ABSTRACT
This study aimed to compare the treatment efficacy and tolerance between drug-eluting beads transarterial chemoembolization (DEB-TACE) and conventional transarterial chemoembolization (cTACE) in hepatocellular carcinoma (HCC) patients with arterioportal fistula (APF). A total of 44 HCC patients with APF scheduled for DEB-TACE (N = 24, as DEB-TACE group) or cTACE (N = 20, as cTACE group) were recruited. Treatment response, hepatic function, and adverse events were assessed or recorded. Besides, progression-free survival (PFS) and overall survival (OS) were calculated. Total treatment response was better in the DEB-TACE group compared with the cTACE group (P = .012). Meanwhile, the objective response rate (87.5% versus 60.0%) was higher (P = .013), while the disease control rate (95.8% versus 85.0%) was similar in the DEB-TACE group compared to the cTACE group (P = .213). Besides, PFS (mean value: 12.2 (95%CI: 9.9–14.6) months versus 7.8 (95%CI: 5.6–10.0) months) (P = .037), but not OS (mean value: 20.0 (95%CI: 18.1–21.9) months versus. 18.6 (95%CI: 15.4–21.8) months) (P = .341) was prolonged in DEB-TACE group compared with cTACE group. Regarding the safety, Child-Pugh stage, albumin level, and bilirubin level after treatment were all similar between the DEB-TACE group and cTACE group (all P > .05); moreover, no difference was found in the occurrence of adverse events during or after treatment between the two groups (all P > .05). Moreover, subsequent analyses found that embolic materials for APF (microspheres) in the DEB-TACE group did not affect the treatment efficacy (all P > .05). DEB-TACE promotes treatment response and PFS compared with cTACE and shows good safety in HCC patients with APF.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer that causes 0.84 million newly diagnosed cases and 0.78 million deaths worldwide each year.Citation1,Citation2 Meanwhile, the burden of HCC is rising partly due to the increasing aging and populations.Citation3 Currently, treatment strategies for HCC consist of curative treatments (such as tumor resection, liver transplantation, and ablation) and palliative treatments (such as transarterial chemoembolization (TACE), targeted therapy, and immunotherapy), among which TACE is recommended as the standard treatment for patients with intermediate-stage HCC.Citation4–6 Besides, TACE is also widely performed as a bridge to liver transplant, for the intention of downstaging HCC burden, or in combination with systematic therapy.Citation7,Citation8
To make matters worse, HCC patients may be complicated with arterioportal fistula (APF), which is an abnormal connection between hepatic artery and portal venous that might lead to portal hypertension, refractory ascites, and hepatic encephalopathy.Citation9,Citation10 When performing conventional TACE (cTACE) in HCC patients with APF, some embolic materials such as gelatin sponge may pass through the fistula, which worsens the treatment efficacy of TACE and raises the risk of hepatic ischemia.Citation11 Therefore, previous studies have used novel embolic materials such as polyvinyl alcohol (PVA) particles and cyanoacrylate glue to perform TACE in HCC patients with APF, which improves the therapeutic effect to some extent.Citation11,Citation12
Drug-eluting beads TACE (DEB-TACE) is a new generation of TACE that uses microspheres as both drug carriers and embolic materials, which is characterized by persistent and stable drug release and is proved to exert better treatment efficacy than cTACE in HCC patients.Citation13–15 Besides, DEB-TACE is also well-tolerated in HCC patients.Citation16,Citation17 Since the size of the microspheres for DEB-TACE is available for a wide range, using microspheres with larger sizes (for instance, 300–500 μm) for DEB-TACE may be a potential solution for treating HCC patients with APF.Citation18 Based on the above information, we hypothesized that DEB-TACE might be an appropriate treatment for HCC patients with APF, however, relevant information was largely unclear. Therefore, we performed the current study and aimed to compare the treatment efficacy and tolerance of DEB-TACE versus cTACE in treating HCC patients with APF.
Patients and methods
Patients
Between September 2018 and May 2020, 44 primary HCC patients with APF treated by DEB-TACE or cTACE were consecutively recruited in this study. The main inclusion criteria were as follows: (1) diagnosed as primary HCC according to clinical and pathological findings; (2) complicated with APF; (3) age above 18 years old; (4) scheduled for DEB-TACE or cTACE treatment; (5) provided the written informed consents. The main exclusion criteria were: (1) secondary liver cancer; (2) contraindications to angiography, embolization procedure, or artery puncture; (3) history of liver transplantation; (4) history of hematological malignancies or other malignant tumors; (5) Child-Pugh grade C; (6) concomitant with severe cardiovascular and cerebrovascular diseases. This study was approved by the Institutional Research Ethics Committee of Beijing Ditan Hospital.
