into the molecular mechanisms involved in the secretion of exosomes from HCC cells. Therefore, lncRNAs may serve as therapeutic target for HCC.Citation141Citation142Citation143Citation144Citation145Citation146
Recent studies have reported that circRNAs can serve as a diagnostic biomarker and a therapeutic target for cancer. CircRNAs are endogenous non-coding RNAs without 5′ to 3′ polarity and contain a covalent closed-loop structure of polyadenylated tail.Citation35 Previous studies have reported that circRNAs can stably bind to miRNAs and regulate gene expression.Citation36 The analysis of the correlation between exosomes and circRNAs may aid in understanding the biological functions of exosomal circRNAs. However, the analysis of exosomal circRNAs is a double-edged sword as exosomes containing circRNAs can transfer biological information to the target cells but also contribute to the clearance of circRNAs.Citation37 At present, the exosomal circRNAs are detected in various cancer cell lines, including the liver, lung, gastric, and breast cancer cell lines.Citation38,Citation39 The differentially expressed circRNA genes promote the tumor-related signaling pathways, suggesting that circRNAs are associated with the occurrence and development of tumors.Citation40 For example, cSMARCA5 (a circRNA derived from exons 15 and 16 of the SMARCA5 gene) binds miR-17-3p and inhibits the proliferation and migration of HCC by promoting TIMP3 expression.Citation41 Huang et al.Citation42 proved that the overexpression of circRNA-100338 activates mTOR signaling pathway in HCC and is correlated with poor prognosis. Zhang et al.Citation43 found that the exosomes secreted by adipocytes contain a circRNA named circ-deubiquitination(circ-DB), which promoted HCC growth and decreased DNA damage via the suppression of miR-34a and the activation of deubiquitination-related USP7. These studies suggest that circRNAs are correlated with the progression and metastasis of HCC. However, the clinical application of exosomal circRNAs is associated with several challenges. circRNAs cannot be easily detected in the exosomes due to their low abundance and complex structures. Thus, the precise evaluation of circRNA expression and function is challenging.Citation37 Future studies must investigate exosomal RNAs in cancer and other diseases to elucidate their functions. Although exosomal RNA function and mechanisms have not been completely elucidated, they can serve as potential biomarkers and novel therapeutic targets for HCC owing to their applicability, specificity, and accessibility.
Compared with those on non-coding RNAs, studies on exosomal mRNAs in tumors are limited. miRNAs are stable in biological fluids. In contrast, mRNAs that are not enclosed within exosomes undergo degradation in the biological fluids.Citation44 However, exosomal mRNAs have a critical role in tumors. A chimeric mRNA called GOLM1-NAA35 is detected in the salivary exosomes of patients with esophageal squamous cell carcinoma (ESCC). The levels of chimeric GOLM1-NAA35 mRNA in salivary exosomes served as a noninvasive biomarker for ESCC detection, postoperative surveillance, therapeutic response, and tumor recurrence.Citation44 The expression of PCA3 mRNA, a typical biomarkers of prostate cancer, is significantly upregulated in PSMA-positive (PSMA: prostate-specific membrane antigen) exosomes.Citation45 Xu et al.Citation46 examined the serum levels of exosomal heterogeneous nuclear ribonucleoprotein H1 (hnRNPH1), a type of RNA-binding protein and splicing factor essential for the development of HCC mRNA in the HCC group were significantly higher than those in the liver cirrhosis, chronic hepatitis B, and healthy control groups. Patients with upregulated exosomal hnRNPH1 mRNA levels exhibited poorer overall survival than those with downregulated hnRNPH1 mRNA levels. Additionally, the expression level of exosomal hnRNPH1 mRNA is correlated with the Child-Pugh classification, portal vein tumor emboli, lymph node metastasis, and TNM stage in patients with HCC.Citation46 These findings suggest that the serum level of exosomal hnRNPH1 mRNA is a prognostic biomarker for HCC. Sasaki et al.Citation47 reported that the copy number of hepcidin mRNA variant was significantly upregulated in the serum exosomes of patients with HCC. This suggested that exosomal hepcidin mRNA may serve as a novel diagnostic biomarker for HCC. These studies offered novel insights into the potential applications of exosomal mRNAs for cancer surveillance and early diagnosis. However, further prospective studies are needed to elucidate the role of exosomal mRNAs.
Role of exosomes in the formation of HCC microenvironment
Approximately 70% of HCC cases exhibit recurrence and metastasis within 5 years after surgery or radiofrequency ablation.Citation48 The mechanisms underlying HCC progression or metastasis must be elucidated to prevent recurrence and metastasis of HCC. The complex TME is critical for various cellular processes, such as maintenance of cell surface structure and adhesion, angiogenesis, cell migration, epithelial-to-stromal transition (EMT), matrix remodeling, and immune regulation.Citation49,Citation50
Matrix cells (such as fibroblasts, macrophages, and T cells), extracellular matrix (ECM; comprising inflammatory cytokines, chemokines, and matrix metalloproteinases), and exosomes constitute the TME, which has a critical role in cancer initiation and progression.Citation51 For example, cancer-related fibroblasts (CAFs) regulate the inflammatory microenvironment, promote lung metastasis of HCC, and induce tumor occurrence, EMT, and chemotherapy resistance.Citation52 The dysfunction of immune cells provides a microenvironment for immunosuppression in tumor cells, which leads to immune tolerance and escape.Citation53 The loss of physiological balance in the microenvironment results in disrupted cell behavior and tumor development. Exosomes are an important component of the TME that transduce signals between cells.Citation54 The exosome-mediated activation of toll-like receptor 3 (TLR3) in hepatic stellate cells (HSCs) exacerbates liver fibrosis via enhancing IL-17A production.Citation55 Wang et al.Citation56 demonstrated that 14-3-3ζ inhibited the anti-tumor functions of tumor-infiltrating T cells in the HCC microenvironment and that 14-3-3ζ can be partially transferred from HCC cells to T cells through the exosomes. These findings suggest exosomes indirectly affect the progression of HCC by influencing the composition of the TME.
