ABSTRACT
Fibroblast growth factors (FGFs) and their receptors (FGFRs) are important for signaling to maintain cancer stem-like cells (CSCs) in esophageal squamous cell carcinoma (ESCC). However, which FGF receptor, 1, 2, 3, 4, and L1, is essential or whether FGFRs have distinct different roles in ESCC-CSCs is still in question. This study shows that FGFR2, particularly the IIIb isoform, is highly expressed in non-CSCs. Non-CSCs have an epithelial phenotype, and such cells are more differentiated in ESCC. Further, FGFR2 induces keratinocyte differentiation through AKT but not MAPK signaling and diminishes CSC populations. Conversely, knockdown of FGFR2 induces epithelial–mesenchymal transition (EMT) and enriches CSC populations in ESCC. Finally, data analysis using The Cancer Genome Atlas (TCGA) dataset shows that expression of FGFR2 significantly correlated with cancer cell differentiation in clinical ESCC samples. The present study shows that each FGFR has a distinct role and FGFR2-AKT signaling is a key driver of keratinocyte differentiation in ESCC. Activation of FGFR2-AKT signaling could be a future therapeutic option targeting CSC in ESCC.
Introduction
Esophageal squamous cell carcinoma (ESCC) is a deadly cancer worldwide.Citation1 Treatment for ESCC, such as surgical resection, chemoradiotherapy, and endoscopic treatments, is evolving, and prognosis for patients with early-stage ESCC shows improvement.Citation2 However, limited treatment options are available for patients with advanced ESCC. Immune checkpoint inhibitors might show promise for drug therapy,Citation3 but no molecularly targeted drugs are approved for ESCC to date.
The existence of cancer stem-like cells (CSCs) is common in various cancers.Citation4 CSCs are characterized by tumorigenicity, self-renewal ability, and pluripotency, leading to maintenance of tumor phenotypes and progression. Further, CSCs also show drug efflux and DNA repair ability, and capacity for relieving oxidative stress. These characteristics may be responsible for therapy resistance and cancer recurrence.Citation5,Citation6 We and other groups showed that CD24l°w/CD44high cells possess CSC features, accompanied with drug resistance and high tumorigenicity.Citation7–9 Also, CD24l°w/CD44high cells present a mesenchymal-like morphology affected by epithelial–mesenchymal transition (EMT) related factors, such as TGF-β, FGF, and Notch signaling.Citation10–12
CSCs display plasticity via repeating differentiation and dedifferentiation.Citation13,Citation14 EMT and mesenchymal–epithelial transition are likely involved in such plasticity. Moreover, ESCC-CSCs also possess an undifferentiated phenotype. Particularly in 3D-culture experiments, differential gradients are clearly observed between non-CSCs and CSCs.Citation15,Citation16
Fibroblast growth factor receptors (FGFRs) are a group of five tyrosine kinases (FGFR1, 2, 3, 4, and L1). FGF ligands regulate various cell activities, such as cell proliferation, differentiation, angiogenesis, and wound healing via binding to the extracellular domain of FGFR.Citation17 Interestingly, FGFR1-3 displays two isoforms (IIIb and IIIc) produced by alternative splicing. These alterations determine the specificity of ligand binding for FGFRs.Citation18 FGFR signaling is involved in tumor development and resistance to therapy, FGF-FGFR signaling is a therapeutic target, and various FGFR inhibitors are in development for clinical application.Citation19,Citation20 We previously reported that FGF-2/FGFR/ERK signaling is essential for maintaining ESCC-CSC.Citation10 However, which FGFRs are essential and whether each FGFR has a distinct role in ESCC-CSCs remain unclear.
We investigated FGFR roles in the regulation of cell differentiation, including CSCs plasticity in ESCC, and show that FGFR2-AKT signaling is crucial for maintaining the differentiated non-CSCs population.
Materials and methods
Cells and reagents
ESCC cell lines (HCE4, TE11, and TE8 cells) were obtained from the Cell Resource Center for Biomedical Research, Tohoku University. Cells were authenticated using the short-tandem repeat-PCR method. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (Moregate Biotec, Bulimba, Australia), 100 U/ml penicillin, and 100 µg/ml streptomycin (WaKo Pure Chemical Industries, Osaka, Japan); cells were incubated at 37°C in 20% O2 and 5% CO2. GDC0068, AZD4547, and trametinib were purchased from Cayman Chemical (Ann Arbor, MI, USA), MK2206 was purchased from Chemscene LLC (Monmouth Junction, NJ, USA) and SC79 was purchased from abcam (Cambridge, UK). These compounds were dissolved in dimethyl sulfoxide (DMSO).
Small interfering RNAs (siRNAs)
siRNAs for FGFR1, FGFR2, FGFR4, AKT1, AKT2, and scramble (control siRNA) were purchased from Qiagen (Hilden, Germany). Sequences for specific siRNAs for FGFR2b and FGFR2c were designed as: (FGFR2b: s; GUAAGGUCUCCAAUUAUAUTT, as; AUAUAAUUGGAGACCUUACAT, FGFR2c: s; CUAUAUUCGGAAUGUAACUUUTT, as; AAAGUUACAUUCCGAAUAUAGAA). siRNA transfection used Lipofectamine RNAi MAX (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The final concentration of siRNAs was 10 nM.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from the cells using a FastGene RNA Basic kit (NIPPON Genetics, Tokyo, Japan) following the manufacturer’s instructions. A total of 500 ng RNA was reverse transcribed to produce cDNA using Prime Script RT Master Mix (Takara, Kusatsu, Japan). PCR was performed in a 20-µl volume containing 10 µl cDNA (15 ng/µl) and 10 µl TB Green Premix Ex Taq (Takara). Following an initial denaturation at 95°C for 20 s, a two-step cycle procedure was used (denaturation at 95°C for 1 s and annealing and extension at 60°C for 20 s) for 40 cycles in a Step One real-time PCR system (Applied Biosystems, Foster City, CA, USA). Gene expression levels were determined using the ddCt method with β-actin as an endogenous control. Data were analyzed with Step One software version 2.2.2 (Applied Biosystems). Primers used for qRT-PCR are listed in Supplementary Table 1.
