ABSTRACT
Evidence suggests that Tripartite Motif Containing 11 (TRIM11) has pro-tumor activity in human non-small cell lung cancer (NSCLC). However, the roles and underlying mechanisms of TRIM11 in NSCLC have not yet been fully elucidated. In this work, human lung cancer cell lines (A549, H446, and H1975) were transfected with siRNA or lentiviruses to knockdown or overexpress TRIM11 and dual-specificity phosphatase 6 (DUSP6). The cell tumor response was assessed by determining the rate of proliferation, apoptosis, the uptake of 2-[N-(7-nitrobenz-2-oxa-1, 3-diaxol-4-yl) amino]-2-deoxyglucose (2-NBDG), and the secretion of lactic acid (LD). Dominant-negative (dn)-MEK1 was used to block the ERK1/2 pathway. The mechanism was investigated by assessing the protein levels of pyruvate kinase isozymes M2 (PKM2) and DUSP6, as well as the activation of ERK1/2 pathway. Our data confirmed the anti-cancer effect of siTRIM11 in human lung cancer by demonstrating inhibition of cancer cell proliferation, induction of apoptosis, prevention of 2-NBDG uptake, suppression of LD production, and prevention of lung cancer cell (A549) tumorigenicity in nude mice. The underlying mechanism involved the up-regulation of DUSP6 and the inhibition of ERK1/2 activity. Overexpression of TRIM11 induced tumorigenesis of NSCLC in vitro, and the activation of ERK1/2 was significantly reversed by DUSP6 overexpression or additional dn-MEK1 treatment. Interestingly, we confirmed TRIM11 as a deubiquitinase that regulated DUSP6 accumulation, indicating that lung cancer progression is regulated via the DUSP6-ERK1/2 pathway. In conclusion, TRIM11 is an oncogene in NSCLC, likely through the DUSP6-mediated ERK1/2 signaling pathway.
Introduction
Lung cancer is recognized as the most common, aggressive, and deadly malignant tumor in humans. The majority (approximately 85%) of lung cancers is non-small cell lung cancer (NSCLC), and have poor prognosis.Citation1,Citation2 Mechanistically, loss of cellular growth control is generally accepted as the fundamental cause of human lung cancer progression, which results in sustained pro-proliferation and enhanced tumor metastatic ability.Citation3 As cancer cells are frequently driven by glycolytic anaerobic pathways, exploring novel targets or drugs based on the regulation of cancer cell glucose metabolism has been considered as a potential means to block NSCLC.Citation4
The Tripartite Motif Containing (TRIM) family protein is characterized by a tripartite motif (a RING domain, B-box motifs, and a coiled-coil region), with most of its members being E3 ubiquitin ligases, including TRIM11.Citation5 Our previous study demonstrated that TRIM11 was up-regulated in lung cancer and predicted poor survival, while knockdown of TRIM11 inhibited lung cancer cell growth, motility, and invasiveness.Citation6 However, whether and how TRIM11 functions in the tumorigenesis of lung cancer remains unclear.
Dual-specificity phosphatase 6 (DUSP6), also known as MKP-3, is a member of the mitogen-activated protein kinase (MAPK) family phosphatase to negatively regulate the MAPK pathway. DUSP6 has shown tumor-suppressive effects in NSCLC,Citation7 and low expression of DUSP6 is usually associated with high tumorigenicity.Citation8 DUSP6 can dephosphorylate and inactivate extracellular signal-regulated kinase (ERK) in the MAPK pathway.Citation7 Indeed, the DUSP6-mediated ERK1/2 pathway has been implicated in the progression of human pancreatic cancer.Citation9 TRIM11 regulates ERK1/2 activity in NSCLC; however, whether the DUSP6-mediated ERK1/2 pathway is involved in TRIM11-induced tumorigenesis in NSCLC remains largely unknown.
Here, we found that TRIM11 functions as an oncogene in human lung cancer in vitro by regulating lung cancer cell growth and glucose metabolism. TRIM11 down-regulates DUSP6, and as a result, the ERK1/2 pathway is activated and promotes the tumorigenesis of human lung cancer cells.
Materials and methods
Immunohistochemistry (IHC) assay
To study the involvement of TRIM11 and DUSP6 in human lung cancer, IHC analysis was performed to assess TRIM11 and DUSP6 expression in ten cases of tumorous and precancerous lung tissue from patients with lung cancer in Northern Jiangsu People’s Hospital and Clinical Medical College of Yangzhou University Hospital.
