ABSTRACT
This real-world study examined the prevalence of programmed death ligand-1 (PD-L1) expression and assessed the frequency of microsatellite instability-high (MSI-H) status and Epstein-Barr virus (EBV) positivity in Japanese patients with advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. This multicenter (5 sites), retrospective, observational study (November 2018–March 2019) evaluated Japanese patients with advanced gastric and GEJ adenocarcinoma after surgical resection (Stage II/III at initial diagnosis) or unresectable advanced cancer (Stage IV). The primary objectives were prevalence of PD-L1 expression (combined positive score [CPS] ≥1), MSI status, and EBV positivity. Tumor specimens of 389/391 patients were analyzed (male, 67.1%; mean age, 67.6 ± 12.2 years); 241/389 (62%) were PD-L1 positive, 24/379 (6.3%) had MSI-H tumors, and 13/389 (3.3%) were EBV positive. PD-L1 expression was higher in tumor-infiltrating immune cells than in tumor cells for lower CPS cutoffs. Among patients with MSI-H tumors and EBV-positive tumors, 19/24 (79.2%) and 9/13 (69.2%), respectively, were PD-L1 positive. A greater proportion of patients with MSI-H tumors (83.3% [20/24]) were PD-L1 positive than those with MSI-low/stable tumors (60.8% [216/355]; p = .0297); similarly, an association was observed between history of H pylori infection and PD-L1 expression. A higher proportion of patients with MSI-H tumors demonstrated PD-L1 expression with a CPS ≥10 (66.7% [16/24]) vs those with MSI-low/stable tumors (24.8% [88/355]; p < .0001). The prevalence of PD-L1 positivity among Japanese patients was comparable to that in previous pembrolizumab clinical trials and studies in gastric cancer. Particularly, higher PD-L1 expression was observed in MSI-H tumors.
Introduction
Gastric cancer (GC) is the fifth most common cancer and accounts for 5.6% of all new cancers reported worldwide in 2020.Citation1
The prevalence of GC in Japan is high and despite diagnosis of GC at an early stage due to regular screening programs, the prevalence of advanced GC and mortality continue to be high. Consequently, surgical options are limited and there exists an unmet need for the treatment of advanced-stage GC.Citation2,Citation3 Therefore, numerous therapeutic options based on molecular characteristics of the tumor are being evaluated to improve GC treatment outcomes.
The Cancer Genome Atlas (TCGA) project proposed a molecular classification based on comprehensive genomic modeling, dividing GC into 4 subtypes: Epstein-Barr virus (EBV)–positive tumors, microsatellite instability-high (MSI-H) tumors, genomically stable tumors, and tumors with chromosomal instability.Citation4 Among them, the correlation between high expression of programmed death ligand-1 (PD-L1) and prognosis in patients with GC has been extensively investigated. Studies report that PD-L1 expression commonly examined in tumor cells (TCs) is mostly associated with a poor prognosis and shorter overall survival (OS) in patients in East Asia,Citation5–7 while a good prognosis was also reported.Citation8,Citation9 However, based on the TCGA classification, an increasing role of EBV-positive and MSI-H subtypes of GC is being observed with PD-L1 expression,Citation10–12 indicating the involvement of the immune microenvironment in the development of GC.Citation8,Citation13 It was reported that PD-L1 expression in immune cells in EBV-positive or MSI-H tumors was associated with favorable prognosis.Citation14,Citation15 However, outcomes based on evaluation of these molecular characteristics are likely to be confounded by choice of assays, scoring methods used, and the stage of tumor. Currently, the combined positive score (CPS) is validated as a sensitive method to score PD-L1 expression in various cancers, including GC,Citation16–19 especially near the low cutoff point.Citation17,Citation18,Citation20,Citation21
For example, with reference to the application of CPS as a diagnostic method, a unique anti–PD-1 inhibitor such as pembrolizumab was approved for the treatment of head and neck squamous cell cancer, esophageal squamous cell carcinoma, and triple-negative breast cancer in Japan.Citation22,Citation23 Pembrolizumab is also indicated for the treatment of adult patients with unresectable or metastatic MSI-H or mismatch repair–deficient solid tumors that have progressed following prior treatment and who have no satisfactory alternative treatment options.Citation24 In Japan, pembrolizumab was approved as monotherapy for MSI-H tumors, including GC/gastroesophageal junction (GEJ) adenocarcinoma, in 2018.Citation25
To date, real-world data on PD-L1 expression determined by CPS have not been evaluated in a Japanese population with advanced GC or GEJ cancer. Consequently, this real-world study examined the prevalence of PD-L1 expression (CPS ≥1) determined using a Food and Drug Administration–approved kit, MSI-H frequency, and EBV positivity in Japanese patients with advanced GC and GEJ adenocarcinoma. The primary objectives of this study were to examine the prevalence of PD-L1 protein expression (CPS ≥1) and assess MSI status and EBV positivity in Japanese patients with advanced gastric and GEJ adenocarcinoma. Other objectives were to characterize the association between PD-L1 expression and biomarker subtypes; determine the association between PD-L1 expression and patients’ clinicopathological features, including site of occurrence, stage of GC, and histological subtype; and summarize PD-L1 protein expression by CPS distribution.
Methods
Patients and study design
This was a multicenter (5 sites in Japan: Kitasato University School of Medicine, Tokai University School of Medicine, Yokohama City University Medical Center, Juntendo University, and Nara Medical University), retrospective, observational study conducted between November 2018 and March 2019 to obtain real-world data from Japanese patients with advanced gastric and GEJ adenocarcinoma ().
Figure 1. Study design
EBV, Epstein-Barr virus; GEJ, gastroesophageal junction; HER-2, human epidermal growth factor receptor-2; H. pylori, Helicobacter pylori; IHC, immunohistochemistry; ISH, insitu hybridization; MSI, microsatellite instability; PCR, polymerase chain reaction; PD-L1, programmed death ligand-1.