Western blotting analysis
The cells were washed with ice-cold PBS (-) and lysed in 1× RIPA buffer (Wako) containing protease inhibitor cocktail (Cell Signaling Technology (CST), Inc., Danvers, MA, USA). Protein concentration was measured with a Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). Ten µg of total protein was mixed with BlotTM 4× LDS sample buffer (Thermo Fisher Scientific) and 2-mercaptoethanol (5% final concentration). Samples were heated at 95°C for 5 min and subjected to SDS-polyacrylamide gel electrophoresis. Separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes using a Trans-Blot turbo transfer pack (Bio Rad, Hercules, CA, USA), and subsequently incubated with TBS with 0.05% Tween 20 containing 5% dried nonfat milk for 30 min at room temperature. Membranes were probed with primary antibodies for FGFR1 (#9740, 1:1000), FGFR2 (#11835, 1:250), FGFR3 (#4574, 1:1000), FGFR4 (#8562, 1:1000), Vimentin (#5741, 1:1000), AKT1 (#2938, 1:1000), AKT2 (#3063, 1:1000), AKT3 (#3788, 1:1000), p-AKT1 (#9018, 1:1000), p-AKT2 (#8599, 1:1000), Snail (#3879, 1:1000) (CST), FGFRL1 (ab95940, 1:1000, Abcam, Cambridge, UK), E-cadherin (#610181, 1:1000, BD Biosciences, Franklin Lakes, NJ, USA), ESRP1 (A14626, 1:1000, ABclonal, Tokyo, Japan), CK13 (sc-390982, 1:1000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA), IVL (sc-21748, 1:1000, Santa Cruz) and β-actin (sc-47778, 1:2000, Santa Cruz), and bound antibodies were detected with peroxidase-labeled rabbit or mouse antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and visualized by Clarity Western ECL Substrate (Bio Rad).
Flow cytometry and fluorescence activated cell sorting (FACS)
A FACSCanto (BD Biosciences) was used for flow cytometry. Cells were suspended in Hank’s balanced salt solution (Invitrogen) containing 0.5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and stained with PE-Cy7-anti-CD24 (Biolegend, San Diego, CA, USA) and APC-anti-CD44 (BD Biosciences) at dilutions of 1:40 on ice for 30 min. Dead cells were eliminated using 7-AAD (BD Biosciences) following the manufacturer’s instructions. Cells were sorted to fractionate CD24High (CD24H) and CD24Low (CD24L).
Cell migration assay
Migration was assessed by seeding 3,000 TE8, TE11, and HCE4 cells on the Radius Cell Migration Assay Kit (Cell biolabs, Inc, San Diego, CA, USA) and incubated overnight. Subsequently, the Radius Gel was removed following the manufacturer’s instructions and cell migration was evaluated after 2 and 48 hr.
3D culture of ESCC cells
3D culture was performed as described previously with modification.Citation12,Citation21 In brief, cells were seeded at 2,000 cells/well in a 24-well plate in 50 µl Matrigel (Corning, Corning, NY, USA). Following solidification, 500 µl of organoid medium (Supplementary Table 2) was added and incubated at 37°C for two weeks. Growth medium was replenished every other day.
Analysis in human ESCCs using TCGA consortium database
Human ESCC gene expression data (total 82 records) were obtained from the TCGA consortium expression database (https://portal.gdc.cancer.gov/). Expression ratios of FGFR2 and FGFR1(FGFR2/FGFR1) were calculated. We defined FGFR2/FGFR1 > 1 as FGFR2-dominant patients, and FGFR2/FGFR1 < 1 as FGFR1-dominant patients, to separate FGFR2-dominant patients from FGFR1-dominant patients. Expression of differentiation and EMT markers were compared between these two patient groups.
Statistical analysis
Data are expressed as mean ± standard deviation. Comparisons of parameters between two groups used two-tailed Student’s t-tests. Comparisons of parameters among groups were performed by one-way analysis of variance, followed by Tukey’s multiple comparison test. Differences were considered significant at p< .05. All statistical data were generated using Prism 8.03 (GraphPad Software, Inc., La Jolla, CA).
Results
Characteristic features of ESCC-CSCs
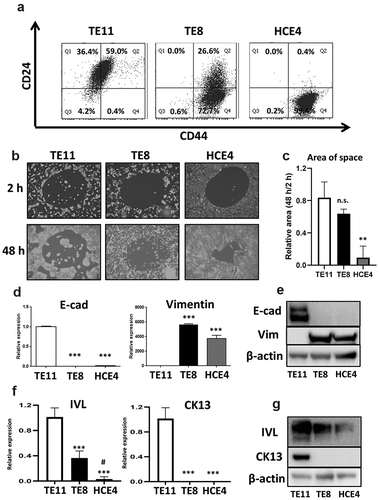
We first examined CSCs population defined by high expression of CD44 and low expression of CD24 (CD44high/CD24l°w) in three ESCC cell lines (TE11, TE8, and HCE4). FACS analyses showed that HCE4 cells contained the highest proportion of CSCs among three cell lines; whereas TE11 cells contained few CSCs and TE8 cells had both CSCs and non-CSCs (). We used migration and 3D-organoid formation assays to confirm the CSC phenotype. As expected, HCE4 cells showed the greatest migration and 3D-organoid formation abilities among the three cell lines (, S1A, B, and C). Further, CD44high/CD24l°w CSCs have mesenchymal characteristics.Citation7,Citation10–12,Citation22 Expression of Vimentin, a major mesenchymal marker, was greater than expression of E-cadherin. This epithelial marker was significantly downregulated in HCE4 and TE8 cells ( respectively). Further, we confirmed the differentiation phenotype of these cell lines. Cytokeratin 13 (CK13) and involucrin (IVL), keratinocyte differentiation markers, were suppressed in HCE4 and TE8 cells, but upregulated in TE11 cells (). HCE4 cells (and partly TE8 cells) display a CSC phenotype accompanied with mesenchymal and undifferentiated features. Conversely, TE11 cells have non-CSCs phenotype accompanied with epithelial and differentiated features.