Data collection
After recruitment, demographic characteristics, clinical features, and laboratory indexes were collected, which included: age, gender, causes of cirrhosis, Eastern Cooperative Oncology Group performance status (ECOG PS) score, portal vein invasion, the grade of APF, tumor distribution, largest nodule diameter, Child-Pugh stage, Barcelona Clinic Liver Cancer (BCLC) stage and levels of albumin as well as bilirubin. The grade of APF was classified into four grades according to the severity of fistulas:Citation12 grade 0, no or limited fistula flow, less than that of the subsegmental portal vein; grade 1, fistula flow regurgitated into the segmental portal vein; grade 2, fistula flow regurgitated into the ipsilateral main portal vein of each lobe; grade 3, fistula flow regurgitated into the contralateral lobe and/or the main portal vein.
Grouping
Patients were assigned to the cTACE group (N = 20) or DEB-TACE group (N = 24) according to the treatment. In the cTACE group, ethiodized poppyseed oil (EPO) (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) mixed with epirubicin hydrochloride (Zhejiang Hisun pharmaceutical Co., Ltd., Zhejiang, China) (60–80 mg) were used for chemoembolization, and the PVA particles (Cook Medical LLC, Bloomington, USA) was used for embolism of APF. In the DEB-TACE group, CalliSpheres® microspheres (100–300 or 300–500 μm CalliSpheres® microspheres according to clinical features of patients) (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) loading with epirubicin (60–80 mg) was used for chemoembolization. Based on the materials used for embolism of APF, the patients in the DEB-TACE group were further divided into two subgroups: (1) DD-TACE group (N = 12): CalliSpheres® microspheres (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu Province, China) were used to embolize the APF; (2) PD-TACE group (N = 12): Polyvinyl Alcohol (PVA) particles (Cook Medical LLC, Bloomington, USA) were used to embolize the APF.
Embolization procedures
In both groups, angiography was performed to detect the feeding artery and location of the tumor, as well as the number, location, and grade of APF, as follows: percutaneous femoral artery intubation was carried out using the Seldinger technique. Then, a 5 F vascular introducer (Boston Scientific, USA) was selectively placed into the hepatic artery, superior mesenteric artery, or diaphragmatic artery, following that iohexol (General Electric Pharmaceutical (Shanghai) Co. LTD, Shanghai, China) was further injected at a speed of 3–6 mL per second with a total amount of 15–30 mL each time. Subsequently, the image was captured at a speed of 4–6 frames per second. After hepatic angiography, based on whether the microcatheter could pass by the region of APF, embolism was performed as follows: (1) if the microcatheter could pass by the region of APF, the feeding artery of the tumor was firstly embolized with drug-loaded microspheres or drug-mixed EPO. After completion of chemoembolization for the tumor, the microcatheter was retracted to the region of APF to embolize the fistula using drug-loaded microspheres or PVA particles; (2) if the microcatheter could not pass by the region of APF, the chemoembolization was performed in an identified region of the feeding artery where the tumor and the APF could be embolized simultaneously. After the embolization, the microcatheter was pulled out, and the wound was pressed for hemostasis and then bandaged. The cycles of chemoembolization that patients received were determined according to clinical needs. In general, the median value of treatment cycles was 3.0 (range: 1.0–7.0) cycles.
Assessment of treatment response
Treatment response was evaluated at 4–6 weeks after the DEB-TACE or cTACE referring to the modified Response Evaluation Criteria in Solid Tumors (mRECIST),Citation19 which was classified as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Besides, the objective response rate (ORR) was calculated as the sum of CR and PR, and the disease control rate (DCR) was calculated as the sum of CR, PR, and SD.
Assessment of safety
At 4–6 weeks after DEB-TACE or cTACE treatment, the hepatic function was evaluated by the Child-Pugh stage; meanwhile, levels of bilirubin, as well as albumin, were shown. In addition, the adverse events during and after the treatment were also recorded.
Assessment of survival
The deadline for follow-up was November 2020, and the median follow-up duration was 12.4 months (range: 3.8–23.0 months). Progression-free survival (PFS) was calculated from the time of operation to the time of disease progression or death. Overall survival (OS) was calculated from the time of operation to the time of death.