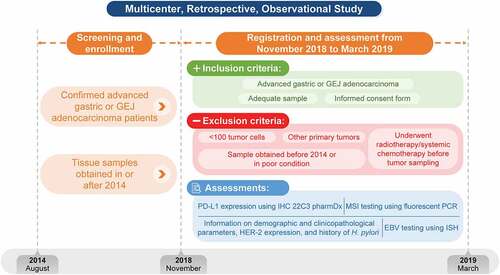
The study enrolled Japanese patients aged ≥20 years (at sampling) with Stage II or III advanced gastric and GEJ adenocarcinoma following surgical resection in or after 2014 and who experienced recurrence at or after 6 months following postoperative adjuvant chemotherapy or who were diagnosed with unresectable advanced cancer (Stage IV) and underwent tumor sampling in or after 2014. Patients were required to have archived tissue specimens adequate to provide 14 paraffin sections. All patients provided informed consent for this study. Patients were excluded if they had other primary tumor types, had undergone radiotherapy and/or systemic chemotherapy prior to tumor sampling, had specimens that had been obtained before 2014, had tissue specimens that had been sectioned >6 months prior to sampling, had <100 TCs, or had tissue specimens that had been preserved poorly. The screening method for registration in this study was to trace back and check the diagnosis date of advanced GC or metastatic GC in medical records of each patient candidate, and among these candidates, patients eligible for this study were registered in order from the newest diagnostic date in each center.
A total of 391 archived tissue specimens from Japanese patients with gastric and GEJ adenocarcinoma who have experienced recurrence or metastasis were tested for PD-L1 protein expression, MSI status, and EBV positivity.
Assessments
PD-L1 expression was assessed using the PD-L1 IHC 22C3 pharmDx kit (Agilent Technologies, Carpinteria, CA, USA), a qualitative immunohistochemistry (IHC) assay using monoclonal mouse anti–PD-L1 clone 22C3, and measured with the EnVision FLEX visualization system on the Autostainer Link 48 (Agilent Technologies, Santa Clara, CA, USA). PD-L1 expression was determined using CPS, which was calculated as the total number of cells stained positive for PD-L1 (TCs, lymphocytes, macrophages) × 100/the total number of viable cells,Citation16 and was approved in Japan as a companion diagnostic (CDx; for head and neck cancer and expanded use for esophageal squamous cell carcinoma) project. To minimize bias during evaluation of PD-L1 expression, participating pathologists were trained on scoring before the study was initiated. A CPS ≥1 indicates positive PD-L1 expression.Citation16
MSI status was evaluated using a fluorescent polymerase chain reaction (PCR)-based assay. The MSI-IVD kit was developed by FALCO (Kyoto, Japan) to assess 5 mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24, and MONO-27) and 2 pentanucleotide repeat markers (Penta C and Penta D). The PCR products were separated by capillary electrophoresis using the Applied Biosystems PRISM® 310 or 3100 or Applied Biosystems™ 3130 or 3130xl Genetic Analyzer (Foster City, CA, USA). The output data were analyzed using the GeneMapper® software (Applied Biosystems, Foster City, CA, USA). EBV testing was performed by in situ hybridization (ISH) at a central laboratory, with the exception of 1 site where the standard protocol was used in-house.
Medical records and test results were used for the collection of demographic information, including Eastern Cooperative Oncology Group performance status (ECOG PS), history of gastrectomy, metastatic location, and number of metastatic sites; clinicopathological data (date of GC diagnosis, site of occurrence [stomach or GEJ], stage of GC based on the Union for International Cancer Control [UICC]/American Joint Committee on Cancer [AJCC] classification 7th edition, histological type and degree of differentiation, metastatic organ, and type of tumor tissue specimen); human epidermal growth factor receptor-2 (HER-2) status obtained from IHC and/or fluorescent in situ hybridization (FISH); and history of Helicobacter pylori (H. pylori) infection. The date of H. pylori testing was recorded on the electronic data capture (EDC), and 106 cases tested for H. pylori infection were included. A positive test result was considered as having a history of infection. As the testing methods had not been specified, tests such as biopsy, serum test, or breath antibody test, which are covered by insurance in Japan, were identified and included.
Statistical analysis
Descriptive analyses were conducted to examine the prevalence of PD-L1 expression and assess MSI status and EBV positivity. Fisher’s exact test was used to differentiate the proportion of patients with PD-L1 expression by other biomarkers. Based on the planned sample size of 400 patients and the results of cohort 1 from the KEYNOTE-059 trial,Citation26,Citation27 which reported PD-L1 protein expression in approximately 57% of patients (CPS ≥1), the results of this study were expected to have a 95% confidence interval (CI) of 52.0%–61.9% for PD-L1 protein expression (CPS ≥1).
Results
Sample and study population
Eligible patients were registered for this study from November 2018 to March 2019. Tumor specimens from 391 Japanese patients with advanced gastric and GEJ adenocarcinoma were obtained. However, tumor specimens from 2 patients were excluded from the study because of violation of inclusion (recurrence during postoperative adjuvant chemotherapy) and exclusion (insufficient [<100] TCs) criteria; therefore, tumor specimens from 389 patients were included in the analysis set.