Figure 1. Characteristic features of CSCs in ESCC cell lines. (a) TE11, TE8, and HCE4 cells were stained with PE-Cy7-anti-CD24 and APC-anti-CD44 antibodies and analyzed by FACS. (b and c) TE11, TE8, and HCE4 cells were seeded on a Radius Cell Migration Assay Kit and incubated overnight. Subsequently Radius Gel was removed, and cell migration was evaluated at 2 and 48 h. The area of space was measured by Image J software (n = 3). **p < .01 vs. TE11 cells. (d) mRNA expression of EMT markers (E-cad and Vimentin) in ESCC cell lines. *** p < .001 vs. TE11 cells. (e) Protein expression of EMT markers (E-cad and Vimentin) in ESCC cell lines. (f) mRNA expression of differentiation markers (IVL and CK13) in ESCC cell lines. ***p < .001 vs. TE11 cells. #p < .05 vs. TE8 cells. (g) Protein expression of differentiation markers (IVL and CK13) in ESCC cell lines. n.s.: not-significant, E-cad: E-cadherin, Vim: Vimentin
Expression of FGFRs is different between non-CSCs and CSCs in ESCC
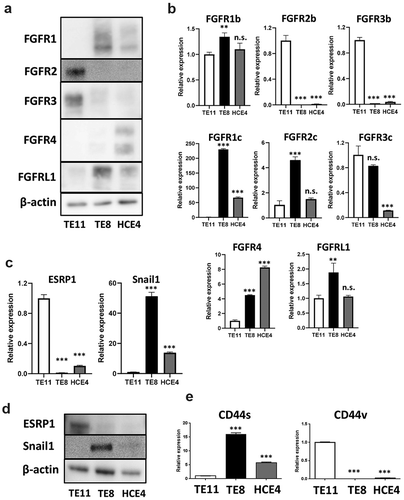
We first confirmed expression levels of FGFRs in ESCC cell lines by western blotting to elucidate which FGFRs (FGFR1, 2, 3, 4, and L1) are expressed in ESCC-CSCs. FGFR2 and 3 were highly expressed in TE11 cells, but FGFR1 and 4 were higher in HCE4 cells (). Subsequently, FGFR1 to 3 have two isoforms generated by splicing variants, one is epithelial (IIIb) and the other mesenchymal (IIIc). We also checked expression by qRT-PCR. Epithelial IIIb isoforms were predominantly expressed in TE11 cells, while mesenchymal IIIc isoforms were predominant in TE8 and HCE4 cells (). Also, expression of ESRP1, a major splicing regulator, and Snail1 (known as ESRP suppressor) were different among TE11, TE8, and HCE4 cells (). CD44, a CSCs marker, also exhibits two isoforms (CD44v is epithelial and CD44s is mesenchymal) similar to FGFRs.Citation23 Predictably, CD44v was higher in TE11 cells, and CD44s higher in TE8 and HCE4 cells ().
Figure 2. Expression levels of FGFRs are different among ESCC cell lines. (a, b) Expression levels of FGFRs in TE11, TE8, and HCE4 cells were examined by western blotting and qRT-PCR. **p < .01, *** p < .001 vs. TE11 cells. (c, d) Expression levels of ESRP1 and Snail1 in TE11, TE8, and HCE4 cells were examined by qRT-PCR and western blotting. ***p < .001 vs. TE11 cells. (e) Expression levels of CD44v and CD44s in TE11, TE8, and HCE4 cells were examined by qRT-PCR. ***p < .001 vs. TE11 cells. n.s.: not-significant
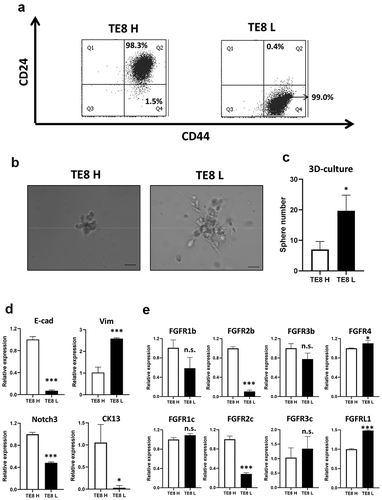
We sorted TE8 cells with high CD24 and CD44 expression (TE8 H cells) or low CD24 and high CD44 expression (TE8 L cells) (), and compared expression of EMT markers, differentiation markers, and each FGFR isoform to validate these results. These cells displayed different characteristics – TE8 H cells were non-CSC like, and TE8 L cells were CSC like. TE8L cells made more colonies in 3D culture () and showed mesenchymal and undifferentiated phenotype (). Interestingly, FGFR2 expression was sharply suppressed in TE8 L cells. Especially, FGFR2b was suppressed in more undifferentiated TE8 L cells (). This finding was also observed among unsorted TE11, TE8, and HCE4 cells (). Expression of FGFR2 might be important to determine keratinocyte differentiation in ESCC.