Lung tissue sections (4–7 μm thick) on slides were incubated with TRIM11 antibody (ab171641, Abcam) and DUSP6 antibody (ab238512, Abcam) at 4°C overnight, followed by incubation with secondary antibodies (D-3004, Long Island Biotech, China) at 25°C for 30 mins. The slides were stained with DAB substrate (Long Island Biotech) and hematoxylin (714094, BASO) for 3 min at room temperature. After being cleared in xylene and mounted using neutral balsam (Beijing Solarbio Technology Co., LTD, Beijing, China). The area of TRIM11- or DUSP6-postive cells was analyzed using a light microscope (Eclipse Ni-E/Ni-U, NIKON) with an image analysis system (DS-Ri2, NIKON).
Cell culture and treatment
A549, H446, and H1975 cells were obtained from ATCC (Manassas, VA, USA), and grown in 89% RPMI-1640 (Hyclone, Logan, UT), penicillin (100 U/ml, Solarbio, Beijing, China), and 10% heat-inactivated fetal bovine serum (Gibco Laboratories) at 37°C under 5% CO2. The cells were passaged when they reached 80% confluency.
TRIM11 was overexpressed in H1975 cells to study the involvement of ERK1/2 and DUSP6 in the carcinogenesis effect of TRIM11 in NSCLC. The cells were treated with lentiviruses expressing DUSP6 or 10 μmol/l of dominant-negative MEK1 (dn-MEK1) (used as an ERK1/2 inhibitor; ADV-118, Cell Biolabs Inc., USA).
Cell proliferation and apoptosis assays
The proliferation of A549, H446, and H1975 cells was assessed at 0, 24, 48, and 72 h with a Cell Counting Kit-8 (CCK-8) method using a Cell Proliferation Assay kit (CP002, SAB). For each well, the absorbance at 450 nm (OD 450) was measured by a plate reader (Bio-Rad).
After treatment for 24 h, the apoptosis of A549, H446, and H1975 cells was determined using am Annexin V-FITC apoptosis detection kit (Beyotime) according to the manufacturer’s instructions. The level of apoptosis was determined by flow cytometry (BD Biosciences).
Colony formation assay
Cells were seeded in 6-well plates (1 × 103 cells per well) and cultured for 14 days at 37°C under 5% CO2. Then, cells were washed twice in PBS, fixed with 4% formaldehyde for 15 min, and stained with GIMSA for 10–30 min. The colonies were counted under a microscope.
Glucose uptake assay
After treatment for 48 h, ECA-109, KYSE140, and TE-1 cells were maintained in low-glucose DMEM (SH30021.01B, Hyclone) (100 μl) for 3 h, and then in no-glucose DMEM (11966-025, GIBCO) (100 μl) supplemented with 100 μM of fluorescent 2-[N-(7-nitrobenz-2-oxa-1, 3-diaxol-4-yl) amino]-2-deoxyglucose (2-NBDG) (CAYMAN, 0467597-16) at 37°C with 5% CO2 for 45 min. A FACSCalibur (Becton Dickinson Immunocytometry Systems, SanJose, CA) flow cytometer was used to determine glucose uptake.
Lactic acid (LD) assessment
The LD content in the culture supernatant of ECA-109, KYSE140, and TE-1 cells was assessed using the Lactic Acid Assay Kit (A019-2; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The OD value at 530 nm was calculated by a plate reader (Bio-Rad).
Production and transfection of TRIM11 and DUSP6 overexpression vectors
The primers of the human DUSP6 gene (NCBI NM_001946.3) were as follows: 5′-GCGAATTCAGGGCTTTTGTGCATGCTA-3′ (forward, with EcoR I on the underline) and 5′-CGGGATCCACTATACTCATAAGAGGTATTGT-3′ (reverse, with BamHI on the underline). Construction and transfection of the DUSP6 or TRIM11 lentivirus were carried out according to a previously reported protocol.Citation6 Cells transfected with lentivirus without DUSP6 or TRIM11 were used as the corresponding controls.