The majority of patients were male (67.1%), with a mean age at diagnosis of 67.6 ± 12.2 years, and most had ECOG PS in the range of 0 (47%) to 1 (26%). The most commonly occurring cancer site was the stomach (94.9%), and 17.2% of patients had a history of gastrectomy. Overall, 93.6% of patients had metastases; the majority of patients (62.7%) had metastasis in the lymph nodes, followed by the peritoneum (38.3%) and liver (30.3%). Most tumor specimens were obtained by biopsy (81.2%), followed by surgical resection (18.8%). The majority of the cancers were poorly differentiated (62.0%), and the most common cancer was of the diffuse type (48.8%). Overall, 50% (53/106) of the tumor specimens of patients tested positive for H. pylori. The prevalence of H. pylori in patients with GC was 50% (53/106). The proportion of men with vs without a history of H. pylori infection was 57% (30/53) vs 66% (35/53), respectively. The proportion of patients with vs without a history of H. pylori infection and having stomach as the tumor site was 98% (52/53) vs 89% (49/53), respectively. When characterized using the Lauren classification, the proportion of patients both with vs without a history of H. pylori infection and diffuse-type tumors was the highest (55% [29/53] vs 62% [33/53]), followed by intestinal-type (43% [23/53] vs 32% [17/53]) and mixed-type (2% [1/53] vs 6% [3/53]) tumors.
Prevalence of PD-L1, MSI status, and EBV positivity
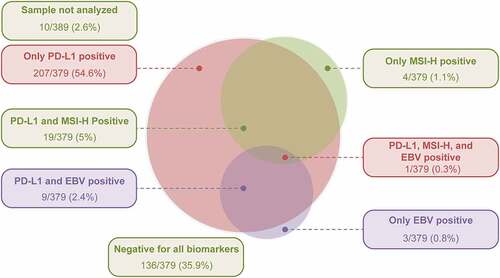
Tumor specimens from 241/389 (62%) patients were positive for PD-L1 expression, with a CPS ≥1. Tumor specimens from 24/379 (6.3%) and 13/389 (3.3%) patients were MSI-H and EBV-positive tumors, respectively (). Tumors that were positive only for PD-L1 were detected in 207/379 (54.6%) patients (), and tumor specimens from 136/379 (35.9%) patients were negative for all biomarkers. Among patients with MSI-H tumors and EBV-positive tumors, 19/24 (79.2%) and 9/13 (69.2%), respectively, were PD-L1 positive ( and ).
Table 1. Prevalence of PD-L1, MSI, and EBV expression
Prevalence of HER-2– and PD-L1–positive tumors based on the Lauren classification
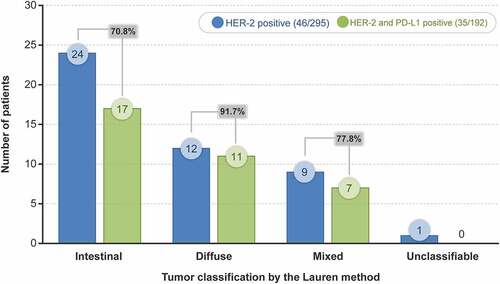
Among 46 patients with HER-2–positive tumors, 35 (76.1%) had PD-L1–positive tumors (). A majority of the patients with HER-2–positive tumors had the intestinal type (24/46 [52.17%]), followed by diffuse type (12/46 [26.09%]), mixed type (9/46 [19.57%]), and unclassifiable type (1/46 [2.17%]). However, the proportion of patients with PD-L1–positive tumors among the HER-2–positive patients was highest for the diffuse type (11/12 [91.7%]), followed by mixed type (7/9 [77.8%]) and intestinal type (17/24 [70.8%]) ().
Table 2. Association of PD-L1 expression with biomarker subtypes
HER-2, human epidermal growth factor receptor-2; PD-L1, programmed death ligand-1.
Association of PD-L1 expression with biomarkers
An association was observed between MSI-H tumors and PD-L1 protein expression, as a greater proportion of patients with MSI-H tumors (83.3% [20/24]) were PD-L1 positive than those with MSI-low/stable tumors (60.8% [216/355]; p = .0297). Similarly, an association was observed between history of H. pylori infection and PD-L1 expression, as a greater proportion of patients with a history of H. pylori infection (83.0% [44/53]) were PD-L1 positive compared with those without a history of H. pylori infection (54.7% [29/53]; p = .0030). However, no association was observed between PD-L1 expression and EBV-positive and HER-2–positive tumors ().
Association of PD-L1 expression with H. pylori infection
An association was observed between history of H. pylori infection and PD-L1 expression, with a greater proportion of patients with a history of H. pylori infection (83.0% [44/53]) being PD-L1 positive compared with those without a history of H. pylori infection (54.7% [29/53]; p = .0030) (). The PD-L1–positive rate was higher in H. pylori–positive cases vs negative cases, and the expression of PD-L1 was more common in the CPS range of 1–<10 among those with vs without a history of H. pylori infection (52.8% [28/53] vs 32.1% [17/53]), i.e., PD-L1 expression at a lower CPS range was associated with positive infiltrating lymphocytes.
Prevalence of PD-L1 expression based on clinicopathological parameters
No association was observed between PD-L1 expression and clinicopathological characteristics (p > .05 for all comparisons; ). However, a greater proportion of PD-L1–positive vs PD-L1–negative patients had mixed type (65.3% vs 34.7%) of tumors, followed by intestinal type (64.2% vs 35.8%), diffuse type (60% vs 40%), and unclassifiable type (53.8% vs 46.2%) ().
Table 3. Prevalence of PD-L1 expression based on clinicopathological characteristics
PD-L1 staining in cells based on CPS cutoffs
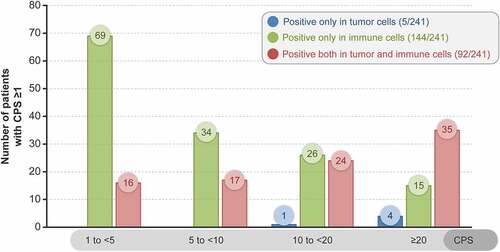
Overall, tumor specimens were PD-L1 positive in immune cells only in 144/241 (59.8%) patients, in both immune cells and TCs in 92/241 (38.2%) patients, and only in TCs in 5/241 (2.1%) patients. Tumor specimens from 56.4% (136/241) of patients had a CPS ranging from 1 to <10 and those from 43.6% (105/241) of patients had a CPS ≥10 (). A representative case of PD-L1 expression using IHC is presented in Supplementary Figure 1.