Recently, exosomes were reported to mediate anti-tumor immune response and immune escape of tumor cell.Citation57 Macrophages, which are abundant in the liver, are involved in the innate immune response. In response to tumor-derived stimuli, macrophages can be polarized into the classical (M1) or alternative (M2) phenotypes. M1 macrophages exhibit anti-tumor activity, whereas M2 macrophages exhibit pro-tumorigenic activity.Citation58 The exosomes derived from HCC cells promote macrophage activation and M2 polarization, which enables tumors to evade immune surveillance.Citation59 LncRNA TUC339, which is upregulated in HCC-derived exosomes, is transferred among HCC cells and promotes HCC growth and metastasis. Furthermore, exosomal lncRNA TUC339 can be transferred to neighboring macrophages where it regulate the M1/M2 polarization and inhibits the anti-tumor immune response in vitro.Citation60 HCC-derived exosomal miR-23a-3p upregulates PD-L1 expression in the macrophages via the STAT3 signaling pathway, which attenuates the anti-HCC immune response in vitro and in vivo.Citation61 The exosomes derived from melatonin-treated HCC cells mitigate the immunosuppressive status by downregulating PD-L1 expression on macrophages in vitro and in vivo.Citation59 Dendritic Cells (DCs) are also involved in initiating innate and adaptive immune responses. Yu et al.Citation62 demonstrated that tumor-derived exosomes promoted immunosuppression by inhibiting DC differentiation and maturation through the IL6-STAT3 signaling pathway. In contrast, Rao et al.Citation63 found that HCC tumor cell-derived exosomes exhibiting various HCC antigens, elicit a strong immune response by activating DCs. The activated DCs increased T lymphocytes and interferon-γ levels and decreased tumor growth factor-β levels in the HCC TME. Another studyCitation64 investigating exosomes from HCC antigen-expressing DCs in different HCC mouse models reported that alpha fetoprotein-enriched DCs-derived exosomes could trigger potent antigen-specific anti-tumor immune responses and remodel the TME in HCC mice. Thus, these exosomes can be a potential immunotherapeutic target for HCC. Natural killer (NK) cells, a critical component of the TME, can be inhibited by exosomal circUHRF1, which results in immunosuppression. Exosomal circUHRF1 can confer resistance to anti-PD1 immunotherapy in patients with HCC. Thus, exosomal circUHRF1 can be a potential therapeutic target for HCC.Citation65 HCC-derived exosomes harbor various non-coding RNAs and proteins that mediate immunoregulation. Therefore, exosomes may serve as prospective diagnostic biomarkers and therapeutic targets for HCC.
EMT is a reversible process of dedifferentiation in which epithelial cells lose the epithelial characteristics (such as polarity and cell–cell junctions) and acquire the typical mesenchymal characteristics (such as increased migratory and invasive abilities).Citation66 However, most tumor cells do not undergo a complete EMT. In these cases, tumor cells acquire partial characteristics of mesenchymal cells with some epithelial characteristics. Moreover, partial EMT (p-EMT) can drive distinct migratory properties and enhance the epithelial-mesenchymal plasticity of cancer cells and cell fate plasticity.Citation67 Yu et al.Citation68 suggested that key p-EMT-related genes (P4HA2, ITGA5, MMP9, MT1X, and SPP1) could serve as prognostic biomarkers and therapeutic targets for HCC. Recent studies have demonstrated the role of exosomes in EMT progression in different types of cancer, including HCC.Citation69 Chen et al.Citation70 revealed that HCC-derived exosomes promoted HCC progression and recurrence by activating EMT through the MAPK/ERK signaling pathway. Rab27a, a small GTPase, regulates exosome secretion by mediating multivesicular endosome docking at the plasma membrane. Zhang et al.Citation71 showed that exosomes derived from CAFs transfered miR-320a to the HCC cells and inhibited EMT. The loss of miR-320a in the CAF-derived exosomes promoted EMT and HCC metastasis. In addition, HCC-derived exosomes transport miR-92-3p to the recipient cells and consequently promote EMT and the conversion of low-metastatic HCC cells into high-metastatic HCC cells via the regulation of the PTEN/Akt pathway. Thus, these exosomes are a biomarker of poor prognosis in patients with HCC.Citation72 HCC cell-derived exosomal miR-21 and miR-10b, which are overexpressed in the acidic microenvironment, promote HCC cell proliferation and metastasis by facilitating the EMT process.Citation73 These findings indicate that EMT and exosomes disrupt the homeostatic balance of the TME. Thus the analysis of EMT and exosomes may contribute to the identification of novel therapeutic targets and prognostic markers, and the development of novel treatment strategies for HCC.
HCC, which is highly vascularized, can secrete exosomes with various ncRNAs and cytokines that promote angiogenesis.Citation42,Citation74 For example, Li et al.Citation75 demonstrated that HCC-derived exosomes promote angiogenesis in the human umbilical vein endothelial cells (HUVECs) by transferring lysyl oxidase-like 4 (LOXL4) through a paracrine mechanism. HCC-derived exosomes harboring angiopoietin-2 (ANGPT2) are endocytosed by HUVECs, which promotes angiogenesis via the Tie2-independent pathway (Tie2: angiopoietin receptor). Additionally, the knockdown of ANGPT2 significantly inhibited angiogenesis.Citation76 However, not all genes and their products exhibiting upregulated expression in the exosomes promote angiogenesis. One study suggested that exosomal miR-200b-3p from HCC suppressed the expression of endothelial transcription factor ERG (erythroblast transformation-specific (ETS)-related gene). The downregulation of miR-200b-3p in HCC cells promoted angiogenesis through the upregulation of endothelial ERG expression.Citation77 These studies provide insights into the novel pathways that may be targeted to increase the efficacy of anti-angiogenic therapies.
Chemoresistance and exosome-related drug-resistance of HCC
Traditional chemotherapeutic strategies are associated with poor prognosis, low efficacy, and increased side effects, which can be attributed due to the nonspecific therapeutic targeting and rapid development of multidrug resistance (MDR).Citation78 MDR is induced in the tumor cells via various mechanisms, including gene mutations, DNA methylation, histone modifications, disrupted membrane transporters, changes in anti-cancer drug targets, and intracellular metabolism of drugs, to escape the cytotoxic effects of chemotherapy drugs.Citation79,Citation80 Genetic heterogeneity among cancer cells is the basis of adaptation to the therapeutic interventions with the most resistant cells surviving against the selective pressure.Citation81 Peitzsch et al.Citation82 indicated that cancer stem cells (CSCs) may serve an important role in tumor drug resistance. Liver CSCs (LCSCs), which are primary stem cells derived from liver cancer, undergo self-renewal and differentiation. Thus, LCSCs contribute to the recurrence and metastasis of HCC. Additionally, LCSCs are resistant to conventional radiotherapy and chemotherapy.Citation83 Yu et al.Citation84 analyzed clinical liver tumor samples and highlighted the importance of the SDC1-PI3K/AKT signaling in cisplatin resistance. Ding et al.Citation85 confirms that CCND1, a protooncogene, is involved in fluorouracil (5-FU) resistance in the hepatoma cell lines. The silencing of CCND1 increases the sensitivity of HCC to 5-FU and inhibits the expression of the DNA repair protein RAD51. This suggested that MDR is regulated at various levels, including genomics, proteomics and TME levels and that the modulation of a single factor cannot completely mitigate drug resistance. Therefore, combined therapy must be used to overcome drug resistance.
Exosomes are involved in conferring resistance to anti-tumor drugs. However, the role of exosomes in inducing drug resistance has not been completely elucidated.Citation86 Current studies suggest that exosomes induce drug resistance through the delivery of cargos from drug-resistant cancer cells to the recipient drug-sensitive cells, which enhances the proliferation, survival, migration, and drug resistance of drug-sensitive cells. Alternatively, exosomes may phagocytose drug molecules and expel them out of cells, which reduces the drug concentration in the cells.Citation87,Citation88 The tumor-derived exosomes are rich in miRNAs, which are associated with the drug resistance phenotype. Some molecules (such as ncRNA) loaded in the exosome interact with molecular receptors in cells and modulate their drug resistance phenotypes.Citation79 Fu et al.Citation89 revealed that exosomes deliver miR-325p from resistant cells to sensitive cells, which results in the activation of the PI3K/Akt pathway. Additionally, these exosomes induce MDR by modulating angiogenesis and EMT. Exosomal miR-199a-3p(Exo-miR-199a-3p), which represses the invasion of cancer cells and stimulate cancer cell apoptosis, was isolated from the HCC cells. Animal experiments revealed that the upregulation of miR-199a-3p mitigated the resistance of HCC to cisplatin and delayed tumor growth in vivo.Citation90 Zhang et al.Citation65 showed that exosomal circUHRF1, which is predominantly secreted by the HCC cells, promotes immunosuppression via inducing NK cell dysfunction. Additionally, circUHRF1 may promote resistance to anti-PD1(Programmed death 1) immunotherapy. These studies indicate that the regulation of exosomal ncRNAs can be a potential therapeutic strategy for HCC.