RNA interference
Two siRNA targeting human TRIM11 mRNA (siRNA-TRIM11-1, 5′-GCUAUUACAAUUCCUCGGA-3′; and siRNA-TRIM11-2, 5′-CUAUUCAUCUUUCCCGAGA-3′) and a nonspecific siRNA (siRNA-NC) were transfected into A549 and H446 cells according to a previously reported protocol.Citation6
Real-time (RT)-PCR
Total RNA from lung tissue or lung cancer cell lines (A549, H446, and H1975) was extracted by Trizol Reagent (1596–026, Invitrogen) and reverse transcribed using a cDNA synthesis kit (Fermentas). The design of the primers for TRIM11, DUSP6, and PKM2 are shown in . The mRNA levels of TRIM11, DUSP6, and PKM2 were determined using SYBR Green PCR Kit (Thermo Fisher) on an ABI7300 system (Applied Biosystem).
Table 1. Primers used in RT-PCR analysis
Western blot analysis
The total protein content in the supernatant of lung tissue (20 mg) or cancer cell (A549, H446, and H1975) lysates was determined using the BCA protein assay kit (Thermo, MA, USA), and 25 μg of the total protein was run on 15% SDS-PAGE. Electrophoretically pure proteins were transferred to PVDF membranes (Millipore, USA) and incubated with primary antibody at 4°C overnight, followed by horseradish peroxidase-conjugated antibodies (Beyotime, Shanghai, China) for 1 h at 25°C. Visualization and quantitative analysis of immunoblotted bands were performed using an ECL system (GE Healthcare/Amersham Biosciences).
The primary antibodies were as follows: anti-TRIM11 antibody (Ab111694, Abcam), anti-DUSP6 antibody (Ab196690, Abcam), anti-PKM2 antibody (Ab137852, Abcam), anti-ERK1/2 antibody (#4695, CST), antibody against p-ERK1/2 (#4730, CST), and antibody against GAPDH (#5174, CST).
In vitro co-immunoprecipitation (Co-IP) and ubiquitination assay
To determine whether TRIM11 was associated with DUSP6, 2 mg of total protein in the supernatant of H1975 cells transfected with TRIM11 was added to protein G-Agarose beads (Roche), and then immune-precipitated with anti-TRIM11 (orb481520), anti-DUSP6 (orb481520), or control IgG antibody overnight at 4°C. TRIM11 and DUSP6 in the immune complex were immunoblotted using anti- TRIM11 (ab111694) and anti-DUSP6 (ab196690), respectively, and 2 mg of the total protein in TRIM11-transfected H1975 cells was reserved as the input control.
To study the effect of TRIM11 on DUSP6 ubiquitination, 100 μg of total protein in the supernatant of siTRIM11-transfected A549 cells was added into Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, sc-2003), and then immune-precipitated with 1 μg of IgG (Proteintech, 30000-0-AP) or anti-DUSP6 antibody overnight at 4°C. Anti-ubiquitin antibody (ab7780) was used to quantify DUSP6 ubiquitin in immune-complexes by western blot.
Xenograft model
Twelve nude mice (Shanghai Laboratory Animal Company) were randomly divided into siNC and siTRIM11 groups. Mice in the siNC group were subcutaneously injected with A549 cells transfected with siNC (5 × 106 cells, 100 μl), and the mice in the siTRIM11 group were injected with siTRIM11 transfected A549 cells (5 × 106 cells, 100 μl). The mice in the two groups were fed separately. The tumor volume (mm3) was calculated every 3 days from the 12th day to the 33rd day; the mice were sacrificed on the 33rd day. The tumor tissue was weighed (g), and histopathological imaging was assessed using hemaoxylin-eosin (HE) staining, according to a previously study.Citation10
TUNEL assay
Apoptosis in mouse lung tissue was calculated using the In Situ Cell Death Detection Kit, POD (Roche) according to the manufacturer’s instructions. The nucleus of apoptotic cells were double dyed using a DBA substrate kit (FL-6001, Long Island, Shanghai, China) and hematoxylin (714094, BASO), and analyzed by the IMS image system (JRDUN, Shanghai, China).
Statistical analysis
Date are presented as mean ± standard error of the mean. The P-value between the two groups was assessed by Student’s t-test with P-values < 0.05 considered statistically significant.