CPS, combined positive score; PD-L1, programmed death ligand-1.
Prevalence of biomarkers by PD-L1 cutoff
A higher proportion (66.7% [16/24]) of patients with MSI-H tumors demonstrated high PD-L1 expression with a CPS ≥10 than those with MSI-low/stable tumors (24.8% [88/355]; p < .0001). No associations were observed between other biomarkers (EBV, HER-2, or H. pylori) and high PD-L1 expression (CPS ≥10) ().
Table 4. Prevalence of biomarkers by CPS ≥10
Discussion
The PD-1/PD-L1 signaling cascade is an inhibitory factor in the cancer-immunity cycle.Citation28 PD-L1 expression is thus assessed on both tumor-infiltrating immune cells (TIICs) and TCs for its predictive value. However, PD-L1 expression on TIICs might be more meaningful in terms of predictive value in specific tumorsCitation18 and response to immunotherapy.Citation8,Citation13 However, differences in molecular characteristics, including PD-L1 expression in the tumor microenvironment, might differ between types of cancers.Citation29–31
This study evaluated the prevalence of PD-L1 expression, MSI-H status, and EBV positivity in Japanese patients with advanced gastric and GEJ adenocarcinoma. Although there have been several reports on the prevalence of PD-L1 in GC, their sample size was generally small, and real-world data for advanced cases are rarely reported.Citation32–35 In this study, tumor specimens from 389 Japanese patients with advanced gastric or GEJ adenocarcinoma were collected consecutively between 2014 and 2019 and evaluated.
The prevalence of PD-L1 positivity (62%) in the real-world setting, assessed using the pharmDx IHC assay (CPS ≥1), is comparable to previously reported results from pembrolizumab clinical trials in GC.Citation26,Citation27,Citation35–38 Among these PD-L1–positive patients (CPS ≥1, 62%), a similar proportion of patients had a CPS between 1 and <10 (56.4%) and ≥10 (43.6%), and this finding is in line with that of other studies in the Japanese population (27%–66%).Citation7,Citation9,Citation33 However, PD-L1 expression was higher in the TIICs than in the TCs for lower CPS cutoffs (1 to <10), supporting the claim that expression on immune cells may be more meaningful as ignoring the expression in TIICs will lower the prevalence, especially for lower CPS cutoffs.
The prevalence of both MSI-H (6.3% by PCR) and EBV positivity (3.3% by ISH) in advanced GC was low in this study. However, of this small number of patients with MSI-H tumors (24/379; 6.3%), a higher proportion showed PD-L1 expression (83.3%), suggesting that MSI-H status is associated with PD-L1 positivity. Furthermore, higher PD-L1 expression (CPS ≥10) was observed in a higher proportion of patients with MSI-H tumors than those with MSI-low/stable tumors. While the proportion of MSI-H tumors was similar to that reported in studies from Japan and East Asia,Citation33,Citation39,Citation40 it was lower than that reported in the TCGA project (stage I–III, 87.8% [259/295]; MSI, 21.2% [55/259] vs stage IV, 6.8% [20/295]; MSI, 10% [2/20]).Citation4,Citation41 These results support evidence from literature pertaining to(1) immune cell staining (CPS scoring) as a key characteristic in GC; (2) the overall, early-stage disease has a higher MSI-H prevalence than later-stage disease,Citation42 and higher proportion of early-stage vs later-stage MSI-H gastric adenocarcinomas in patients with gastric/GEJ cancers screened in the clinical trial of pembrolizumab;Citation43 (3) observations from studies reporting that the EBV-positive and MSI-H subtypes of GC are more likely to express PD-L1 when immune cells demonstrate a tumor-infiltrating pattern,10−12 indicating the involvement of the immune microenvironment in the development of GC;Citation8,Citation13 and (4) the role of MSI as a predictor for anti–PD-1/PD-L1 immunotherapy efficacy as demonstrated across tumor types.Citation29,Citation30 No relationship was observed when HER-2 status, EBV status, and histological subtype of GC per the Lauren classification were stratified by PD-L1 expression status (, , Supplementary Figure 1).
Retrospectively, we also observed that a history of H. pylori infection might be associated with PD-L1 expression, and this warrants further investigation as it might not reflect the current biological disease state. H. pylori, the most common cause of GC, predominantly in East Asia,Citation44,Citation45 also induces PD-L1 expression in the gastric epithelium, increasing the risk of developing GC.Citation46 The positive/negative test results for H. pylori were available only in patients with the test date recorded in the medical chart. As the testing method is not clear (blood, breath, or biopsy), a positive result (a history of infection) is not always indicative of the presence of H. pylori. Similarly, a negative test result (no history of infection) cannot differentiate whether it is just a decrease in the quantity of antibodies or if the patient was uninfected. Because 99% of Japanese patients with GC have a history of H. pylori infection,Citation47 most individuals who tested negative in the present study should ideally have a history of infection. Patients may test negative depending on the quantity of antibodies and the timing of infection.Citation48,Citation49 Therefore, an H. pylori–negative status is dependent on the diagnostic method and represents the phase of infection over time,Citation49 as a positive case is considered to have active inflammation and a negative case is considered to have less inflammation. Thus, our results may only perceive differences in the expression of PD-L1 among the different stages of inflammation. In the future, we hope that the effects of H. pylori on the immune milieu will be further clarified by examining PD-L1 expression in the inflamed phase or by examining the expression of PD-L1 in uninfected cases.