Figure 3. Expression levels of FGFRs are different between CD24H cells and CD24L cells. (a) High CD24 and high CD44 (CD24H; TE8H) or low CD24 and high CD44 (CD24L; TE8L) cells were sorted by FACS inTE8 cells. (b) Representative colonies of 3D-culture of TE8H and TE8L cells. (c) Counts of sphere (≧100 µm) of TE8H and TE8L cells (n = 3) *p < .05 vs. TE8H cells. (d) mRNA expression of EMT markers and differentiation markers in TE8H and TE8L cells. *p < .05, ***p < .001 vs. TE8H cells. (e) mRNA expression of FGFRs in TE8H and TE8L cells. *p < .05, ***p < .001 vs. TE8H cells. n.s.: not-significant, E-cad: E-cadherin, Vim: Vimentin
FGFR2 drives keratinocyte differentiation and maintains non-CSCs in a differentiated state
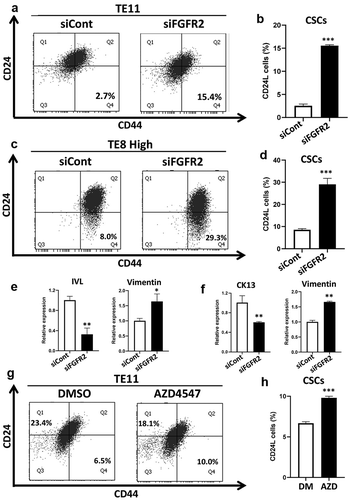
We next silenced FGFR2 expression using siRNA. FACS analysis showed that knockdown of FGFR2 increased CSC populations in both TE11 and TH8 H cells (). Further, specific knockdown of the FGFR2b isoform highly increased CSC populations compared to the FGFR2c isoform (Figure S2). IVL and CK13 expression were suppressed and Vimentin expression was upregulated by FGFR2 knockdown (). Consistently, the FGFR inhibitor, AZD4547, increased CSC populations in TE11 cells (). FGFR2 thus drives keratinocyte differentiation and keeps non-CSCs in a differentiated state in ESCC.
Figure 4. FGFR2 expression is important for the maintenance of CD24H cells. (a, c) FGFR2 was knocked down using siRNA in TE11 and TE8H cells at a concentration of 10 nM for 48 h, and CSCs populations were analyzed by FACS. (b, d) Statistical analyses of CSCs population (CD24L cells) (n = 3) ***p < .00 vs. siCont cells. (e, f) mRNA expression of EMT markers and differentiation markers in FGFR2 knocked down TE11 (e) and TE8L cells (f) *p < .05, **p < .01 vs. siCont cells. (g, h) TE11 cells were treated with or without AZD4547 (2 µM) for 48 h, and the expression levels of CSCs markers were examined by FACS. ***p < .001 vs. DMSO (control). siCont: siControl (scramble siRNA)
AKT plays an important role for keratinocyte differentiation in ESCC
We previously reported that FGF/FGFR signaling supports the maintenance of CSCs in ESCC via the MAPK(RAS/MEK/ERK) pathway.Citation10 MEK inhibitor (trametinib) or knockdown of FGFR1 and FGFR4 decreased CSC populations in HCE4 cells (Figure S3). Therefore, we focused on and explored the AKT pathway since AKT is a well-known FGFR downstream pathway and plays various roles for modulating CSC characteristics and cell differentiation.Citation24,Citation25 AKT consists of three isoforms (AKT1-3), and we then checked their expression and activation status in TE8H and TE8L cells. AKT1 and AKT2 were expressed in both cells; however, interestingly AKT1 was only activated in TE8H cells (). Subsequently, we silenced AKT1 or AKT2 expression by siRNA and examined the changes in CSC populations. Knockdown of either AKT1 or AKT2 resulted in significant increases in CSC populations in TE11 cells (). Knockdown efficiencies were confirmed by qRT-PCR (). Consistently, AKT specific inhibitors, MK2206 and GDC0068, also increased CSC populations in TE11 cells (). Moreover, Notch3, a key transcription factor that induces terminal differentiation in keratinocytes, and CK13 expression were suppressed by AKT inhibition (Figure S4). Conversely, AKT activator (SC79) decreased CSC populations and induced differentiation in TE8 cells (). AKT activation was confirmed by Western blotting (). Both AKT1 and AKT2 were important to drive keratinocyte differentiation in ESCC and maintain non-CSCs in a differentiated state in ESCC. The FGFR2-AKT pathway has the opposite function to FGFR1 and FGFR4-MAPK pathways.