Exosomes may clear drugs and metabolites from the tumor cells and consequently mitigate the therapeutic effect of drugs through various transporters.Citation91 Meena et al.Citation92 indicated that paclitaxel promoted exosome releasing from HCC cells, which conferred drug resistance to ajacent cells as the exosomes transported the drug out of the cells. This process was partly mediated by the ATP-binding cassette (ABC) transporter family. The ABC transporter family, which contributes to MDR in cancer cells, is subdivided into seven subfamilies (A to G).Citation93 The relatively active proteins include ABC subfamily B member 1 (ABCB1, also known as MDR1 and P-glycoprotein or P-gp), ABC subfamily C member 1 (ABCC1, also known as multidrug resistance-associated protein 1), and ABC subfamily G member 2 (ABCG2, also known as breast cancer resistance protein [BRCP]).Citation94 The exosomal delivery of ABCB1, which is the most studied transporter, confers chemoresistance in HCC. In particular, ABCB1 expression is upregulated in HCC cells that are resistant to paclitaxel, epirubicin, and doxorubicin.Citation91 The other efflux pumps ABCB2 and ABCA3 also contribute to chemoresistance by transferring exosomal drugs.Citation94 This is a potential novel mechanism of chemoresistance in cancer cells. Thus, exosome-mediated drug transportation must be considered to overcome MDR.
Exosomes and HCC targeting therapy
The identification of drug targets can aid in the development of targeted therapy to improve the efficacy of therapeutics and reduce the toxic and side effects. However, the development of targeted therapy for tumors is challenging.Citation95 Recently, several potential molecular targets (such as PD-1/PD-L1, cytotoxic T lymphocyte antigen 4 (CTLA-4), vascular endothelial growth factor (VEGF) pathway, tumor suppressor p53, skin cell adhesion molecule (EpCAM), and Wnt/β-Catenin) and therapeutics (such as nivolumab, pembrolizumab, and lenvatinib) have been widely used for the clinical treatment of HCC.Citation95–97 However, most target molecules for HCC exhibit poor clinical performance. Approximately 25% of HCC cases exhibit resistance to the currently used drugs.Citation98 Thus, there is a need to develop methods to accurately deliver anti-cancer drugs to the tumor and specially kill the cancer cells.
EVs can be a potential drug delivery system to mitigate the side effects of chemotherapy and enhance treatment effect.Citation99 Zhang et al.Citation100 presented the challenges of EV-based drug delivery, including the selection and production of vesicles and cargos and the methods to load the cargo into the vesicles, modify the vesicle surface, and prolong the half-life of vesicles in the circulation. The membrane permeability, biocompatibility, and nontoxic immunogenicity of exosomes can be an advantage for transferring drugs, proteins, and nucleic acids.Citation101 Exosomes are characterized by low immunogenicity and high stability in the tissues and circulation. Thus, exosomes may be a better drug delivery vehicle in cancer therapies than previously reported compounds, such as liposomes.Citation102 For example, Hood et al.Citation103 loaded superparamagnetic iron oxide nanoparticles (SPIONs) in exosomes through electroporation to obtain exosomes loaded with 5 nm iron nanoparticles, which can be used for diagnosis or treatment. To achieve the targeted delivery to the tumor cells and improve the applicability of treatment, the exosomal surface was modified. One studyCitation104 transfected EV-producing cells with vectors encoding anti-epidermal growth factor receptor nanobodies, which served as targeting ligands for tumor cells, fused to glycosylphosphatidylinositol (GPI) anchor signal peptides. EVs were isolated using ultrafiltration/size-exclusion liquid chromatography. The analysis of EV-tumor cell interaction revealed that nanobodies can be anchored on the surface of EVs via GPI, which alters their cell-targeting behavior. Tian et al.Citation105 engineered immature DCs (imDCs) to express a well-characterized exosomal membrane protein (Lamp2b) fused to αv integrin-specific iRGD peptide (CRGDKGPDC) to facilitate tumor targeting. The purified exosomes from imDCs were loaded with Dox via electroporation with an encapsulation efficiency of up to 20%. iRGD exosomes were highly efficient for the targeted delivery Dox to αv integrin-positive breast cancer cells in vitro and in vivo. These studies demonstrate the potential of exosomes for anti-tumor drug delivery. However, there are limited studies on exosome-based drug delivery to liver cancer. Future studies must examine exosomal chemotaxis to lay the foundation for the application of exosome-directed drug delivery to the liver, which can improve tumor characterization and optimize personalized treatment for patients with HCC.
Conclusions and future perspectives
Immunotherapies, such as nivolumab, pembrolizumab, and ipilimumab, have been used for the clinical treatment of cancers.Citation106 However, the objective response rate of anti-PD1 immunotherapy is only 15%~20%.Citation107 The unique immune response in the liver is reported to promote drug tolerance, which is a major challenge for the application of conventional immunotherapy in patients with HCC.Citation108 The liver, which is a major immunological organ, is exposed to antigen-enriched blood from the gut via the portal vein.Citation109 Therefore, the uninflamed liver provides a tolerogenic microenvironment and suppresses both innate and adaptive immunity under homeostasic conditions to prevent prolonged inflammation and tissue damage.Citation110 This underscores the need to develope novel therapeutic strategies for HCC. Exosomes, which serve a pivotal role in intercellular commnication and TME, are a prospective therapeutic target for HCC. The cargos, including functional proteins, RNAs, and anti-tumor drugs, in the exosomes can serve as diagnostic markers and regulate various physiological and pathological processes.Citation65,Citation111 Recent studies have improved our understanding of the role of exosomes in cancers, such as gastrointestinal and liver cancers.Citation112 Additionally, exosomes can serve as a potential therapeutic target for cancer. Exosomes can also be potentially used as drug carriers for cancer treatments.Citation113 The modification of the exosomal membrane can increase the chemotaxis of exosomes to specific lesions.Citation114,Citation115 Thus, exosomes can deliver anti-tumor drugs directly and effectively to the HCC tissues and prevent the progression of HCC. However, further studies are needed for the clinical application of exosomes for the diagnosis and treatment of HCC.
Nores on contributors
DX wrote and reviewed the manuscript. DX, YL and JH designed the figures and table. YP and HT critically revised the manuscript. DX, YP and HT are responsible for confirming the authenticity of the data. All authors read and approved the final version of the manuscript.