In our study, PD-L1 expression is observed more frequently in MSI-H tumors than in MSI-low/stable tumors without any significant relation to histological subtype. Higher tumor PD-L1 expression was reported in most MSI-H GC using CPS compared with MSI-stable GC.Citation50 In the CheckMate 649 study, 4% (18/473) and 3% (16/482) of patients with a PD-L1 CPS of ≥5 had MSI-H tumors in the nivolumab plus chemotherapy and chemotherapy alone groups, respectively,Citation51 and some MSI-H patients may not express PD-L1 at high levels or may have a CPS of 1–5.Citation50,Citation52 However, we must note that differences exist in antibody IHC assays (22C3 and 28-8Ab), definitions and cutoffs of CPS, and methods for detecting MSI (IHC vs PCR).
A recent meta-analysis of randomized controlled trials has shown that patients with MSI-H GC should be considered as a specific population that is highly immunosensitive.Citation53 Hence, immunotherapy could be an option in this subset of patients. Furthermore, a correlation between MSI-H tumors and immune checkpoint ligands such as PD-L1 has been indicatedCitation54 due to the increased number of neoantigens present in the MSI-H subtype, leading to the stimulation of PD-L1 through the secretion of interferon gamma by T lymphocytes.Citation55
Additionally, an analysis of MSI status could help assess the tumor microenvironment and provide insights into the most appropriate treatment option.Citation39 Therefore, patients with MSI-H tumors could benefit from an immunotherapeutic approach. In concordance with this hypothesis, an evaluation of the KEYNOTE-158 and KEYNOTE-164 studies showed robust antitumor activity of pembrolizumab in heavily pretreated patients with MSI-H cancers.Citation56,Citation57 Pembrolizumab is approved for the treatment of MSI-H solid tumors after failure of current standard therapy.Citation58 It is known that the EBV subtype accounts for 8.8% of all GC subtypes and is associated with a better prognosis.Citation41,Citation59 Interestingly, the prevalence of EBV (3.3%) was low in our study compared with that in studies in Asia and Latin America (7.7%)Citation60 and Japan (approximately 5.3%Citation61 and 5.1%Citation32). This could be attributable to the difference in the ISH protocol used for this analysis. The EBV subtype of GC usually has specific histological and clinical features such as intra- or peritumoral lymphocytic infiltration (carcinoma with lymphoid stroma).Citation60–62 None of the tumor specimens obtained in our study were of the carcinoma with lymphoid stroma type. This unusual histological finding could be due to a bias in sampling, since only advanced lesions were collected for this study. Additional research is therefore required to further evaluate the biological behavior of EBV-positive adenocarcinoma without lymphoid stroma.
No association between PD-L1 expression and EBV-infected tumor type was observed, possibly due to the small cohort of patients with EBV-positive tumors. However, previous studies have demonstrated an overexpression of PD-L1 in EBV-positive GC.Citation55,Citation63 Indeed, a combined analysis of 4 studies involving 1307 Japanese patients indicated a significant association between EBV positivity and PD-L1 expression (p < .0001).Citation10 Similar findings have also been reported in studies from Japan.Citation11,Citation32
Our study did not show an association between HER-2 and PD-L1 expression. To date, contrasting results have been reported indicating PD-L1 expression in HER-2–negativeCitation64 and HER-2–positive tumors,Citation65 and further research is therefore warranted to confirm this association. When HER-2 status was characterized by histological subtype, HER-2 positivity was most common in the intestinal subtype of GC, which is in line with that reported in previous studies.Citation66,Citation67 Interestingly, 91.7% of diffuse HER-2–positive tumors were PD-L1 positive.
Further, we did not observe any association between PD-L1 expression using CPS and patient clinicopathological characteristics/parameters. However, a recent study by Kawazoe and colleaguesCitation33 reported an association between PD-L1 expression and clinicopathological parameters (TCs: mismatch repair deficient, PIK3CA mutation, and KRAS mutation; immune cells: EBV positivity and lymph node metastasis) using the IHC 22C3 pharmDx kit in metastatic GC, but IC and TC were scored separately in the study. Other characteristics such as tumor size and lymph node status have been associated with PD-L1 positivity.Citation68 Overall, 47.3% (114/241) of all PD-L1–positive specimens were of the diffuse cancer subtype; this finding is in line with a retrospective study that reported an association between the diffuse type GC and PD-L1 positivity, indicating that histological characteristics should be considered when selecting patients who may benefit from anti–PD-L1 therapy.Citation69
To our knowledge, this is the first real-world study that used the CPS method to evaluate PD-L1 expression in Japanese patients with gastric and GEJ adenocarcinoma. A number of studies have stressed the importance of evaluating PD-L1 expression not only on TCs but also on TIICs.Citation8,Citation9,Citation13,Citation70,Citation71 PD-L1 expression on TIICs has a stronger relationship with the cancer immune response than PD-L1 expression only on TCs.Citation8 Most responders to pembrolizumab were identified when tumor and immune cell PD-L1 expression were combined,Citation18 making CPS a more sensitive measure/scoring system compared with tumor proportion score (TPS).Citation17–19 However, there are certain limitations of this study. This was a retrospective analysis that used archived tissue. In addition, since this was a multisite study, selection bias between the study sites may have existed.
Conclusion
The prevalence of PD-L1 positivity (62%) in the real-world setting using the pharmDx IHC assay (CPS ≥1) was comparable to previously reported results from pembrolizumab clinical trials and other exploratory studies in GC.Citation26,Citation27,Citation33,Citation35–38,Citation72 PD-L1 staining was observed more frequently in TIICs than in TCs for a lower CPS cutoff. The rate of MSI-H and EBV-positive tumors in advanced GC was 6.3% and 3.3%, respectively. We observed that the MSI-H status is associated with PD-L1 positivity, suggesting high number of tumor neoantigens. We also observed that a history of H. pylori infection might be associated with PD-L1 expression; however, further investigation is warranted to confirm this finding.