Figure 5. AKT activation is important for the maintenance of CD24H cells. (a) Expression and activation levels of AKTs in TE8H and TE8L cells were examined by western blotting. (b) Knockdown efficiency of AKT1 and AKT2 were confirmed by qRT-PCR in TE11 cells. (c) AKT1 or AKT2 was knocked down using siRNA in TE11 cells at a concentration of 10 nM for 48 h, and CSCs populations were analyzed by FACS. (d) Statistical analyses of CSCs population (CD24L cells) (n = 3) ***p < .001 vs. siCont cells. (e, f) TE11 cells were treated with or without GDC0068 (2.5 µM) or MK2206 (2.5 µM) for 48 h, and the expression of CSCs markers were examined by FACS. ***p < .001 vs. DMSO (control)
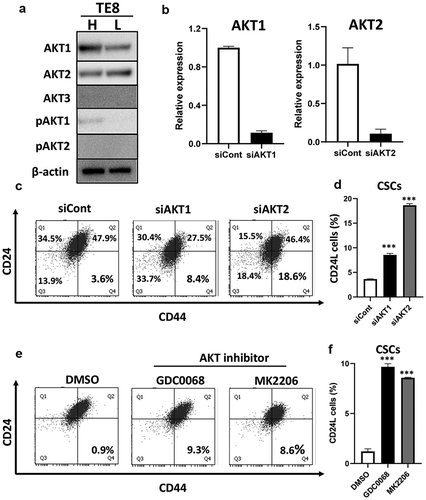
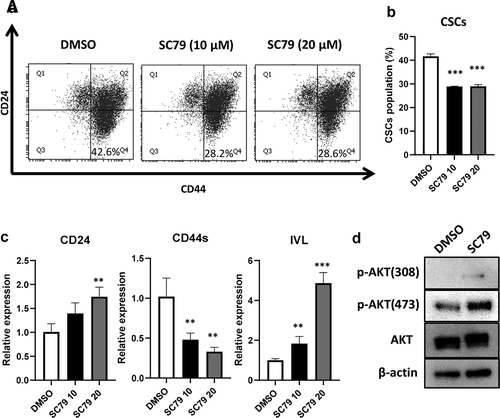
Figure 6. AKT activation decreased CD24L cells. (a, b) TE8 cells were treated with SC79 (0, 10, and 20 µM) for 48 h, and the expression of CSCs markers were examined by FACS. ***p ≤ 0.001 vs. DMSO (control). (c) mRNA expression of CD24, CD44s and IVL were examined by qRT-PCR in SC79 treated TE8 cells. **p ≤ 0.01 vs. DMSO (control), ***p ≤ 0.001 vs. DMSO. (d) TE8 cells were pre-incubated serum free media for 3 h and treated with or without SC79 (10 µM) for 30 min. Activation levels of AKT by SC79 were examined by western blotting
The expression levels of EMT and differentiation markers in FGFR1 or FGFR2-dominant ESCC patients
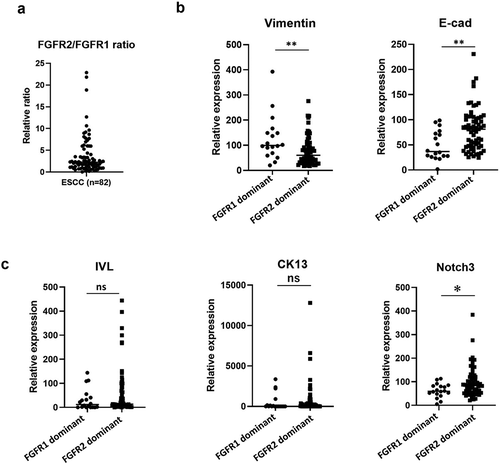
Finally, we examined expression levels of keratinocyte differentiation markers (CK13, IVL, and Notch3) and EMT related markers (Vimentin and E-cad) in clinical ESCC samples using The Cancer Genome Atlas (TCGA) dataset (n = 82). We separated FGFR2-dominant patients from FGFR1-dominant patients by FGFR2/FGFR1 ratio (). Expression of E-cad and differentiation markers, CK13, IVL, and Notch3 were upregulated in FGFR2-dominant ESCC patients. Conversely, expression of Vimentin, a mesenchymal marker, was significantly suppressed in FGFR2-dominant ESCC patients (). The FGFR2 pathway as a driver for keratinocyte differentiation is confirmed in clinical ESCC samples.
Figure 7. Epithelial and differentiation markers are upregulated in FGFR2-dominant ESCC patients. (a). The FGFR2/FGFR1 expression ratio of all 82 ESCC patients from TCGA database. Eighteen patients were FGFR1-dominant, and 64 FGFR2-dominant. (b, c) The expression of EMT markers and differentiation markers in FGFR2 or FGFR1-dominant ESCC patients. *p < .05, **p < .01 vs. FGFR1 dominant patients. n.s.: not-significant, E-cad: E-cadherin
Discussion
We focused on unique features of FGFRs in regulating cell differentiation, including CSCs plasticity in ESCC and demonstrated that (1) FGFR2, particularly IIIb isoform, is highly expressed in non-CSCs in ESCC; (2) FGFR2-AKT signaling drives keratinocyte differentiation and maintains non-CSCs in a differentiated state; (3) FGFR2 dominant ESCC patients show more differentiated ESCC.
Several studies demonstrate that FGF/FGFR signaling is involved in malignant progression in ESCC. Ueno et al. revealed that enhanced expression of FGFR3IIIb promotes ESCC proliferation.Citation26 Shi et al. showed that FGFR2 is highly expressed in ESCC tissue and plays an important role in cell survival and proliferation via the AKT/mTOR pathway.Citation27 Takase et al. reported that N-CAM and FGF2 mediate FGFR1 signaling that regulates survival and migration of tumor-associated macrophages and cancer cells in esophageal cancer.Citation28 Also, protein tyrosine kinase 7, a catalytically defective receptor protein tyrosine kinase, binds and activates FGFR1 in a ligand-independent manner in ESCC.Citation29 However, which FGFRs are essential and whether each FGFR has a distinct role in ESCC-CSCs remains to be elucidated.
We addressed these questions and found that the FGFR2(IIIb)-AKT pathway is important for keratinocyte differentiation and pivotal for the maintenance of differentiated non-CSCs cells in ESCC. The FGFR2(IIIb)-AKT pathway may have the opposite function of FGFR1 and FGFR4-MAPK pathways in ESCC (, 6 and S3). Cell differentiation state, including CSCs plasticity, is controlled by the coordination of various FGFR expression in ESCC. Therefore, applying pan-FGFR inhibitors might not be ideal for treatment of ESCC. Rather it might be necessary to develop specific inhibitors for each FGFR.