Acknowledgments
Not applicable.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Dong G, Zhang S, Shen S, Sun L, Wang X, Wang H, Wu J, Liu T, Wang C, Wang H, et al. 2020. Spats2, negatively regulated by mir-145-5p, promotes hepatocellular carcinoma progression through regulating cell cycle. Cell Death Dis. 11(10):837. doi:10.1038/s41419-020-03039-y.
- Ozakyol A. 2017. Global epidemiology of hepatocellular carcinoma (hcc epidemiology). J Gastrointest Cancer. 48(3):238–240. doi:10.1007/s12029-017-9959-0.
- Qin Z, Xiang C, Zhong F, Liu Y, Dong Q, Li K, Shi W, Ding C, Qin L, He F. 2019. Transketolase (tkt) activity and nuclear localization promote hepatocellular carcinoma in a metabolic and a non-metabolic manner. J Exp Clin Cancer Res. 38(1):154. doi:10.1186/s13046-019-1131-1.
- Vibert E, Schwartz M, Olthoff KM. 2020. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 72(2):262–276. doi:10.1016/j.jhep.2019.11.017.
- Liang B, Hu X, Ding Y, Liu M. 2020. Tumor-derived exosomes in the pd-1/pd-l1 axis: significant regulators as well as promising clinical targets. J Cell Physiol. doi:10.1002/jcp.30197.
- Gougelet A. 2018. Exosomal micrornas as a potential therapeutic strategy in hepatocellular carcinoma. World J Hepatol. 10(11):785–789. doi:10.4254/wjh.v10.i11.785.
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. doi:10.1016/S0021-9258(18)48095-7.
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. 2012. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 1820(7):940–948. doi:10.1016/j.bbagen.2012.03.017.
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (misev2018): a position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J Extracell Vesicles. 7(1):1535750. doi:10.1080/20013078.2018.1535750.
- Li C, Hou X, Zhang P, Li J, Liu X, Wang Y, Guan Q, Zhou Y. 2020. Exosome-based tumor therapy: opportunities and challenges. Curr Drug Metab. 21(5):339–351. doi:10.2174/1389200221666200515103354.
- Shehzad A, Islam SU, Shahzad R, Khan S, Lee YS. 2021. Extracellular vesicles in cancer diagnostics and therapeutics. Pharmacol Ther. 223:107806. doi:10.1016/j.pharmthera.2021.107806.
- Takasugi M. 2018. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 17(2):e12734. doi:10.1111/acel.12734.
- Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. 2018. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 188:1–11. doi:10.1016/j.pharmthera.2018.02.013.
- Jabalee J, Towle R, Garnis C. 2018. The role of extracellular vesicles in cancer: cargo, function, and therapeutic implications. Cells. 7(8):93. doi:10.3390/cells7080093.
- Yang L, Peng X, Li Y, Zhang X, Ma Y, Wu C, Fan Q, Wei S, Li H, Liu J. 2019. Long non-coding rna hotair promotes exosome secretion by regulating rab35 and snap23 in hepatocellular carcinoma. Mol Cancer. 18(1):78. doi:10.1186/s12943-019-0990-6.
- Zou W, Lai M, Zhang Y, Zheng L, Xing Z, Li T, Zou Z, Song Q, Zhao X, Xia L, et al. 2019. Exosome release is regulated by mtorc1. Adv Sci (Weinh). 6(3):1801313. doi:10.1002/advs.201801313.
- Lal CV, Olave N, Travers C, Rezonzew G, Dolma K, Simpson A, Halloran B, Aghai Z, Das P, Sharma N, et al. Exosomal microrna predicts and protects against severe bronchopulmonary dysplasia in extremely premature infants. JCI Insight. 2018;3(5). doi:10.1172/jci.insight.93994.
- Anel A, Gallego-Lleyda A, De Miguel D, Naval J, Martinez-Lostao L. 2019. Role of exosomes in the regulation of t-cell mediated immune responses and in autoimmune disease. Cells. 8(2):154. doi:10.3390/cells8020154.
- Almeida VH, Rondon AMR, Gomes T, Monteiro RQ. 2019. Novel aspects of extracellular vesicles as mediators of cancer-associated thrombosis. Cells. 8(7):716. doi:10.3390/cells8070716.
- Rao PSS, O’Connell K, Finnerty TK. 2018. Potential role of extracellular vesicles in the pathophysiology of drug addiction. Mol Neurobiol. 55(8):6906–6913. doi:10.1007/s12035-018-0912-4.
- Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. 2016. Exosomes derived from hcc cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 35(1):159. doi:10.1186/s13046-016-0430-z.
- Srivastava A, Amreddy N, Pareek V, Chinnappan M, Ahmed R, Mehta M, Razaq M, Munshi A, Ramesh R. 2020. Progress in extracellular vesicle biology and their application in cancer medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 12(4):e1621. doi:10.1002/wnan.1621.
- Sasaki R, Kanda T, Yokosuka O, Kato N, Matsuoka S, Moriyama M. 2019. Exosomes and hepatocellular carcinoma: from bench to bedside. Int J Mol Sci. 20(6):1406. doi:10.3390/ijms20061406.
- Aparicio S, Mardis E. 2014. Tumor heterogeneity: next-generation sequencing enhances the view from the pathologist’s microscope. Genome Biol. 15(9):463. doi:10.1186/s13059-014-0463-6.
- Kohama I, Kosaka N, Chikuda H, Ochiya T. 2019. An insight into the roles of micrornas and exosomes in sarcoma. Cancers (Basel). 11(3):428. doi:10.3390/cancers11030428.
- Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. 2019. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 18(1):75. doi:10.1186/s12943-019-0991-5.
- Ji Q, Zhang C, Sun X, Li Q. 2018. Circular rnas function as competing endogenous rnas in multiple types of cancer. Oncol Lett. 15(1):23–30. doi:10.3892/ol.2017.7348.
- Tutar L, Ozgur A, Tutar Y. 2018. Involvement of mirnas and pseudogenes in cancer. Methods Mol Biol. 1699:45–66. doi:10.1007/978-1-4939-7435-1_3.
- Tang H, Zhao H, Yu Z-Y, Feng X, Fu B-S, Qiu C-H, Zhang J-W. 2019. MicroRNA-194 inhibits cell invasion and migration in hepatocellular carcinoma through PRC1-mediated inhibition of Wnt/β-catenin signaling pathway. Dig Liver Dis. 51(9):1314–1322. doi:10.1016/j.dld.2019.02.012.
- Sun JF, Zhang D, Gao CJ, Zhang YW, Dai QS. 2019. Exosome-mediated mir-155 transfer contributes to hepatocellular carcinoma cell proliferation by targeting pten. Med Sci Monit Basic Res. 25:218–228. doi:10.12659/MSMBR.918134.
- Sohn W, Kim J, Kang SH, Yang SR, Cho J-Y, Cho HC, Shim SG, Paik Y-H. 2015. Serum exosomal micrornas as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 47(9):e184. doi:10.1038/emm.2015.68.
- Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, Shinden Y, Iguchi T, Eguchi H, Shirabe K, et al. 2015. Identification of a bona fide microrna biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 112(3):532–538. doi:10.1038/bjc.2014.621.