Results
Expression of TRIM11 and USP6 in NSCLC
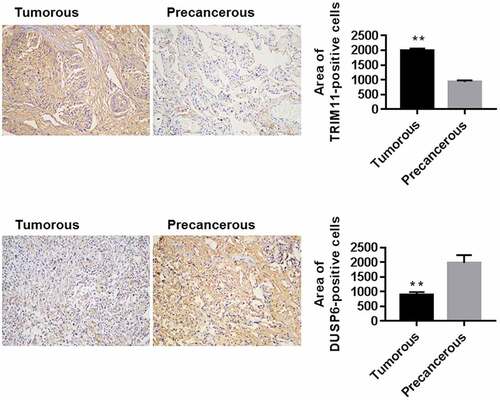
The expression of TRIM11 and USP6 was determined by IHC in ten cases of tumorous and none-tumorous colorectal tissue from patients with NSCLC. TRIM11 was up-regulated and DUSP6 was significantly decreased in tumorous lung tissue in NSCLC compared to the corresponding none-tumorous tissue (), suggesting the participation of TRIM11 and DUSP6 in human NSCLC.
Figure 1. Expression of TRIM11 and DUSP6 in the lung tissue of patients with NSCLC, assessed by IHC assay. Ten cases of tumorous and precancerous lung tissues were analyzed. The representative images and the quantification of positive staining are shown. Magnification: ×200. **P < .01 vs. precancerous lung tissue
Knockdown of TRIM11 suppressed the tumorigenesis of lung cancer cells
Our previous report demonstrated that TRIM11 was highly expressed in A549 and H446 cell lines; however, TRIM11 had low expression in the H1975 cell line. Therefore, we chose A549, H446, and H1975 for the current study.
A549 and H446 cells were transfected with siTRIM11. shows that the mRNA and protein level of TRIM11 in A549 and H446 cells was significantly decreased in the siTRIM11 group when compared with siNC, suggesting successful silencing of TRIM11 in these two cell lines. After transfection, the tumorigenesis of A549 and H446 cells was assessed through measuring cell proliferation and apoptosis, 2-NBDG uptake ability, and the secretion of LD. Our results showed that knockdown of TRIM11 time-dependently inhibited lung cancer cell proliferation (), significantly suppressed colony formation (), enhanced cell apoptosis (), reduced lung cancer cell uptake of 2-NBDG (), and reduced the concentration of LD in the cell culture supernatant (). These findings demonstrate that knockdown of TRIM11 ameliorates the tumorigenesis of human lung cancer cells.
Figure 2. Knockdown of TRIM11 inhibited the tumorigenesis of NSCLC cells, up-regulated DUSP6, and inhibited ERK1/2 activity. (a) mRNA levels of TRIM11, measured by RT-PCR. (b) Proliferation, (c) colony formation, and (d) apoptosis of A549 and H446 cells, assessed by CCK8, colony formation assay, and flow cytometry, respectively. (e and f) siTRIM11 inhibited the glucose metabolism of A549 and H446 cells by decreasing the secretion of LD and the uptake of 2-NBDG. (g) Western blot analysis showed that siTRIM11 remarkably down-regulated TRIM11, PKM2, and ERK1/2, and up-regulated DUSP6 in A549 and H446 cells. **P < .01 vs. siNC
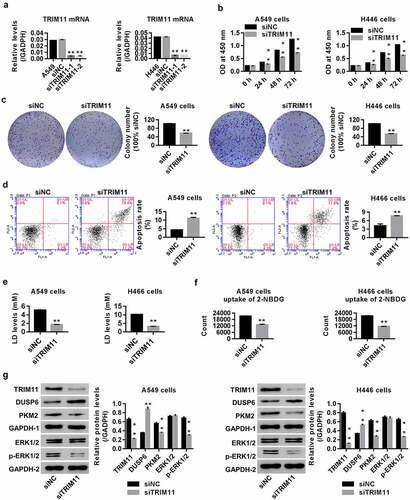
The protein levels of DUSP6, p-ERK1/2, and PKM2 were measured to evaluate the mechanism of the observed anti-tumor effects. The data showed that siTRIM11 obviously down-regulated p-ERK1/2 and PKM2, and up-regulated DUSP6 in lung cancer cells ().