PI3K/AKT/mTOR signaling promotes survival, maintenance of stemness, and tumorigenicity in many cancers.Citation24,Citation30 However, studies which focus on the role of AKTs in CSCs are insufficient and controversial. For instance, PD-L1 sustains AKT activation and promotes the expression of stem cell-related genes, OCT4 and Nanog, in breast cancer stem cells.Citation31 TGF-β induces stemness via the AKT pathway in oral squamous cell carcinoma.Citation32 Tenascin-C (TNC), an extracellular matrix protein, promotes cancer stemness via the AKT/HIF1α axis in ESCC-CSCs.Citation33 The AKT/c-MYC axis, Hsp27/AKT/HK2 pathway, and AKT/β-catenin axis have also been reported to promote CSC in ESCC.Citation34–36 Conversely, FOXO3a that is mainly suppressed by AKTs, is key for CSC in various cancers. Kumazoe et al. reported that FOXO3 is essential for CD44 expression in pancreatic cancer cells.Citation37 Naka et al. revealed that TGF-β-FOXO pathway maintains CSCs in chronic myeloid leukemia.Citation38 Thus, AKTs could play opposite roles on CSCs. In this study, we demonstrate that AKT suppressed CSC populations in ESCC ( and 6).
Esophageal squamous differentiation progresses from basal keratinocytes in the proliferative zone to luminal surface epithelial cells. CK5 and CK14 are basal cell markers, and CK13 and IVL are expressed in the suprabasal layer and regarded as differentiation markers.Citation39,Citation40 Differentiation state in ESCC is related to tumor development. For instance, Xiong et al. showed that Pax9, a transcription factor, regulates differentiation and carcinogenesis in oro-esophageal epithelium.Citation41 Luo et al. reported that the expression of differentiation-associated genes is reduced during malignant transformation in ESCC.Citation42 Also, our group previously demonstrated that Notch signaling is essential for esophageal squamous differentiation and inhibition of that signaling raises malignant cellular potential.Citation43,Citation44 Consistently, the expression level of Notch3 in this study was higher in differentiated (non-CSCs) than undifferentiated ESCC (CSCs) (). More interestingly, AKT inhibitors suppressed Notch3 expression (Figure S4), and the FGFR2-AKT pathway could be an upstream signal for Notch3. Further study is required to elucidate detailed regulation mechanisms of Notch3 expression by AKT.
Differentiation therapy could be a novel therapeutic strategy targeting CSCs in various cancers. In 1990, Castaigne et al. reported that all-trans retinoic acid (ATRA) achieved high complete response rate in acute promyelocytic leukemia (APL) patients.Citation45 ATRA induces terminal differentiation in APL cells and now ATRA is a standard treatment option for APL patients. It has been also reported that ATRA induced differentiation in CD133 positive glioblastoma CSCs and sensitized the glioblastoma cells to conventional anti-cancer treatments in vitro and in vivo studies.Citation46 Bone morphogenetic protein 7 (BMP7) also can induce differentiation in glioblastoma CSCsCitation47 and González-Gómez P et al. have reported the effectiveness of microspheres loaded with BMP7 in glioblastoma treatment.Citation48 In the present study, we have shown for the first time that FGFR2-AKT axis induces keratinocyte differentiation in ESCC CSCs. Although we have not discovered how to activate this pathway specifically and efficiently, specific activators of FGFR2-AKT pathway could shed light on a new differentiation therapy in ESCC.
In conclusion, each FGFR has a distinct role in ESCC. FGFR2-AKT signaling is a driver of keratinocyte differentiation. Activation of FGFR2-AKT signaling could be a future therapeutic option for targeting CSC in ESCC.
Abbreviations
| CSCs | = | Cancer stem-like cells |
| DMEM | = | Dulbecco’s modified Eagle’s medium |
| DMSO | = | Dimethyl sulfoxide |
| EMT | = | Epithelial-mesenchymal transition |
| ESCC | = | Esophageal squamous cell carcinoma |
| FACS | = | Fluorescence activated cell sorting |
| FGF | = | Fibroblast growth factor |
| LDS | = | Lithium dodecyl sulfate |
| PBS | = | phosphate-buffered saline |
| SDS | = | Sodium dodecyl sulfate |
| TBS | = | Tris-buffered saline. |
| TCGA | = | The cancer genome atlas |
Supplemental Material
Download Zip (904 KB)Disclosure statement
No potential conflicts of interest were disclosed.
Supplementary materials
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah M, Lagergren P, Cunningham D. Oesophageal cancer. Nat Rev Dis Prim. 2017;3. doi:10.1038/nrdp.2017.48.
- Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi:10.1016/S0140-6736(17)31462-9.
- Kato K, Cho BC, Takahashi M, Okada M, Lin C, Chin K, Kadowaki S, Ahn M, Hamamoto Y, Doki Y, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. doi:10.1016/S1470-2045(19)30626-6
- Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–1134. doi:10.1038/nm.4409.
- Najafi M, Mortezaee K, Ahadi R. Cancer stem cell (a)symmetry & plasticity: tumorigenesis and therapy relevance. Life Sci. 2019;231:116520. doi:10.1016/j.lfs.2019.05.076.
- Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. doi:10.1016/j.lfs.2019.116781.
- Natsuizaka M, Kinugasa H, Kagawa S, Whelan KA, Naganuma S, Subramanian H, Chang S, Nakagawa K, Rustgi N, Kita Y, et al. IGFBP3 promotes esophageal cancer growth by suppressing oxidative stress in hypoxic tumor microenvironment. Am J Cancer Res. 2014;4(1):29–41. http://www.ncbi.nlm.nih.gov/pubmed/24482736 Accessed 2020 Jul 21.