- Cao SQ, Zheng H, Sun BC, Wang ZL, Liu T, Guo DH, Shen ZY. 2019. Long non-coding rna highly up-regulated in liver cancer promotes exosome secretion. World J Gastroenterol. 25(35):5283–5299. doi:10.3748/wjg.v25.i35.5283.
- Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, et al. 2015. Cd90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing h19 lncrna. Mol Cancer. 14(1):155. doi:10.1186/s12943-015-0426-x.
- Wilusz JE, Sharp PA. 2013. Molecular biology. A circuitous route to noncoding rna. Sci. 340(6131):440–441. doi:10.1126/science.1238522.
- Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. 2013. Natural rna circles function as efficient microrna sponges. Nat. 495(7441):384–388. doi:10.1038/nature11993.
- Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, Wang G, Wu P, Wang H, Jiang L, et al. 2019. Exosomal circrnas: biogenesis, effect and application in human diseases. Mol Cancer. 18(1):116. doi:10.1186/s12943-019-1041-z.
- Chen LL, Yang L. 2015. Regulation of circrna biogenesis. RNA Biol. 12(4):381–388. doi:10.1080/15476286.2015.1020271.
- Wang G, Liu W, Zou Y, Wang G, Deng Y, Luo J, Zhang Y, Li H, Zhang Q, Yang Y, et al. 2019. Three isoforms of exosomal circPTGR1 promote hepatocellular carcinoma metastasis via the miR449a–MET pathway. EBioMedicine. 40:432–445. doi:10.1016/j.ebiom.2018.12.062.
- Ahmed I, Karedath T, Andrews SS, Al-Azwani IK, Mohamoud YA, Querleu D, Rafii A, Malek JA. 2016. Altered expression pattern of circular rnas in primary and metastatic sites of epithelial ovarian carcinoma. Oncotarget. 7(24):36366–36381. doi:10.18632/oncotarget.8917.
- Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma JZ, Sun SH, Yang F, Zhou WP. 2018. Circular rna csmarca5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 68(6):1214–1227. doi:10.1016/j.jhep.2018.01.012.
- Huang X-Y, Huang Z-L, Huang J, Xu B, Huang X-Y, Xu Y-H, Zhou J, Tang Z-Y. 2020. Exosomal circrna-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J Exp Clin Cancer Res. 39(1):20. doi:10.1186/s13046-020-1529-9.
- Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, et al. 2019. Exosome circrna secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related usp7. Oncogene. 38(15):2844–2859. doi:10.1038/s41388-018-0619-z.
- Lin Y, Dong H, Deng W, Lin W, Li K, Xiong X, Guo Y, Zhou F, Ma C, Chen Y, et al. 2019. Evaluation of salivary exosomal chimeric golm1-naa35 rna as a potential biomarker in esophageal carcinoma. Clin Cancer Res. 25(10):3035–3045. doi:10.1158/1078-0432.CCR-18-3169.
- Li P, Yu X, Han W, Kong Y, Bao W, Zhang J, Zhang W, Gu Y. 2019. Ultrasensitive and reversible nanoplatform of urinary exosomes for prostate cancer diagnosis. ACS Sensors. 4(5):1433–1441. doi:10.1021/acssensors.9b00621.
- Xu H, Dong X, Chen Y, Wang X. 2018. Serum exosomal hnrnph1 mrna as a novel marker for hepatocellular carcinoma. Clin Chem Lab Med. 56(3):479–484. doi:10.1515/cclm-2017-0327.
- Sasaki K, Kohgo Y, Ohtake T. 2019. Splicing variant of hepcidin mrna. Vitam Horm. 110:131–141. doi:10.1016/bs.vh.2019.01.006.
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. 2016. Hepatocellular carcinoma. Nat Rev Dis Primers. 2:16018. doi:10.1038/nrdp.2016.18.
- Lambert AW, Pattabiraman DR, Weinberg RA. 2017. Emerging biological principles of metastasis. Cell. 168(4):670–691. doi:10.1016/j.cell.2016.11.037.
- Yang L-Y, Luo Q, Lu L, Zhu -W-W, Sun H-T, Wei R, Lin Z-F, Wang X-Y, Wang C-Q, Lu M, et al. 2020. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 13(1):3. doi:10.1186/s13045-019-0836-0.
- Leonardi GC, Candido S, Cervello M, Nicolosi D, Raiti F, Travali S, Spandidos DA, Libra M. 2012. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol. 40(6):1733–1747. doi:10.3892/ijo.2012.1408.
- Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, et al. 2018. Tumor-derived exosomal mir-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 9(1):191. doi:10.1038/s41467-017-02583-0.
- Zamarron BF, Chen W. 2011. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 7(5):651–658. doi:10.7150/ijbs.7.651.
- Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, Forshew T, Appella E, Gorgoulis VG, Harris CC. 2018. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal mir-1246. Nat Commun. 9(1):771. doi:10.1038/s41467-018-03224-w.
- Seo W, Eun HS, Kim SY, Yi H-S, Lee Y-S, Park S-H, Jang M-J, Jo E, Kim SC, Han Y-M, et al. 2016. Exosome-mediated activation of toll-like receptor 3 in stellate cells stimulates interleukin-17 production by γδ T cells in liver fibrosis. Hepatology. 64(2):616–631. doi:10.1002/hep.28644.
- Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, Jin K, Zhuo H, Zhang Z, Wang J, et al. 2018. 14-3-3zeta delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating t lymphocytes. Cell Death Dis. 9(2):159. doi:10.1038/s41419-017-0180-7.
- Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, Zhou F. 2020. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol. 17(4):323–334. doi:10.1038/s41423-020-0391-1.
- Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, Luo X, Chen R, Chen T. 2018. Long non-coding rna cox-2 prevents immune evasion and metastasis of hepatocellular carcinoma by altering m1/m2 macrophage polarization. J Cell Biochem. 119(3):2951–2963. doi:10.1002/jcb.26509.
- Cheng L, Liu J, Liu Q, Liu Y, Fan L, Wang F, Yu H, Li Y, Bu L, Li X, et al. 2017. Exosomes from melatonin treated hepatocellularcarcinoma cells alter the immunosupression status through stat3 pathway in macrophages. Int J Biol Sci. 13(6):723–734. doi:10.7150/ijbs.19642.
- Li X, Lei Y, Wu M, Li N. Regulation of macrophage activation and polarization by hcc-derived exosomal lncrna tuc339. Int J Mol Sci. 2018;19(10). doi:10.3390/ijms19102958.
- Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, Wang F, Li X, Liu Q, Li Y, et al. 2019. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology. 70(1):241–258. doi:10.1002/hep.30607.
- Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, et al. 2007. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 178(11):6867–6875. doi:10.4049/jimmunol.178.11.6867.
- Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. 2016. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 64(2):456–472. doi:10.1002/hep.28549.
- Lu Z, Zuo B, Jing R, Gao X, Rao Q, Liu Z, Qi H, Guo H, Yin H. 2017. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 67(4):739–748. doi:10.1016/j.jhep.2017.05.019.
- Zhang P-F, Gao C, Huang X-Y, Lu J-C, Guo X-J, Shi G-M, Cai J-B, Ke A-W. 2020. Cancer cell-derived exosomal circuhrf1 induces natural killer cell exhaustion and may cause resistance to anti-pd1 therapy in hepatocellular carcinoma. Mol Cancer. 19(1):110. doi:10.1186/s12943-020-01222-5.