Statistical analysis
Statistical analysis was performed using SPSS 24.0 statistical software (SPSS Inc., Chicago, USA), and figures were plotted by GraphPad Prism 7.01 software (GraphPad Software Inc., San Diego, USA). Shapiro-Wilk test was used to determine the normality of quantitative data. Normal distributed quantitative data were shown as mean ± standard deviation (SD). Non-normal distributed quantitative data were described as median (25th-75th quantiles). Qualitative data were presented as count (percentage). Comparison of normal distributed quantitative data between the two groups was determined by Student’s t-test. Comparisons of non-normal distributed quantitative data and ordered qualitative data between two groups were determined by the Wilcoxon rank-sum test. Comparison of disordered qualitative data between two groups was determined by Chi-square test or Yate’s corrected Chi-square test. PFS and OS were displayed using the Kaplan-Meier curve, and comparisons of PFS and OS between two groups were determined by Log-rank (Mantel-Cox) test. P-value <0.05 was considered significant.
Results
Comparison of basic characteristics between DEB-TACE group and cTACE group
The cTACE group consisted of 1 (5.0%) female and 19 (95.0%) males with a mean age of 58.0 ± 9.9 years, and the DEB-TACE group consisted of 1 (4.2%) female and 23 (95.8%) males with a mean age of 58.4 ± 9.6 years. Comparison analyses showed that no difference was found in age, gender, cause of cirrhosis, ECOG PS score, the occurrence of portal vein invasion, APF grade, tumor distribution, the median level of largest nodule diameter, Child-Pugh stage, BCLC stage, albumin level of bilirubin level between the two groups (all P > .05) ().
Table 1. Demographic and clinical characteristics of HCC patients with APF
Comparison of treatment response between DEB-TACE group and cTACE group
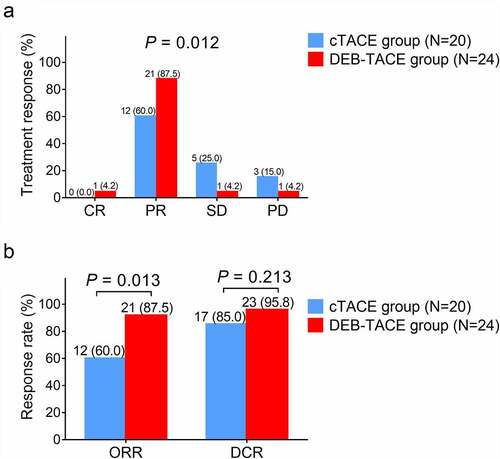
Total treatment response was better in the DEB-TACE group compared with the cTACE group (P = .012) ()). Meanwhile, ORR was increased in the DEB-TACE group compared with the cTACE group (87.5% vs. 60.0%) (P = .013), while DCR remained similar between the DEB-TACE group and cTACE group (95.8% vs. 85.0%) (P = .213) ()).
Figure 1. Treatment response in DEB-TACE group and cTACE group. A: Comparison of total treatment response between DEB-TACE group and cTACE group; B: Comparison of ORR and DCR between DEB-TACE group and cTACE group. DEB-TACE: drug-eluting beads transarterial chemoembolization; cTACE: conventional transarterial chemoembolization; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: objective response rate; DCR: disease control rate.
Comparison of PFS and OS between DEB-TACE group and cTACE group
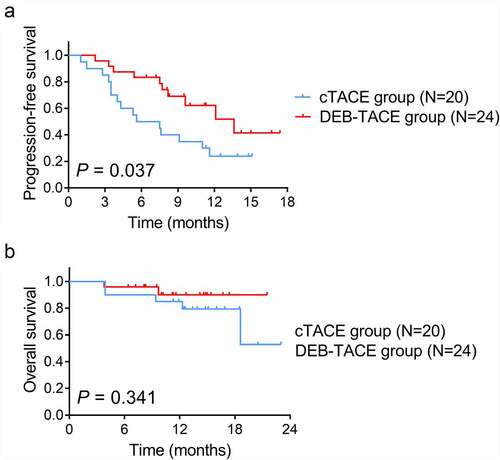
PFS was increased in the DEB-TACE group compared with cTACE group (mean value: 12.2 (95% CI: 9.9–14.6) months vs. 7.8 (95% CI: 5.6–10.0) months) (P = .037) ()). However, OS did not vary between the DEB-TACE group and cTACE group (mean value: 20.0 (95% CI: 18.1–21.9) months vs. 18.6 (95% CI: 15.4–21.8) months) (P = .341) ()).