Author contributions
Tsutomu Yoshida, Mikiko Tanabe, Chiho Ohbayashi, Mizutomo Azuma, Yoichi Akazawa, Soji Ozawa, Sohei Matsumoto, Takayoshi Suzuki, Akira Mitoro, Tetsu Fukunaga, and Takashi Yao contributed towards acquisition of the data and critically reviewing or revising the manuscript for important intellectual content.
Go Ogura contributed towards acquisition of the data, analysis of data, and critically reviewing or revising the manuscript for important intellectual content.
Takuo Hayashi contributed towards acquisition of the data, analysis of data, interpretation of results, and critically reviewing or revising the manuscript for important intellectual content.
Chikara Kunisaki contributed towards acquisition of the data, interpretation of results, and critically reviewing or revising the manuscript for important intellectual content.
Akiko Shimizu contributed towards conception, design, or planning of the study; interpretation of results; and drafting the manuscript.
Go Fujimoto contributed towards conception, design, or planning of the study, and critically reviewing or revising the manuscript for important intellectual content.
All authors provided final approval and are accountable for all aspects of the work.
Human rights statement and informed consent
Ethics committee approval was taken from each investigation site prior to initiating the study. Informed consent was obtained from patients, and an opt-out option promulgated by the (Japanese) Ethical Guidelines for Medical and Health Research Involving Human Subjects was availed in the case of deceased patients.
Supplemental Material
Download MS Word (8.2 MB)Acknowledgments
This study was sponsored and funded by MSD K.K., Tokyo, Japan. The sponsor was involved in the study design, data analysis, interpretation of the data, writing of the manuscript, and decision to submit for publication. Editorial support, in the form of medical writing, assembling tables and creating high-resolution images based on authors’ detailed directions, collating author comments, copyediting, fact checking, and referencing, was provided by Shaleen Multani, PhD, and Annirudha Chillar, MD, PhD, of Cactus Life Sciences (part of Cactus Communications) and funded by MSD K.K., Tokyo, Japan.
Disclosure statement
The institutions of T. Yo, GO, MT, TH, CO, MA, CK, YA, SO, SM, TS, AM, TF, and T. Ya have received research support for this study from MSD K.K., Tokyo, Japan. AS and FG are employees of MSD K.K., Tokyo, Japan.
Supplementary Material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
References
- International Agency for Research on Cancer. Globocan 2020. [accessed 2020 Jan 11]. http://gco.iarc.fr/ (Japan: https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-sheets.pdf.)
- Eto K, Ida S, Watanabe M, Baba H. Treatment strategy for metastatic gastric cancer in Japan. J Cancer Metastasis Treat. 2018;4(5):23. doi:10.20517/2394-4722.2017.73.
- Ministry of Health, Labour and Welfare. Cancer control promotion basic plan. [ accessed 2021 Jan, 11]. https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000183313.html.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi:10.1038/nature13480.
- Eto S, Yoshikawa K, Nishi M, Higashijima J, Tokunaga T, Nakao T, Kashihara H, Takasu C, Iwata T, Shimada M . Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer. 2016;19 (2):466–471 doi:10.1007/s10120-015-0519-7.
- Liu YX, Wang XS, Wang YF, Hu XC, Yan JQ, Zhang YL, Wang W, Yang R-J, Feng Y-Y, Gao S-G, et al. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. Onco Targets Ther. 2016;9:2649–2654. doi:10.2147/OTT.S102616.
- Tamura T, Ohira M, Tanaka H, Muguruma K, Toyokawa T, Kubo N, Sakurai K, Amano R, Kimura K, Shibutani M, et al. Programmed death-1 ligand-1 (PDL1) expression is associated with the prognosis of patients with stage II/III gastric cancer. Anticancer Res. 2015;35(10):5369–5376.
- Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T, Wang Q, Jiang J. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget. 2017;8(38):64066–64082. doi:10.18632/oncotarget.19318.
- Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee J-O, Kim YJ, Lee HS, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19(1):42–52. doi:10.1007/s10120-014-0440-5.
- Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X . PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0182692. doi:10.1371/journal.pone.0182692.
- Saito R, Abe H, Kunita A, Yamashita H, Seto Y, Fukayama M. Overexpression and gene amplification of PD-L1 in cancer cells and PD-L1(+) immune cells in Epstein-Barr virus-associated gastric cancer: the prognostic implications. Mod Pathol. 2017;30(3):427–439. doi:10.1038/modpathol.2016.202.
- Kelly RJ. Immunotherapy for esophageal and gastric cancer. Am Soc Clin Oncol Educ Book. 2017;37(37):292–300. doi:10.1200/EDBK_175231.
- Koh J, Ock C-Y, Kim JW, Nam SK, Kwak Y, Yun S, Ahn S-H, Park DJ, Kim H-H, and Kim WH, et al. Clinicopathologic implications of immune classification by PD-L1 expression and CD8-positive tumor-infiltrating lymphocytes in stage II and III gastric cancer patients. Oncotarget. 2017;8(16):26356–26367. doi:10.18632/oncotarget.15465.
- Kim DH, Bae GE, Suh KS, Ryuman D, Song KS, Kim JS, Lee SI, and Yeo MK. Clinical significance of tumor and immune cell PD-L1 expression in gastric adenocarcinoma. In Vivo. 2020;34(6):3171–3180. doi:10.21873/invivo.12152.
- Choi E, Chang MS, Byeon SJ, Jin H, Jung KC, Kim H, Lee KL, Kim W, Park JH, and Kim KH, et al. Prognostic perspectives of PD-L1 combined with tumor-infiltrating lymphocytes, Epstein-Barr virus, and microsatellite instability in gastric carcinomas. Diagn Pathol. 2020 ;15(1):69. doi:10.1186/s13000-020-00979-z.
- Agilent Technologies. Interpretation Manual - Gastric or Gastroesophageal Junction Adenocarcinoma. PD-L1 IHC 22C3 pharmDx is FDA-approved for in vitro diagnostic use. [ accessed 2021 Jan 11]. https://www.agilent.com/cs/library/usermanuals/public/29219_pd-l1-ihc-22C3-pharmdx-gastric-interpretation-manual_us.pdf.