- Tian X, Zhou D, Chen L, Tian Y, Zhong B, Cao Y, Dong Q, Zhou M, Yan J, Wang Y, et al. 2018. Polo-like kinase 4 mediates epithelial-mesenchymal transition in neuroblastoma via pi3k/akt signaling pathway. Cell Death Dis. 9(2):54. doi:10.1038/s41419-017-0088-2.
- Aiello NM, Kang Y. 2019. Context-dependent emt programs in cancer metastasis. J Exp Med. 216(5):1016–1026. doi:10.1084/jem.20181827.
- Lei Y, Yan W, Lin Z, Liu J, Tian D, Han P. 2020. Comprehensive analysis of partial epithelial mesenchymal transition-related genes in hepatocellular carcinoma. J Cell Mol Med. doi:10.1111/jcmm.16099.
- Li S, Liu Y, Bai Y, Chen M, Cheng D, Wu M, Xia J. 2020. Rhof promotes hepatocellular carcinoma metastasis by altering the metabolic status of cancer cells via rab3d. Hepatology. doi:10.1002/hep.31641.
- Chen L, Guo P, He Y, Chen Z, Chen L, Luo Y, Qi L, Liu Y, Wu Q, Cui Y, et al. 2018. Hcc-derived exosomes elicit hcc progression and recurrence by epithelial-mesenchymal transition through mapk/erk signalling pathway. Cell Death Dis. 9(5):513. doi:10.1038/s41419-018-0534-9.
- Zhang Z, Li X, Sun W, Yue S, Yang J, Li J, Ma B, Wang J, Yang X, Pu M, et al. 2017. Loss of exosomal mir-320a from cancer-associated fibroblasts contributes to hcc proliferation and metastasis. Cancer Lett. 397:33–42. doi:10.1016/j.canlet.2017.03.004.
- Yang B, Feng X, Liu H, Tong R, Wu J, Li C, Yu H, Chen Y, Cheng Q, Chen J, et al. 2020. High-metastatic cancer cells derived exosomal mir92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating pten/akt pathway in hepatocellular carcinoma. Oncogene. 39(42):6529–6543. doi:10.1038/s41388-020-01450-5.
- Tian XP, Wang CY, Jin XH, Li M, Wang FW, Huang WJ, Yun JP, Xu RH, Cai QQ, Xie D. 2019. Acidic microenvironment up-regulates exosomal mir-21 and mir-10b in early-stage hepatocellular carcinoma to promote cancer cell proliferation and metastasis. Theranostics. 9(7):1965–1979. doi:10.7150/thno.30958.
- Lin X-J, Fang J-H, Yang X-J, Zhang C, Yuan Y, Zheng L, Zhuang S-M. 2018. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids. 11:243–252. doi:10.1016/j.omtn.2018.02.014.
- Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, Yang X, Fang F, Xia Q, Zhang Z, et al. 2019. Exosome-mediated secretion of loxl4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 18(1):18. doi:10.1186/s12943-019-0948-8.
- Xie JY, Wei JX, Lv LH, Han QF, Yang WB, Li GL, Wang PX, Wu SB, Duan JX, Zhuo WF, et al. 2020. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal. 18(1):46. doi:10.1186/s12964-020-00535-8.
- Moh-Moh-Aung A, Fujisawa M, Ito S, Katayama H, Ohara T, Ota Y, Yoshimura T, Matsukawa A. 2020. Decreased mir-200b-3p in cancer cells leads to angiogenesis in hcc by enhancing endothelial erg expression. Sci Rep. 10(1):10418. doi:10.1038/s41598-020-67425-4.
- Xiao L, Hou Y, He H, Cheng S, Hou Y, Jin H, Song X, Nie G, Hou Y. 2020. A novel targeted delivery system for drug-resistant hepatocellular carcinoma therapy. Nanoscale. 12(32):17029–17044. doi:10.1039/d0nr01908a.
- Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K, Sun B, Chen B, Xiao Z. 2020. Engineered exosomes for targeted co-delivery of mir-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 18(1):10. doi:10.1186/s12951-019-0563-2.
- Kachalaki S, Ebrahimi M, Mohamed Khosroshahi L, Mohammadinejad S, Baradaran B. 2016. Cancer chemoresistance; biochemical and molecular aspects: a brief overview. Eur J Pharm Sci. 89:20–30. doi:10.1016/j.ejps.2016.03.025.
- Bagrodia S, Smeal T, Abraham RT. 2012. Mechanisms of intrinsic and acquired resistance to kinase-targeted therapies. Pigment Cell Melanoma Res. 25(6):819–831. doi:10.1111/pcmr.12007.
- Peitzsch C, Tyutyunnykova A, Pantel K, Dubrovska A. 2017. Cancer stem cells: the root of tumor recurrence and metastases. Semin Cancer Biol. 44:10–24. doi:10.1016/j.semcancer.2017.02.011.
- Nio K, Yamashita T, Kaneko S. 2017. The evolving concept of liver cancer stem cells. Mol Cancer. 16(1):4. doi:10.1186/s12943-016-0572-9.
- Yu L, Xu H, Zhang S, Chen J, Yu Z. 2020. Sdc1 promotes cisplatin resistance in hepatic carcinoma cells via pi3k-akt pathway. Hum Cell. 33(3):721–729. doi:10.1007/s13577-020-00362-6.
- Ding H, Wang Y, Zhang H. 2020. Ccnd1 silencing suppresses liver cancer stem cell differentiation and overcomes 5-fluorouracil resistance in hepatocellular carcinoma. J Pharmacol Sci. 143(3):219–225. doi:10.1016/j.jphs.2020.04.006.
- Wu Q, Zhou L, Lv D, Zhu X, Tang H. 2019. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 12(1):53. doi:10.1186/s13045-019-0739-0.
- Wang J, Yeung BZ, Cui M, Peer CJ, Lu Z, Figg WD, Guillaume Wientjes M, Woo S, Au JL. 2017. Exosome is a mechanism of intercellular drug transfer: application of quantitative pharmacology. J Control Release. 268:147–158. doi:10.1016/j.jconrel.2017.10.020.
- Sharma A. 2017. Chemoresistance in cancer cells: exosomes as potential regulators of therapeutic tumor heterogeneity. Nanomedicine (Lond). 12(17):2137–2148. doi:10.2217/nnm-2017-0184.
- Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, Yang Y, Wang S, Wang J, et al. 2018. Exosomal microrna-32-5p induces multidrug resistance in hepatocellular carcinoma via the pi3k/akt pathway. J Exp Clin Cancer Res. 37(1):52. doi:10.1186/s13046-018-0677-7.
- Zhang K, Shao CX, Zhu JD, Lv XL, Tu CY, Jiang C, Shang MJ. Exosomes function as nanoparticles to transfer mir-199a-3p to reverse chemoresistance to cisplatin in hepatocellular carcinoma. Biosci Rep. 2020;40(7). doi:10.1042/BSR20194026.
- Jena BC, Mandal M. 2021. The emerging roles of exosomes in anti-cancer drug resistance and tumor progression: an insight towards tumor-microenvironment interaction. Biochim Biophys Acta Rev Cancer. 1875(1):188488. doi:10.1016/j.bbcan.2020.188488.