Overexpression of TRIM11 contributes to the tumorigenesis of lung cancer cells
H1975 cells were transfected with the TRIM11 overexpression vector. show that the mRNA level of TRIM11 in H1975 cells was significantly increased in the TRIM11 overexpression group compared with the control vector, suggesting successful TRIM11 overexpression in H1975 cells. After transfection, cell proliferation, colony formation, cell apoptosis, 2-NBDG uptake ability, and secretion of LD were assessed. Our results showed that TRIM11 overexpression time-dependently promoted lung cancer cell proliferation (), significantly enhanced colony formation (), reduced cell apoptosis (), increased LD secretion (), and increased the uptake of 2-NBDG by H1975 cells (). These findings demonstrate that TRIM11 overexpression favors the tumorigenesis of human lung cancer cells.
Figure 3. Overexpression of TRIM11 promoted the tumorigenesis of NSCLC cells, down-regulated DUSP6, and promoted ERK1/2 activation. H1975 cells were infected with lentivirus overexpressing TRIM11. (a) RT-PCR showing the mRNA levels of TRIM11. (b) CCK8 for proliferation assay; (c and d) flow cytometry for apoptosis assay; (e) LD secretion, assessed by a commercial Kit; and (f) 2-NBDG uptake, measured by a biochemical analysis method. (g) Western blot analysis showed that TRIM11 remarkably up-regulated TRIM11, PKM2, ERK1/2, and down-regulated DUSP6 in H1975 cells. (h) RT-PCR showing the mRNA levels of TRIM11, PKM2, and DUSP6. ##P < .01 vs. vector
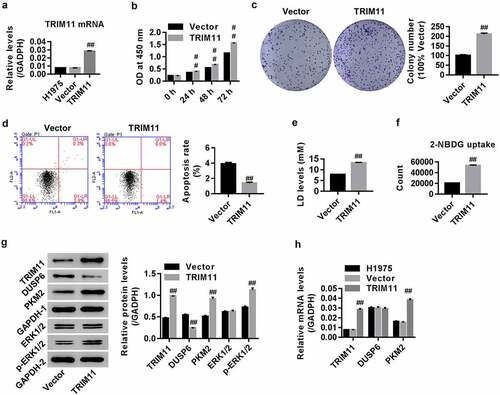
The effects of TRIM11 overexpression on protein expression of DUSP6, p-ERK1/2, and PKM2 were also measured. We found that TRIM11 overexpression obviously enhanced p-ERK1/2 and PKM2 expression, and reduced DUSP6 expression in human lung cancer cells (). TRIM11 overexpression enhanced PKM2 mRNA expression, but had no effect on DUSP6 expression ().
TRIM11 regulates tumorigenesis of lung cancer cells, probably via the DUSP6-mediated ERK1/2 signaling pathway
H1975 cells were transfected with the vector/USP6N overexpression vector. shows that both DUSP6 mRNA and protein expression were significantly enhanced in the DUSP6 group when compared with the vector. This finding suggests successful establishment of DUSP6 overexpression within H1975 cells.
Figure 4. The DUSP6 and ERK1/2 inhibitor dn-MEK1 attenuated TRIM11-induced tumorigenesis of H1975 cells. (a) Successful DUSP6 overexpression in H1975 cells. Both DUSP6 and dn-MEK1 significantly inhibited (b) the secretion of LD, (c) the uptake of 2-NBDG, and (d) the proliferation of H1975, but decreased (e) H1975 apoptosis. (f) Western blot analysis showed that DUSP6 and dn-MEK1 remarkably down-regulated PKM2 and p-ERK1/2 in H1975 cells transfected with TRIM11. ##P < .01 vs. vector, ++P < .01 vs. TRIM11
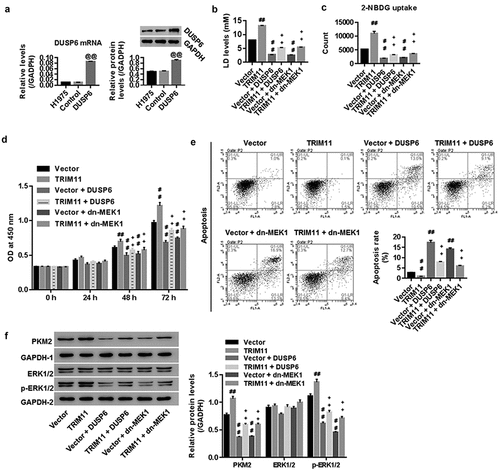
Next, the mechanisms by which TRIM11 regulate the tumorigenesis of lung cancer cells was studied. To this end, H1975 cells with TRIM11 overexpression were treated with dominant-negative MEK1 (an inhibitor of the ERK1/2 pathway), or H1975 cells were transfected with both TRIM11 and DUSP6 overexpression. Our data show that DUSP6 and dn-MEK1 significantly reduced the concentration of LD in the cell culture supernatant (), decreased the uptake of 2-NBDG by H1975 cells (), inhibited cell proliferation in a time-depended manner (), induced apoptosis (), and down-regulated the protein levels of PKM2 and p-ERK1/2 in H1975 cells transfected with vector or TRIM11 overexpression. Given the roles of TRIM11 in regulating DUSP6 and ERK1/2, our data suggest that TRIM11 influences the tumorigenesis of NSCLC cells via DUSP6 and the ERK1/2 pathway.