- Emancipator K, Huang L, Aurora-Garg D, Bal T, Cohen EEW, Harrington K, Soulières D, Le Tourneau C, Licitra L, and Burtness B, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol. 2020;34(3):532–541. doi:10.1038/s41379-020-00710-9.
- Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, Jansson M, Shah S, Hanks D, Wang J, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. doi:10.5858/arpa.2018-0043-OA
- Jiang Y, Lo AWI, Wong A, Chen W, Wang Y, Lin L, Xu J. Prognostic significance of tumor-infiltrating immune cells and PD-L1 expression in esophageal squamous cell carcinoma. Oncotarget. 2017;8(18):30175–30189. doi:10.18632/oncotarget.15621.
- Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, Doi T, Moriwaki T, Kim S-B, Lee S-H, et al. KEYNOTE-181 Investigators. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–4148. doi:10.1200/JCO.20.01888.
- Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim S-B, Tajika M, Kim HT, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: the phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5(4):546–550. doi:10.1001/jamaoncol.2018.5441
- U.S. Food and Drug Administration. KEYTRUDA® (pembrolizumab) FDA prescribing information. [ accessed 2021 Jan 11]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf.
- Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, Keegan P, McKee AE, Pazdur R. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24(1):103–109. doi:10.1634/theoncologist.2018-0221.
- U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. [ accessed 2020 Jan 5]. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication.
- Japanese gastric cancer treatment guidelines. [ accessed 2020 Jan 5]. http://www.jgca.jp/guide_mag.html.
- Bang Y-J, Kang Y-K, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, and Jalal SI, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828–837. doi:10.1007/s10120-018-00909-5.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4(5):e180013. doi:10.1001/jamaoncol.2018.0013.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012.
- Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12(1):54. doi:10.1186/s13045-019-0738-1.
- Oliveira AF, Bretes L, Furtado I. Review of PD-1/PD-L1 inhibitors in metastatic dMMR/MSI-H colorectal cancer. Front Oncol. 2019;9:396. doi:10.3389/fonc.2019.00396.
- Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8(19):31347–31354. doi: 10.18632/oncotarget.15532.
- Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–415. doi:10.1007/s10120-016-0631-3.
- Kawazoe A, Shitara K, Kuboki Y, Bando H, Kojima T, Yoshino T, Ohtsu A, Ochiai A, Togashi Y, Nishikawa H, et al. Clinicopathological features of 22C3 PD-L1 expression with mismatch repair, Epstein-Barr virus status, and cancer genome alterations in metastatic gastric cancer. Gastric Cancer. 2019;22(1):69–76. doi:10.1007/s10120-018-0843-9.
- Shitara K, Van Cutsem E, Bang Y-J, Fuchs C, Wyrwicz L, Lee K-W, Kudaba I, Garrido M, Chung HC, and Lee J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–1580. doi:10.1001/jamaoncol.2020.3370
- Shitara K, Özgüroğlu M, Bang Y-J, Di Bartolomeo M, Mandalà M, Ryu M-H, Fornaro L, Olesiński T, Caglevic C, Chung HC, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi:10.1016/S0140-6736(18)31257-1.
- Muro K, Fuchs CS, Jang RW-J, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges J-P, and Garrido M, et al. KEYNOTE-059 cohort 1: pembrolizumab (pembro) monotherapy in previously treated advanced gastric or gastroesophageal junction (G/GEJ) cancer in patients (Pts) with PD-L1+ tumors—Asian subgroup analysis. J Clin Oncol. 2018;36(4_suppl):723. doi:10.1200/JCO.2018.36.4_suppl.723.
- Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–726. doi:10.1016/S1470-2045(16)00175-3.
- Tabernero J, Cutsem EV, Bang Y-J, Fuchs CS, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Salguero HRC, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase III KEYNOTE-062 study. J Clin Oncol. 2019;37(18_suppl):LBA4007. doi:10.1200/JCO.2019.37.18_suppl.LBA4007.
- Morihiro T, Kuroda S, Kanaya N, Kakiuchi Y, Kubota T, Aoyama K, Tanaka T, Kikuchi S, Nagasaka T, Nishizaki M, et al. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer. Sci Rep. 2019;9(1):4633. doi:10.1038/s41598-019-41177-2.
- Kim J-Y, Shin NR, Kim A, Lee H-J, Park W-Y, Kim J-Y, Lee, C-H, Huh, G-Y, Park, DY, et al. Microsatellite instability status in gastric cancer: a reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J Pathol. 2013;47(1):28–35. doi:10.4132/KoreanJPathol.2013.47.1.28.
- Lin X, Zhao Y, Song WM, Zhang B. Molecular classification and prediction in gastric cancer. Comput Struct Biotechnol J. 2015;13:448–458. doi:10.1016/j.csbj.2015.08.001.
- Lorenzi M, Amonkar M, Zhang J, Mehta S,Liaw K-L. Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review. J Oncol. 2020;20(1807929):1–20. doi:10.1155/2020/1807929.
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi:10.1126/science.aan6733.
- Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017;20(S1):3–7. doi:10.1007/s10120-016-0658-5.
- Goh LY, Leow AH, Goh KL. Observations on the epidemiology of gastrointestinal and liver cancers in the Asia-Pacific region. J Dig Dis. 2014;15(9):463–468. doi:10.1111/1751-2980.12164.
- Holokai L, Chakrabarti J, Broda T, Chang J, Hawkins JA, Sundaram N, Wroblewski LE, Peek Jr RM, Wang J, Helmrath M, et al. Increased programmed death-ligand 1 is an early epithelial cell response to helicobacter pylori infection. PLoS Pathog. 2019;15(1):e1007468. doi:10.1371/journal.ppat.1007468.