- Meena AS, Sharma A, Kumari R, Mohammad N, Singh SV, Bhat MK. 2013. Inherent and acquired resistance to paclitaxel in hepatocellular carcinoma: molecular events involved. PLoS One. 8(4):e61524. doi:10.1371/journal.pone.0061524.
- Khamisipour G, Jadidi-Niaragh F, Jahromi AS, Zandi K, Hojjat-Farsangi M. 2016. Mechanisms of tumor cell resistance to the current targeted-therapy agents. Tumour Biol. 37(8):10021–10039. doi:10.1007/s13277-016-5059-1.
- Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. 2018. Revisiting the role of abc transporters in multidrug-resistant cancer. Nat Rev Cancer. 18(7):452–464. doi:10.1038/s41568-018-0005-8.
- Huang M, He M, Guo Y, Li H, Shen S, Xie Y, Li X, Xiao H, Fang L, Li D, et al. 2020. The influence of immune heterogeneity on the effectiveness of immune checkpoint inhibitors in multifocal hepatocellular carcinomas. Clin Cancer Res. 26(18):4947–4957. doi:10.1158/1078-0432.CCR-19-3840.
- Llovet JM, Montal R, Sia D, Finn RS. 2018. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 15(10):599–616. doi:10.1038/s41571-018-0073-4.
- El Dika I, Makki I, Abou-Alfa GK. Hepatocellular carcinoma, novel therapies on the horizon. Chin Clin Oncol. 2021;10(1). doi:10.21037/cco-20-113.
- Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, et al. 2015. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 47(5):505–511. doi:10.1038/ng.3252.
- Sun N, Lee YT, Zhang RY, Kao R, Teng PC, Yang Y, Yang P, Wang JJ, Smalley M, Chen PJ, et al. 2020. Purification of hcc-specific extracellular vesicles on nanosubstrates for early hcc detection by digital scoring. Nat Commun. 11(1):4489. doi:10.1038/s41467-020-18311-0.
- Zhang YF, Shi JB, Li C. 2019. Small extracellular vesicle loading systems in cancer therapy: current status and the way forward. Cytotherapy. 21(11):1122–1136. doi:10.1016/j.jcyt.2019.10.002.
- Kooijmans SA, Vader P, Van Dommelen SM, Van Solinge WW, Schiffelers RM. 2012. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine. 7:1525–1541. doi:10.2147/IJN.S29661.
- Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, et al. 2015. Applying extracellular vesicles based therapeutics in clinical trials - an isev position paper. J Extracell Vesicles. 4(1):30087. doi:10.3402/jev.v4.30087.
- Hood JL, Scott MJ, Wickline SA. 2014. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 448:41–49. doi:10.1016/j.ab.2013.12.001.
- Kooijmans SA, Aleza CG, Roffler SR, Van Solinge WW, Vader P, Schiffelers RM. 2016. Display of gpi-anchored anti-egfr nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 5(1):31053. doi:10.3402/jev.v5.31053.
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. 2014. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 35(7):2383–2390. doi:10.1016/j.biomaterials.2013.11.083.
- Ruf B, Heinrich B, Greten TF. 2020. Immunobiology and immunotherapy of hcc: spotlight on innate and innate-like immune cells. Cell Mol Immunol. doi:10.1038/s41423-020-00572-w.
- Zhang Q, Chen Y, Bai X, Liang T. 2020. Immune checkpoint blockade therapy for hepatocellular carcinoma: clinical challenges and considerations. Front Oncol. 10:590058. doi:10.3389/fonc.2020.590058.
- Longo V, Gnoni A, Casadei Gardini A, Pisconti S, Licchetta A, Scartozzi M, Memeo R, Palmieri VO, Aprile G, Santini D, et al. 2017. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget. 8(20):33897–33910. doi:10.18632/oncotarget.15406.
- Heymann F, Tacke F. 2016. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 13(2):88–110. doi:10.1038/nrgastro.2015.200.
- Bottcher JP, Knolle PA, Stabenow D. 2011. Mechanisms balancing tolerance and immunity in the liver. Dig Dis. 29(4):384–390. doi:10.1159/000329801.
- Tkach M, Thery C. 2016. Communication by extracellular vesicles: where we are and where we need to go. Cell. 164(6):1226–1232. doi:10.1016/j.cell.2016.01.043.
- Colombo M, Raposo G, Thery C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 30(1):255–289. doi:10.1146/annurev-cellbio-101512-122326.
- Slomka A, Mocan T, Wang B, Nenu I, Urban SK, Gonzales-Carmona M, Igh S-W, Lukacs-Kornek V, Strassburg CP, Sparchez Z, et al. 2020. Evs as potential new therapeutic tool/target in gastrointestinal cancer and hcc. Cancers (Basel). 12(10):3019. doi:10.3390/cancers12103019.
- Ciullo A, Biemmi V, Milano G, Bolis S, Cervio E, Fertig ET, Gherghiceanu M, Moccetti T, Camici GG, Vassalli G, et al. 2019. Exosomal expression of cxcr4 targets cardioprotective vesicles to myocardial infarction and improves outcome after systemic administration. Int J Mol Sci. 20(3):468. doi:10.3390/ijms20030468.
- Khan N, Maurya S, Bammidi S, Jayandharan GR. 2020. Aav6 vexosomes mediate robust suicide gene delivery in a murine model of hepatocellular carcinoma. Mol Ther Methods Clin Dev. 17:497–504. doi:10.1016/j.omtm.2020.03.006.
- Fu Q, Zhang Q, Lou Y, Yang J, Nie G, Chen Q, Chen Y, Zhang J, Wang J, Wei T, et al. 2018. Primary tumor-derived exosomes facilitate metastasis by regulating adhesion of circulating tumor cells via smad3 in liver cancer. Oncogene. 37(47):6105–6118. doi:10.1038/s41388-018-0391-0.
- Zhang J, Lu S, Zhou Y, Meng K, Chen Z, Cui Y, Shi Y, Wang T, He QY. 2017. Motile hepatocellular carcinoma cells preferentially secret sugar metabolism regulatory proteins via exosomes. Proteomics. 17:13–14. doi:10.1002/pmic.201700103.
- He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, Ngai SM, Chan TF, Wong N. 2015. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and rnas. Carcinogenesis. 36(9):1008–1018. doi:10.1093/carcin/bgv081.
- Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature. 527(7578):329–335. doi:10.1038/nature15756.
- Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, Wang L, Li L, Wang B, Shen J, et al. 2018. Horizontal transfer of exosomal cxcr4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 676:101–109. doi:10.1016/j.gene.2018.07.018.
- Wang S, Xu M, Li X, Su X, Xiao X, Keating A, Zhao RC. 2018. Exosomes released by hepatocarcinoma cells endow adipocytes with tumor-promoting properties. J Hematol Oncol. 11(1):82. doi:10.1186/s13045-018-0625-1.
- Gai X, Tang B, Liu F, Wu Y, Wang F, Jing Y, Huang F, Jin D, Wang L, Zhang H. 2019. Mtor/ mir-145-regulated exosomal golm1 promotes hepatocellular carcinoma through augmented gsk-3beta/mmps. J Genet Genomics. 46(5):235–245. doi:10.1016/j.jgg.2019.03.013.