Figure 2. PFS and OS in DEB-TACE group and cTACE group. A: Comparison of PFS between DEB-TACE group and cTACE group; B: Comparison of OS between DEB-TACE group and cTACE group. DEB-TACE: drug-eluting beads transarterial chemoembolization; cTACE: conventional transarterial chemoembolization; PFS: progression-free survival; OS: overall survival.
Comparison of liver function after treatment between DEB-TACE group and cTACE group
No difference was found in the Child-Pugh stage (P = .273), albumin level (P = .176), or bilirubin level (P = .248) after treatment between the DEB-TACE group and cTACE group ().
Table 2. Analysis of hepatic function after treatment
Comparison of adverse events between DEB-TACE group and cTACE group
The most commonly occurring adverse events in the DEB-TACE group and cTACE group were fever, myelosuppression, and abdominal distension. It was of note that the occurrences of fever, myelosuppression, abdominal distension, abdominal pain, nausea, liver abscess, anorexia, hepatic failure, and upper gastrointestinal hemorrhage were all comparable between the DEB-TACE group and cTACE group (all P > .05) ().
Table 3. Adverse events
Comparison of treatment response and survival between DD-TACE group and PD-TACE group
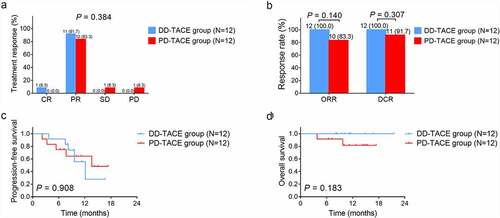
Total treatment response ()), ORR, and DCR ()) were all similar between the DD-TACE group and PD-TACE group (all P > .05). Besides, PFS (P = .908) ()) and OS (P = .183) ()) did not vary between the two groups either.
Figure 3. Treatment response and survival in the DD-TACE group and PD-TACE group. A: Comparison of total treatment response between DD-TACE group and PD-TACE group; B: Comparison of ORR and DCR between DD-TACE group and PD-TACE group; C: Comparison of PFS between DD-TACE group and PD-TACE group; D: Comparison of OS between DD-TACE group and PD-TACE group. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: objective response rate; DCR: disease control rate; PFS: progression-free survival; OS: overall survival.
Discussion
The main findings of this study could be summarized as follows: (1) DEB-TACE improved treatment response compared with cTACE in HCC patients with APF; (2) DEB-TACE prolonged PFS but not OS compared with cTACE in HCC patients with APF; (3) DEB-TACE had comparable safety profile to cTACE in HCC patients with APF; (4) DD-TACE and PD-TACE shared similar effect on treatment response and survival in HCC patients with APF.
The occurrence of APF in HCC patients is reported to be 28.8%–63.2%, and there exist potential concerns in performing TACE in these patients since some embolic materials may pass through the APF due to the diameter and further causes unfavorable treatment effect.Citation9,Citation11 Since TACE is a standard treatment for intermediate-stage HCC patients (although a previous meta-analysis suggests that TACE is not superior to transarterial embolizationCitation20), searching for feasible approaches to perform TACE in HCC patients with APF is urgent.Citation5 Therefore, previous studies have focused on novel embolic materials with different sizes available, which include PVA particles and cyanoacrylate glue particles, and these studies have achieved certain progress on performing TACE in HCC patients with APF.Citation11,Citation12 Regarding DEB-TACE, it can be performed by using microspheres of varied sizes, which means that the microspheres with large sizes for DEB-TACE may not easily pass through the fistula.Citation18 Besides, DEB-TACE also possesses a more durable and stable drug release compared with cTACE, which enhances its treatment efficacy and safety in HCC patients.Citation15–17 Although previous randomized, controlled trials indicate that no difference is found in tumor response, 1-year or 2-year survival rate between patients receiving DEB-TACE or TACE,Citation21,Citation22 a recent meta-analysis reveals that patients undergoing DEB-TACE achieve higher ORR, DCR, and better PFS than those undergoing cTACE.Citation23 However, studies investigating the treatment efficacy of DEB-TACE in HCC patients with APF are quite rare. Therefore, we performed this study and found that DEB-TACE improved total treatment response and ORR in HCC patients with APF. Possible explanations for these data might be that: (1) the microspheres used for DEB-TACE released drug more stably and persistently in HCC patients with APF, which resulted in a higher level of locoregional drug concentration to increase the cytotoxic effect of the drug on HCC cells;Citation13 (2) the microspheres used for DEB-TACE might achieve better embolization effect in HCC patients with APF, which improved the ischemic effect on HCC;Citation15 however, further studies should be conducted to verify that. Therefore, increased total treatment response and ORR was observed in patients who received DEB-TACE than those who received cTACE.