TRIM11 regulates DUSP6 ubiquitination
Co-IP was performed to explore whether TRIM11 was associated with DUSP6. We found that TRIM11 bound DUSP6 within H1975 cells ().
Figure 5. TRIM11 was associated with DUSP6, and regulated the ubiquitinoylation of DUSP6 in human H1975 cells transfected with TRIM11. Following co-immunoprecipitation with (a) anti-DUSP6 antibody and (b) anti-TRIM11 antibody, the presence of TRIM11 and DUSP was measured by western blot. (c) The presence of DUSP6 in TRIM11-transfected HCT116 cells was immunoprecipitated with DUSP6 antibodies and immunoblotted with anti-ubiquitin antibodies
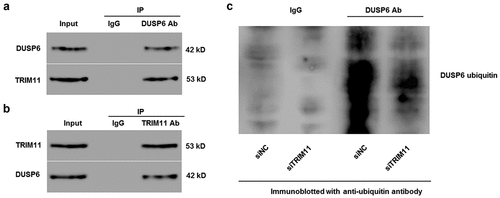
To examine whether TRIM11 controls DUSP6 ubiquitination, we immunoprecipitated DUSP6 from siTRIM11 transfected H1975 cells, and then immunoblotted DUSP6 ubiquitin by western blot. Our data suggest that ubiquitinated DUSP6 was significantly reduced in siTRIM11 when compared with siNC (), demonstrating that TRIM11 may be an ubiquitinase that enhances DUSP6 ubiquitination.
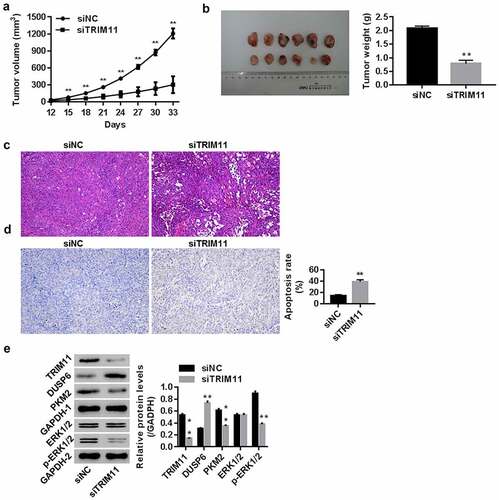
Knockdown of TRIM11 suppresses the tumorigenicity of A549 cells in nude mice
After injecting nude mice with siNC/siTRIM11-transfected A549 cells, the tumor volume (from the 12th to 33rd day), tumor weight (on the 33th day), and tumor histopathology were examined. Our data show that siTRIM11 significantly inhibited both the tumor volume and tumor weight (, all P < .01), attenuated histomorphological changes related to tumorigenesis (), and promoted apoptosis in tumor tissues (). These effects were associated with a reduction in TRIM11, PKM2, and p-ERK1/2, and an increase in DUSP6 when compared with the siNC group (). These findings suggest the involvement of TRIM11 and its potential association with the ERK1/2 pathway and DUSP6 in lung cancer in vivo.