- Kinugasa H, Whelan KA, Tanaka K, Natsuizaka M, Long A, Guo A, Chang S, Kagawa S, Srinivasan S, Guha M, et al. Mitochondrial SOD2 regulates epithelial-mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene. 2015;34(41):5229–5239. doi:10.1038/onc.2014.449
- Kagawa S, Natsuizaka M, Whelan KA, Facompre N, Naganuma S, Ohashi S, Kinugasa H, Egloff A, Basu D, Gimotty P, et al. Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities. Oncogene. 2015;34(18):2347–2359. doi:10.1038/onc.2014.169
- Maehara O, Suda G, Natsuizaka M, Ohnishi S, Komatsu Y, Sato F, Nakai M, Sho T, Morikawa K, Ogawa K, et al. Fibroblast growth factor-2-mediated FGFR/Erk signaling supports maintenance of cancer stem-like cells in esophageal squamous cell carcinoma. Carcinogenesis. 2017;38(11):1073–1083. doi:10.1093/carcin/bgx095
- Sato F, Kubota Y, Natsuizaka M, Maehara O, Hatanaka Y, Marukawa K, Terashita K, Suda G, Ohnishi S, Shimizu Y, et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol Ther. 2015;16(6):933–940. doi:10.1080/15384047.2015.1040959
- Natsuizaka M, Whelan KA, Kagawa S, Tanaka K, Giroux V, Chandramouleeswaran P, Long A, Sahu V, Darling D, Que J, et al. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nat Commun. 2017;8(1):1–16. doi:10.1038/s41467-017-01500-9
- Shimokawa M, Ohta Y, Nishikori S, Matano M, Takano A, Fujii M, Date S, Sugimoto S, Kanai T, Sato T. Visualization and targeting of LGR5 + human colon cancer stem cells. Nature. 2017;545(7653):187–192. doi:10.1038/nature22081.
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi:10.1038/nature12624.
- Kijima T, Nakagawa H, Shimonosono M, Chandramouleeswaran P, Hata T, Sahu V, Kasagi Y, Kikuchi O, Tanaka K, Giroux V, et al. Three-dimensional organoids reveal therapy resistance of esophageal and oropharyngeal squamous cell carcinoma cells. Cell Mol Gastroenterol Hepatol. 2019;7(1):73–91. doi:10.1016/j.jcmgh.2018.09.003
- Whelan KA, Muir AB, Nakagawa H. Esophageal 3D culture systems as modeling tools in esophageal epithelial pathobiology and personalized medicine. Cell Mol Gastroenterol Hepatol. 2018;5(4):461–478. doi:10.1016/j.jcmgh.2018.01.011.
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–129. doi:10.1038/nrc2780.
- Holzmann K, Grunt T, Heinzle C, Sampl S, Steinhoff H, Reichmann N, Kleiter M, Hauck M, Marian B. Alternative splicing of fibroblast growth factor receptor IgIII loops in cancer. J Nucleic Acids. 2012;2012:12. doi:10.1155/2012/950508.
- Touat M, Ileana E, Postel-Vinay S, André F, Soria JC. Targeting FGFR signaling in cancer. Clin Cancer Res. 2015;21(12):2684–2694. doi:10.1158/1078-0432.CCR-14-2329.
- Zhou Y, Wu C, Lu G, Hu Z, Chen Q, Du X. FGF/FGFR signaling pathway involved resistance in various cancer types. J Cancer. 2020;11(8):2000–2007. doi:10.7150/jca.40531.
- Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, Sharma M, Wang J, Benitez A, DeMarshall M, Tobias J, et al. The esophageal organoid system reveals functional interplay between notch and cytokines in reactive epithelial changes. Cell Mol Gastroenterol Hepatol. 2018;5(3):333–352. doi:10.1016/j.jcmgh.2017.12.013
- Natsuizaka M, Ohashi S, Wong GS, Ahmadi A, Kalman R, Budo D, Klein-Szanto A, Herlyn M, Diehl J, Nakagawa H. Insulin-like growth factor-binding protein-3 promotes transforming growth factor-b1-mediated epithelial-to-mesenchymal transition and motility in transformed human esophageal cells. Carcinogenesis. 2010;31(8):1344–1353. doi:10.1093/carcin/bgq108.
- Prochazka L, Tesarik R, Turanek J. Regulation of alternative splicing of CD44 in cancer. Cell Signal. 2014;26(10):2234–2239. doi:10.1016/j.cellsig.2014.07.011.
- Xia P, Xu XY. PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res. 2015;5(5):1602–1609. https://pubmed.ncbi.nlm.nih.gov/26175931/.
- Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34(2):280–300. doi:10.1002/med.21288.
- Ueno N, Shimizu A, Kanai M, Iwaya Y, Ueda S, Nakayama J, Seo M. Enhanced expression of fibroblast growth factor receptor 3 IIIc promotes human esophageal carcinoma cell proliferation. J Histochem Cytochem. 2016;64(1):7–17. doi:10.1369/0022155415616161.
- Shi Y, Liu X, Fredimoses M, Song M, Chen H, Liu K, Lee M, Dong Z. FGFR2 regulation by picrasidine Q inhibits the cell growth and induces apoptosis in esophageal squamous cell carcinoma. J Cell Biochem. 2018;119(2):2231–2239. doi:10.1002/jcb.26385.
- Takase N, Koma Y, Urakawa N, Nishio M, Arai N, Akiyama H, Shigeoka M, Kakeji Y, Yokozaki H. NCAM- and FGF-2-mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor-associated macrophages and cancer cells. Cancer Lett. 2016;380(1):47–58. doi:10.1016/j.canlet.2016.06.009.
- Shin WS, Lee HW, Lee ST. Catalytically inactive receptor tyrosine kinase PTK7 activates FGFR1 independent of FGF. FASEB J. 2019;33(11):12960–12971. doi:10.1096/fj.201900932R.