As to the long-term treatment efficacy of DEB-TACE in HCC patients with APF, it is reported that DEB-TACE slightly increases the cumulative survival rate than PVA-TACE in HCC patients with APF.Citation18 In the present study, it was observed that DEB-TACE improved PFS in HCC patients with APF. These data could be explained by that: the persistently and stably high drug concentration, as well as the good embolization effect of DEB-TACE, induced higher apoptosis and necrosis in HCC, which hindered the progression of HCC. However, DEB-TACE did not vary OS in HCC patients with APF. Possible explanations might be that (1) the relatively small sample size of this study resulted in lower statistical power; (2) the follow-up duration was not long enough to observe the significant difference in OS of the two groups. Moreover, according to the material that embolized the APF, patients receiving DEB-TACE were further divided into the DD-TACE group and PD-TACE group. Data showed that treatment response and survival did not vary between these two groups. A possible explanation might be that: the difference in the materials used for embolism of APF mainly influenced the embolization of APF but had little effect on that of HCC itself.
Regarding the safety of DEB-TACE, one previous study suggests that DEB-TACE is tolerable in HCC patients with APF.Citation18 Part in line with that previous study, we found that DEB-TACE did not vary Child-Pugh stage, albumin level, and bilirubin level compared with cTACE in HCC patients with APF. These data suggested that DEB-TACE did not change liver function in HCC patients with APF compared with cTACE. Regarding the adverse events, HCC patients who receive TACE (including cTACE and DEB-TACE) often occur fever, abdominal pain as well as nausea and vomiting.Citation16,Citation17 In this study, we found that fever was the most commonly occurring adverse event in both patients who received DEB-TACE or cTACE. Notably, the occurrence of adverse events was comparable in patients who received DEB-TACE or cTACE, which could be explained by that: DEB-TACE is characterized by stable and durable locoregional drug level, thus it might have less systematic or hepatic toxicity;Citation13 however, the relatively small sample size of this study might cause low statistical power; therefore, we failed to observe the difference in the occurrences of adverse events caused by DEB-TACE and cTACE in HCC patients with APF. Our data further indicated that DEB-TACE and cTACE had equal safety profiles in HCC patients with APF.
It could be mentioned that in the current study when performing the embolization procedure, the size of embolic material was not directly determined by APF grade, but there was an indirect association between the size of embolic material and APF grade. If the microcatheter could pass through the area of APF, then embolic materials with smaller sizes would be chosen to seek complete embolization. Conversely, if the microcatheter could not pass through the area of APF, then embolic materials with larger size would be chosen to realize the simultaneous embolization of APF and tumor, thus avoiding ectopia embolization. Generally, according to our clinical experience, the higher the APF grade, the lower the probability of microcatheter crossing the APF region. Meanwhile, according to our previous clinical experience, both cTACE and DEB-TACE could be applied to patients with different APF grades, therefore, we did not consider APF grades in the grouping, and the correlation of embolization techniques and APF grades was not assessed. Meanwhile, according to , no difference was found in APF grade between the cTACE group and the DEB-TACE group. Thus, APF grades did not affect grouping and the comparative analyses between the two groups.
Several limitations in this study should be clarified. Firstly, the sample size of this study was relatively small, which may lead to low statistical power; therefore, further studies should enroll more HCC patients with APF who received DEB-TACE or cTACE for verifying the data of this present study. Secondly, the follow-up duration of this study was relatively short, which did not support us to observe the statistical significance in the OS between DEB-TACE and cTACE groups; thus, further studies with a longer follow-up duration might be conducted. Third, this study was non-randomized, which might induce some biases and confounding factors; therefore, further randomized, controlled trials should be conducted for verification of these data.
To be conclusive, DEB-TACE enhances treatment response and PFS compared with cTACE and shows good tolerance in HCC patients with APF. Therefore, DEB-TACE may be an acceptable strategy for these patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi:10.1038/nrdp.2016.18.
- Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156(2):477–91 e1. doi:10.1053/j.gastro.2018.08.065.
- de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62(4):1190–1200. doi:10.1002/hep.27969.
- Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han K-H, Chawla YK, Shiina S, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi:10.1007/s12072-017-9799-9.