Figure 6. Knockdown of TRIM11 inhibited the tumorigenicity of A549 cells in a xenograft model. Nude mice were injected with siNC/siTRIM11-transfected A549 cells (5 × 106 cells, 100 μl) (n = 6 in each group). (a) Tumor volume (mm3) from the 12th to 33rd day; (b) tumor weight (g) on the 33rd day; (c) histopathology images of the tumor determined using HE, and (d) apoptosis in the tumor was analyzed by TUNEL staining. (e) Protein levels of TRIM11, DUSP6, PKM2, and ERK1/2 in tumors, assessed by western blot. **P < .01 vs. siNC
Discussion
Herein, by analyzing the protein expression of TRIM11 and DUSP6 in lung cancer tissue using IHC assay (), we found that TRIM11 was elevated, and DUSP6 was reduced in human lung cancer. Previous evidence has suggested that siTRIM11 mediates the inhibition of ERK1/2 activation, which is usually tracked in tandem with the promoted levels of DUSP6 in human lung cancer.Citation6 Therefore, we studied the roles of TRIM11 in human NSCLC, and its association with DUSP6 in this process.
In the present study, the functions of TRIM11 in lung cancer were further investigated. Our data suggest that knockdown of TRIM11 regulates glucose metabolism by inhibiting 2-NBDG uptake and reducing the secretion of LD, both of which are associated with cell proliferation and increased apoptosis (). On the contrary, TRIM11 exerted the opposite effect (), substantiating that TRIM11 is an oncogene in human lung cancer. We also confirmed that deletion of TRIM11 in human lung cancer tissue improves the pathological conditions of the tumor lung tissue ().
PKM2 is known as an isoenzyme of the glycolytic enzyme pyruvate kinase. Up-regulation of PKM2 promotes tumor growth and is responsible for the high glucose metabolism in human lung cancer,Citation11,Citation12 but its mechanism of action remains unclear. A previous report has demonstrated an inverse correlation between TRIM25 and PKM2 expressions (P = .026) in NSCLC.Citation13 In this study, we found that knockdown of TRIM11 significantly decreased PKM2; however, the overexpression of TRIM11 obviously enhanced PKM2 in human lung cancer cells, suggesting that TRIM family members play different roles in regulating PKM2 expression.
The ERK pathway is frequently over-activated in human lung cancer and can promote lung cancer cell tumorigenesis.Citation14,Citation15 The inhibition of ERK1/2 is often tracked in tandem with the promoted levels of DUSP6, an ERK1/2-specific phosphatase that negatively mediates ERK activity in human lung cancer.Citation16 Unlike TRIM11, we found that DUSP6 exerted tumor-suppressive effects in human lung cancer cells by regulating tumor cell proliferation and apoptosis, as well as 2-NBDG uptake and LD secretion; this process was associated with inactivation of the ERK1/2 pathway.
Our data show that TRIM11 suppresses DUSP6 at the protein level, yet, but has no clear effect on DUSP6 at the mRNA level (). As DUSP6 can be degraded by ubiquitination,Citation17 we studied whether and how the ubiquitin E3 ligase TRIM11 interacts with DUSP6. Our results firstly suggest that siTRIM11 inhibits the ubiquitination of DUSP6, thus favoring the accumulation of DUSP6. Given the roles of DUSP6 in regulating the ERK1/2 pathway, we can deduce that TRIM11 regulates the tumorigenesis of lung cancer cells, probably via the DUSP6-mediated ERK1/2 signaling pathway.
In conclusion, we confirmed the oncogenic activities of TRIM11 in human lung cancer both in vitro and in vivo. Moreover, increased PKM2 expression and promotion of the DUSP6-mediated ERK1/2 pathway activation were the underlying mechanisms of the observed carcinogenesis. TIRM11 likely activates the ERK1/2 pathway by promoting DUSP6 ubiquitination. Our research suggests that targeting TRIM11 may represent a novel therapeutic target in human NSCLC treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors’ contributions
Yu-sheng Shu and Hong-can Shi, designed the study; Xiao-lin Wang performed the experiment and entered the data; Shi-chun Lu, Chao Sun, and Wei-guo Jin performed data collection/entry/analysis; Yi-wei Fan and Ling-feng Min drafted the manuscript and gave final approval of the version to be published
Ethics approval and consent to participate
The use of CRC patients was permitted by the Human Ethics Committee of Northern Jiangsu People’s Hospital and Clinical Medical College of Yangzhou University Hospital, and written informed consent was obtained from the patients. The use of mice was approved by the Animal Ethics Committee in Northern Jiangsu People’s Hospital and Clinical Medical College of Yangzhou University.
Acknowledgments
The study was supported by grant (from the Natural Science Foundation of Yangzhou (YZ2016108) and The 13th 5-year-plan of strengthening scientific medical technology in Yangzhou city (ZDRC201812).