- Huang A, Dong J, Li S, Wang C, Ding H, Li H, Su X, Ge X, Sun L, Bai C, et al. 2015. Exosomal transfer of vasorin expressed in hepatocellular carcinoma cells promotes migration of human umbilical vein endothelial cells. Int J Biol Sci. 11(8):961–969. doi:10.7150/ijbs.11943.
- Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, Zhang T, Cao Y, Pan H, Zhang L, et al. 2018. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J Immunother Cancer. 6(1):145. doi:10.1186/s40425-018-0451-6.
- Alzahrani FA, El-Magd MA, Abdelfattah-Hassan A, Saleh AA, Saadeldin IM, El-Shetry ES, Badawy AA, Alkarim S. 2018. Potential effect of exosomes derived from cancer stem cells and mscs on progression of den-induced hcc in rats. Stem Cells Int. 2018:8058979. doi:10.1155/2018/8058979.
- Zhao S, Li J, Zhang G, Wang Q, Wu C, Zhang Q, Wang H, Sun P, Xiang R, Yang S. 2019. Exosomal mir-451a functions as a tumor suppressor in hepatocellular carcinoma by targeting lpin1. Cell Physiol Biochem. 53(1):19–35. doi:10.33594/000000118.
- Wang G, Zhao W, Wang H, Qiu G, Jiang Z, Wei G, Li X. 2019. Exosomal mir-744 inhibits proliferation and sorafenib chemoresistance in hepatocellular carcinoma by targeting pax2. Med Sci Monit. 25:7209–7217. doi:10.12659/MSM.919219.
- Cui Y, Xu HF, Liu MY, Xu YJ, He JC, Zhou Y, Cang SD. 2019. Mechanism of exosomal microrna-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J Gastroenterol. 25(15):1890–1898. doi:10.3748/wjg.v25.i15.1890.
- Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, Shi X. 2018. Hepatocellular carcinoma-derived exosomal mirna-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 37(1):324. doi:10.1186/s13046-018-0965-2.
- Qu Z, Wu J, Wu J, Ji A, Qiang G, Jiang Y, Jiang C, Ding Y. 2017. Exosomal mir-665 as a novel minimally invasive biomarker for hepatocellular carcinoma diagnosis and prognosis. Oncotarget. 8(46):80666–80678. doi:10.18632/oncotarget.20881.
- Yugawa K, Yoshizumi T, Mano Y, Itoh S, Harada N, Ikegami T, Kohashi K, Oda Y, Mori M. 2021. Cancer-associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR-150-3p. Eur J Surg Oncol. 47(2):384–393. doi:10.1016/j.ejso.2020.08.002.
- Tang J, Li Y, Liu K, Zhu Q, Yang WH, Xiong LK, Guo DL. 2018. Exosomal mir-9-3p suppresses hbgf-5 expression and is a functional biomarker in hepatocellular carcinoma. Minerva Med. 109(1):15–23. doi:10.23736/S0026-4806.17.05167-9.
- Fang J-H, Zhang Z-J, Shang L-R, Luo Y-W, Lin Y-F, Yuan Y, Zhuang S-M. 2018. Hepatoma cell-secreted exosomal microrna-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology. 68(4):1459–1475. doi:10.1002/hep.29920.
- Xiong L, Zhen S, Yu Q, Gong Z. 2017. Hcv-e2 inhibits hepatocellular carcinoma metastasis by stimulating mast cells to secrete exosomal shuttle micrornas. Oncol Lett. 14(2):2141–2146. doi:10.3892/ol.2017.6433.
- Han Q, Zhao H, Jiang Y, Yin C, Zhang J. 2019. Hcc-derived exosomes: critical player and target for cancer immune escape. Cells. 8(6):6. doi:10.3390/cells8060558.
- Xue X, Wang X, Zhao Y, Hu R, Qin L. 2018. Exosomal mir-93 promotes proliferation and invasion in hepatocellular carcinoma by directly inhibiting timp2/tp53inp1/cdkn1a. Biochem Biophys Res Commun. 502(4):515–521. doi:10.1016/j.bbrc.2018.05.208.
- Nakano T, Chen IH, Wang CC, Chen PJ, Tseng HP, Huang KT, Hu TH, Li LC, Goto S, Cheng YF. 2019. et al. Circulating exosomal mir-92b: its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am J Transplant. 19(12):3250–3262. doi:10.1111/ajt.15490.
- Yu Y, Min Z, Zhihang Z, Linhong M, Tao R, Yan L, Song H. 2019. Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via mir-1273f transfer. Exp Cell Res. 385(1):111649. doi:10.1016/j.yexcr.2019.111649.
- Liu H, Chen W, Zhi X, Chen EJ, Wei T, Zhang J, Shen J, Hu LQ, Zhao B, Feng XH, et al. 2018. Tumor-derived exosomes promote tumor self-seeding in hepatocellular carcinoma by transferring mirna-25-5p to enhance cell motility. Oncogene. 37(36):4964–4978. doi:10.1038/s41388-018-0309-x.
- Wang D, Xing N, Yang T, Liu J, Zhao H, He J, Ai Y, Yang J. 2020. Exosomal lncrna h19 promotes the progression of hepatocellular carcinoma treated with propofol via mir-520a-3p/limk1 axis. Cancer Med. 9(19):7218–7230. doi:10.1002/cam4.3313.
- Yao Z, Jia C, Tai Y, Liang H, Zhong Z, Xiong Z, Deng M, Zhang Q. 2020. Serum exosomal long noncoding rnas lnc-fam72d-3 and lnc-epc1-4 as diagnostic biomarkers for hepatocellular carcinoma. Aging (Albany NY). 12(12):11843–11863. doi:10.18632/aging.103355.
- Li Y, Zhang X, Zheng Q, Zhang Y, Ma Y, Zhu C, Yang L, Peng X, Wang Q, Wang B, et al. 2020. Yap1 inhibition in huvecs is associated with released exosomes and increased hepatocarcinoma invasion and metastasis. Mol Ther Nucleic Acids. 21:86–97. doi:10.1016/j.omtn.2020.05.021.
- Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al. 2014. A long noncoding rna activated by tgf-beta promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 25(5):666–681. doi:10.1016/j.ccr.2014.03.010.
- Su Y, Lv X, Yin W, Zhou L, Hu Y, Zhou A, Qi F. 2019. CircRNA Cdr1as functions as a competitive endogenous RNA to promote hepatocellular carcinoma progression. Aging (Albany NY). 11(19):8182–8203. doi:10.18632/aging.102312.
- Chen W, Quan Y, Fan S, Wang H, Liang J, Huang L, Chen L, Liu Q, He P, Ye Y. 2020. Exosome-transmitted circular rna hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 475:119–128. doi:10.1016/j.canlet.2020.01.022.
- Lai Z, Wei T, Li Q, Wang X, Zhang Y, Zhang S. 2020. Exosomal circfblim1 promotes hepatocellular carcinoma progression and glycolysis by regulating the mir-338/lrp6 axis. Cancer Biother Radiopharm. doi:10.1089/cbr.2020.3564.