- Lanza E, Donadon M, Poretti D, Pedicini V, Tramarin M, Roncalli M, Rhee H, Park YN, Torzilli G. Transarterial Therapies for Hepatocellular Carcinoma. Liver Cancer. 2016;6(1):27–33. doi:10.1159/000449347.
- Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi:10.1016/j.ctrv.2018.11.002.
- Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–1195. doi:10.1016/j.jhep.2015.02.010.
- Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol. 2015;62(1):S144–56. doi:10.1016/j.jhep.2015.02.007.
- Choi JW, Lee JM, Kim HC, Lee M, Hur S, Jae HJ, Chung JW. Iatrogenic Arterioportal Fistula Caused by Radiofrequency Ablation of Hepatocellular Carcinoma: clinical Course and Treatment Outcomes. J Vasc Interv Radiol. 2020;31(5):728–736. doi:10.1016/j.jvir.2019.10.020.
- Luo MY, Shan H, Jiang ZB, Liang WW, Zhang JS, Li LF. Capability of multidetector CT to diagnose hepatocellular carcinoma-associated arterioportal shunt. World J Gastroenterol. 2005;11(17):2666–2669. doi:10.3748/wjg.v11.i17.2666.
- Liu QS, Mei QL, Li YH. Polyvinyl alcohol terminal chemoembolization for hepatocellular carcinoma with hepatic arteriovenous shunts: safety, efficacy, and prognostic factors. Eur J Radiol. 2017;89:277–283. doi:10.1016/j.ejrad.2016.04.016.
- Shi HB, Yang ZQ, Liu S, Zhou WZ, Zhou CG, Zhao LB, Xia J-G, Li L-S. Transarterial embolization with cyanoacrylate for severe arterioportal shunt complicated by hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(2):412–421. doi:10.1007/s00270-012-0410-4.
- Melchiorre F, Patella F, Pescatori L, Pesapane F, Fumarola E, Biondetti P, Brambillasca P, Monaco C, Ierardi AM, Franceschelli G, et al. DEB-TACE: a standard review. Future Oncol. 2018;14(28):2969–2984. doi:10.2217/fon-2018-0136.
- Zhu D, Yuan D, Wang Z, Chen S. Efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) combined with radiofrequency ablation versus DEB-TACE alone in Chinese hepatocellular carcinoma patients. Medicine (Baltimore). 2019;98(26):e15682. doi:10.1097/MD.0000000000015682.
- Zhang X, Zhou J, Zhu DD, Huang J, Sun JH, Li TF, Shi C-S, Sun Z-C, Hou Q-M, Peng Z-Y, et al. CalliSpheres(R) drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol. 2019;21(2):167–177. doi:10.1007/s12094-018-1902-8.
- Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17(8):510–517. doi:10.1111/1751-2980.12380.
- Luz JH, Luz PM, Martin HS, Gouveia HR, Levigard RB, Nogueira FD, Rodrigues BC, de Miranda TN, Mamede MH. DEB TACE for Intermediate and advanced HCC - Initial Experience in a Brazilian Cancer Center. Cancer Imaging. 2017;17(1):5. doi:10.1186/s40644-017-0108-6.
- Xiao YD, Ma C, Zhang ZS, Liu J. Safety and efficacy assessment of transarterial chemoembolization using drug-eluting beads in patients with hepatocellular carcinoma and arterioportal shunt: a single-center experience. Cancer Manag Res. 2019;11:1551–1557. doi:10.2147/CMAR.S193948.
- Liang B, Xiang H, Ma C, Xiong B, Ma Y, Zhao C, et al. Comparison of chemoembolization with CalliSpheres((R)) microspheres and conventional chemoembolization in the treatment of hepatocellular carcinoma: a multicenter retrospective study. Cancer Manag Res. 2020;12:941–956. doi:10.2147/CMAR.S187203.
- Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, et al. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: a meta-analysis of randomized trials. United European Gastroenterol J. 2017;5(4):511–518. doi:10.1177/2050640616673516.
- Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–264. doi:10.1038/bjc.2014.199.
- Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi:10.1007/s00270-009-9711-7.
- Liang B, Makamure J, Shu S, Zhang L, Sun T, Zheng C. Treatment Response, Survival, and Safety of Transarterial Chemoembolization With CalliSpheres((R)) Microspheres Versus Conventional Transarterial Chemoembolization in Hepatocellular Carcinoma: a Meta-Analysis. Front Oncol. 2021;11:576232. doi:10.3389/fonc.2021.576232.