- Rivas S, Gómez-Oro C, Antón I, Wandosell F. Role of Akt isoforms controlling cancer stem cell survival, phenotype and self-renewal. Biomedicines. 2018;6(1):29. doi:10.3390/biomedicines6010029.
- Almozyan S, Colak D, Mansour F, Alaiya A, Al-Harazi O, Qattan A, Al-Mohanna F, Al-Alwan M, Ghebeh H. PD-L1 promotes OCT4 and Nanog expression in breast cancer stem cells by sustaining PI3K/AKT pathway activation. Int J Cancer. 2017;141(7):1402–1412. doi:10.1002/ijc.30834.
- Li K, Yang L, Li J, Guan C, Zhang S, Lao X, Ouyang D, Zheng G, Gao S, Wang D, et al. TGFβ induces stemness through non-canonical AKT-FOXO3a axis in oral squamous cell carcinoma. EBioMedicine. 2019;48:70–80. doi:10.1016/j.ebiom.2019.09.027.
- Yang Z, Zhang C, Feng Y, Qi W, Cui Y, Xuan Y. Tenascin-C is involved in promotion of cancer stemness via the Akt/HIF1ɑ axis in esophageal squamous cell carcinoma. Exp Mol Pathol. 2019;109:104239. doi:10.1016/j.yexmp.2019.03.007.
- Zhang HF, Wu C, Alshareef A, Gupta N, Zhao Q, Xu X, Jiao J, Li E, Xu L, Lai R. The PI3K/AKT/c-MYC axis promotes the acquisition of cancer stem-like features in esophageal squamous cell carcinoma. Stem Cells. 2016;34(8):2040–2051. doi:10.1002/stem.2395.
- Wang W, He S, Zhang R, Peng J, Guo D, Zhang J, Xiang B, Li L. ALDH1A1 maintains the cancer stem-like cells properties of esophageal squamous cell carcinoma by activating the AKT signal pathway and interacting with β-catenin. Biomed Pharmacother. 2020;125:109940. doi:10.1016/j.biopha.2020.109940.
- Liu C, Chou K, Hsu J, Lin J, Hsu T, Yen D, Hung S, Hsu H. High metabolic rate and stem cell characteristics of esophageal cancer stem-like cells depend on the Hsp27–AKT–HK2 pathway. Int J Cancer. 2019;145(8):2144–2156. doi:10.1002/ijc.32301.
- Kumazoe M, Takai M, Bae J, Hiroi S, Huang Y, Takamatsu K, Won Y, Yamashita M, Hidaka S, Yamashita S, et al. FOXO3 is essential for CD44 expression in pancreatic cancer cells. Oncogene. 2017;36(19):2643–2654. doi:10.1038/onc.2016.426
- Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-Β-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463(7281):676–680. doi:10.1038/nature08734.
- Viaene AI, Baert JH. Expression of cytokeratin-mRNAs in squamous-cell carcinoma and balloon-cell formation of human oesophageal epithelium. Histochem J. 1995;27(1):69–78. doi:10.1007/BF00164174.
- Banks-Schlegel S, Green H. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol. 1981;90(3):732–737. doi:10.1083/jcb.90.3.732.
- Xiong Z, Ren S, Chen H, Liu Y, Huang C, Zhang Y, Odera J, Chen T, Kist R, Peters H, et al. PAX9 regulates squamous cell differentiation and carcinogenesis in the oro-oesophageal epithelium. J Pathol. 2018;244(2):164–175. doi:10.1002/path.4998
- Luo A, Yu X, Li G, Ma G, Chen H, Ding F, Li Y, Liu Z. Differentiation-associated genes regulated by c-Jun and decreased in the progression of esophageal squamous cell carcinoma. PLoS One. 2014;9(5). doi:10.1371/journal.pone.0096610.
- Ohashi S, Natsuizaka M, Naganuma S, Kagawa S, Kimura S, Itoh H, Kalman R, Nakagawa M, Darling D, Basu D, et al. A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res. 2011;71(21):6836–6847. doi:10.1158/0008-5472.CAN-11-0846
- Ohashi S, Natsuizaka M, Yashiroohtani Y, Kalman R, Nakagawa M, Wu L, Kleinszanto A, Herlyn M, Diehl J, Katz J, et al. NOTCH1 and NOTCH3 coordinate esophageal squamous differentiation through a csl-dependent transcriptional network. Gastroenterology. 2010;139(6):2113–2123. doi:10.1053/j.gastro.2010.08.040
- Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood 1990 Nov 1;76(9):1704–1709. doi:10.1182/blood.V76.9.1704.1704.
- Campos B, Wan F, Farhadi M, Ernst A, Zeppernick F, Tagscherer KE, Ahmadi R, Lohr J, Dictus C, Gdynia G, et al. Differentiation therapy exerts antitumor effects on stem-like glioma cells. Clin Cancer Res. 2010 May 15;16(10):2715–2728. doi:10.1158/1078-0432.CCR-09-1800.
- Tso JL, Yang S, Menjivar JC, Yamada K, Zhang Y, Hong I, Bui Y, Stream A, McBride WH, Liau LM, et al. Bone morphogenetic protein 7 sensitizes O6-methylguanine methyltransferase expressing-glioblastoma stem cells to clinically relevant dose of temozolomide. Mol Cancer. 2015 Nov 6;14:189. doi:10.1186/s12943-015-0459-1.
- González-Gómez P, Crecente-Campo J, Zahonero C, De La Fuente M, Hernández-Laín A, Mira H, Sánchez-Gómez P, Garcia-Fuentes M. Controlled release microspheres loaded with BMP7 suppress primary tumors from human glioblastoma. Oncotarget. 2015 May 10;6(13):10950–10963. doi:10.18632/oncotarget.3459.