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi:10.1016/j.ejca.2018.07.005.
- Cho WC, Yip TT, Cheng WW, Au JS. Serum amyloid A is elevated in the serum of lung cancer patients with poor prognosis. Br J Cancer. 2010;102(12):1731–1735. doi:10.1038/sj.bjc.6605700.
- Li XF, Ma Y. Preclinical visualization of hypoxia, proliferation and glucose metabolism in non-small cell lung cancer and its metastasis. Boston (MA): Springer; 2014.
- Giatromanolaki A, Sivridis E, Arelaki S, Koukourakis MI. Expression of enzymes related to glucose metabolism in non-small cell lung cancer and prognosis. Exp Lung Res. 2017;43(4–5):167–174. doi:10.1080/01902148.2017.1328714.
- Sanchez JG, Okreglicka K, Chandrasekaran V, Welker JM, Sundquist WI, Pornillos O. The tripartite motif coiled-coil is an elongated antiparallel hairpin dimer. Proc Natl Acad Sci U S A. 2014;111(7):2494–2499. doi:10.1073/pnas.1318962111.
- Wang X, Shi W, Shi H, Lu S, Wang K, Sun C, He J, Jin W, Lv X, Zou H, et al. TRIM11 overexpression promotes proliferation, migration and invasion of lung cancer cells. J Exp Clin Cancer Res. 2016;35(1):100. doi:10.1186/s13046-016-0379-y.
- Ahmad MK, Abdollah NA, Shafie NH, Yusof NM, Razak SRA. Dual-specificity phosphatase 6 (DUSP6): a review of its molecular characteristics and clinical relevance in cancer. Cancer Biol Med. 2018;15:14–28. doi:10.20892/j.2095-3941.2017.0107.
- Okudela K, Yazawa T, Woo T, Sakaeda M, Ishii J, Mitsui H, Shimoyamada H, Sato H, Tajiri M, Ogawa N, et al. Down-regulation of DUSP6 expression in lung cancer: its mechanism and potential role in carcinogenesis. Am J Pathol. 2009;175(2):867–881. doi:10.2353/ajpath.2009.080489.
- Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol. 2003;162(6):1807–1815. doi:10.1016/S0002-9440(10)64315-5.
- Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-beta1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15(1):97. doi:10.1186/s12885-015-1119-y.
- Sun H, Zhu A, Zhang L, Zhang J, Zhong Z, Wang F. Knockdown of PKM2 suppresses tumor growth and invasion in lung adenocarcinoma. Int J Mol Sci. 2015;16(10):24574–24587. doi:10.3390/ijms161024574.
- Liu Y, He C, Huang X. Metformin partially reverses the carboplatin-resistance in NSCLC by inhibiting glucose metabolism. Oncotarget. 2017;8(43):75206–75216. doi:10.18632/oncotarget.20663.
- Jing HZ, Qiu F, Chen SZ, Su L, Qu C. Tripartite-motif protein 25 and pyruvate kinase M2 protein expression in non-small cell lung cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:437–441.
- Vicent S, Lopez-Picazo JM, Toledo G, Lozano MD, Torre W, Garcia-Corchon C, Quero C, Soria JC, Martin-Algarra S, Manzano RG, et al. ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumours. Br J Cancer. 2004;90(5):1047–1052. doi:10.1038/sj.bjc.6601644.
- Wang J, Huang S. Fisetin inhibits the growth and migration in the A549 human lung cancer cell line via the ERK1/2 pathway. Exp Ther Med. 2018;15(3):2667–2673. doi:10.3892/etm.2017.5666.
- Zhang Z, Kobayashi S, Borczuk AC, Leidner RS, Laframboise T, Levine AD, Halmos B. Dual specificity phosphatase 6 (DUSP6) is an ETS-regulated negative feedback mediator of oncogenic ERK signaling in lung cancer cells. Carcinogenesis. 2010;31(4):577–586. doi:10.1093/carcin/bgq020.
- Xie XL, Nie X, Wu J, Zhang F, Zhao LL, Lin YL, Yin YJ, Liu H, Shu YN, Miao SB, et al. Smooth muscle 22alpha facilitates angiotensin II-induced signaling and vascular contraction. J Mol Med (Berl). 2015;93(5):547–558. doi:10.1007/s00109-014-1240-4.