- Matsuo T, Ito M, Takata S, Tanaka S, Yoshihara M, Chayama K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter. 2011;16(6):415–419. doi:10.1111/j.1523-5378.2011.00889.x.
- Tsuda M, Asaka M, Kato M, Matsushima R, Fujimori K, Akino K, Kikuchi S, Lin Y, Sakamoto N. Effect on Helicobacter pylori eradication therapy against gastric cancer in Japan. Helicobacter. 2017;22(5):e12415. doi:10.1111/hel.12415.
- Miftahussurur M, Yamaoka Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: critical importance of indirect test validation. Biomed Res Int. 2016;2016:4819423. doi:10.1155/2016/4819423.
- Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi:10.1038/s41591-018-0101-z.
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2.
- Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. 2019;7(1):24. doi:10.1186/s40425-019-0514-3.
- Pietrantonio F, Randon G, Di Bartolomeo M, Luciani A, Chao J, Smyth EC, Petrelli F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: a meta-analysis of randomized clinical trials. ESMO Open. 2021;6(1):100036. doi:10.1016/j.esmoop.2020.100036.
- Kim JH, Park HE, Cho N-Y, Lee HS, Kang GH . Characterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer. 2016;115(4):490–496. doi:10.1038/bjc.2016.211.
- Derks S, Liao X, Chiaravalli AM, Xu X, Camargo MC, Solcia E, Sessa F, Fleitas T, Freeman GJ, Rodig SJ, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget. 2016;7(22):32925–32932. doi:10.18632/oncotarget.9076.
- Diaz LA, Marabelle A, Delord J-P, Shapira-Frommer R, Geva R, Peled N, Kim TW, Andre T, Van Cutsem E, Guimbaud R, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. [Abstract]. 2017;35:3071.
- Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, Geva R, Gottfried M, Penel N, and Hansen AR, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/JCO.19.02105.
- Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–3758. doi:10.1158/1078-0432.CCR-18-4070.
- Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJY, Herrera-Goepfert R, Meneses-Gonzalez F, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63(2):236–243. doi:10.1136/gutjnl-2013-304531.
- Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, Corvalan AH, Carrascal E, Abdirad A, Anwar M, et al. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105(1):38–43. doi:10.1038/bjc.2011.215.
- Koriyama C, Akiba S, Corvalan A, Carrascal E, Itoh T, Herrera-Goepfert R, Eizuru Y, Tokunaga M. Histology-specific gender, age and tumor-location distributions of Epstein-Barr virus-associated gastric carcinoma in Japan. Oncol Rep. 2004;12(3):543–547.
- Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K, Suehiro Y, et al. Clinical importance of Epstein-Barr virus-associated gastric cancer. Cancers (Basel). 2018;10(6):167. doi:10.3390/cancers10060167.
- Nakano H, Saito M, Nakajima S, Saito K, Nakayama Y, Kase K, Yamada L, Kanke Y, Hanayama H, Onozawa H, et al. PD-L1 overexpression in EBV-positive gastric cancer is caused by unique genomic or epigenomic mechanisms. Sci Rep. 2021;11(1):1982. doi:10.1038/s41598-021-81667-w.
- Wang L, Zhang Q, Ni S, Tan C, Cai X, Huang D, Sheng W . Programmed death-ligand 1 expression in gastric cancer: correlation with mismatch repair deficiency and HER2-negative status. Cancer Med. 2018;7(6):2612–2620. doi:10.1002/cam4.1502.
- Oki E, Okano S, Saeki H, Umemoto Y, Teraishi K, Nakaji Y, Ando K, Zaitsu Y, Yamashita N, Sugiyama M, et al. Protein expression of programmed death 1 ligand 1 and HER2 in gastric carcinoma. Oncology. 2017;93(6):387–394. doi:10.1159/000479231.
- Van Cutsem E, Bang Y-J, Feng-Yi F, Xu JM, Lee K-W, Jiao SC, Chong JL, López-Sanchez RI, Price T, and Gladkov O, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18(3):476–484. doi:10.1007/s10120-014-0402-y.
- Irkkan C, Balci S, Güler Tezel G, Akinci B, Yalcin B, Güler G. Comparison of clinicopathologic parameters and survivals between Epstein-Barr virus-positive and Her2-positive gastric cancers. Appl Immunohistochem Mol Morphol. 2017;25(9):609–614. doi:10.1097/PAI.0000000000000353.
- Zhang M, Dong Y, Liu H, Wang Y, Zhao S, Xuan Q, Wang Y, Zhanga Q, et al. The clinicopathological and prognostic significance of PD-L1 expression in gastric cancer: a meta-analysis of 10 studies with 1,901 patients. Sci Rep. 2016;6(1):37933. doi:10.1038/srep37933.
- Li Z, Lai Y, Sun L, Zhang X, Liu R, Feng G, Zhou L, Jia L, Huang X, Kang Q, et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol. 2016;55:182–189. doi:10.1016/j.humpath.2016.05.012.
- Wang Y, Zhu C, Song W, Li J, Zhao G, Cao H. PD-L1 expression and CD8+ T cell infiltration predict a favorable prognosis in advanced gastric cancer. J Immunol Res. 2018;2018:4180517. doi:10.1155/2018/4180517.
- Yamashita K, Iwatsuki M, Harada K, Eto K, Hiyoshi Y, Ishimoto T, Nagai Y, Iwagami S, Miyamoto Y, Yoshida N, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23(1):95–104. doi:10.1007/s10120-019-00999-9.
- Kim HS, Wu T, Kim H, Chung HC, Ajani JA, Ku GY, Kelsen DP, Zhu X, Savage MJ, Koshiji M, et al. PD-L1 expression in patients with metastatic gastric cancer in South Korea. ABSTRACT J Clin Oncol. 2017;35(15_suppl):1571. doi:10.1200/JCO.2017.35.15_suppl.1571.