ABSTRACT
Screening strategies have demonstrated their potential for decreasing the incidence and mortality of cancers, particularly that of colorectal cancer (CRC). Another strategy that has been developed to reduce CRC occurrence is the use of chemoprevention agents. Among them, aspirin is the most promising. Aspirin acts in colorectal tumourigenesis through several mechanisms, either directly in tumor cells or in their microenvironment, such as through its anti-inflammatory activity or its effect on the modulation of platelet function. Many retrospective studies, as well as follow-up of large cohorts from trials with primary cardiovascular end points, have shown that long-term treatment with daily low-dose aspirin decreases the incidence of adenomas and colorectal cancers. Therefore, aspirin is currently recommended by the United States Preventive Services Task Force (USPSTF) for primary prevention of CRC in all patients aged 50 to 59 with a 10-y risk of cardiovascular events greater than 10%. Furthermore, several studies have also reported that long-term aspirin treatment taking after CRC resection decreases recurrence risk and increases overall survival, especially in patients with PIK3CA-mutated tumors. This review summarizes current knowledge on the pathophysiological mechanisms of aspirin chemoprevention, discusses the primary clinical results on CRC prevention and highlights the potential biomarkers identified to predict aspirin efficacy.
KEYWORDS:
Introduction
Colorectal cancer (CRC) is a worldwide public health problem. Every year, more than 1.8 million CRCs are diagnosed, and 800,000 persons die from this disease.Citation1 Screening strategies for CRC have also made it possible to decrease both the incidence and mortality by detecting and removing advanced precancerous lesions, as well as by detecting cancerous lesions at an early stage. Chemoprevention has the potential to decrease the occurrence of cancer, as well as delay its onset. Numerous agents, such as metabolic agents, vitamin and minerals, non-steroidal anti-inflammatory (NSAIDs) drugs or aspirin, have been reported for CRC chemoprevention.Citation2 The latter is likely the most promising agent that will be able to meet this aim. Aspirin, also known as acetylsalicylic acid (ASA), is primarily known for its analgesic, antipyretic actions but also as an agent for cardiovascular prophylaxis. It has been known for several years as a protective factor against cancer development, especially CRC. However, chemoprevention by aspirin remains controversial. This review closely details the mechanisms of action by which aspirin exerts its anti-tumor effects, as well as the primary clinical results of chemoprevention on neoplastic colorectal lesions. It also provides an overview of the biomarkers most likely to predict aspirin efficacy.
I. Actions of aspirin on CRC tumourigenesis
Aspirin is a well-known protective factor against several cancers, especially CRC.Citation3 However, its molecular mechanisms are incompletely understood. It exerts both direct mechanisms on CRC cells and indirect mechanisms on the tumor microenvironment ().
Figure 1. Actions of aspirin on colorectal tumourigenesis. This inhibitory action occurs through direct mechanisms on tumor cells, either by inhibiting the synthesis of prostaglandins and associated intracellular pathways, such as WNT (green), EGFR/PIK3CA/AKT/PPARδ (Orange) and Ras/Raf/MAP kinase (MEK)/ERK pathways (purple), or through an inhibition on prostaglandin-independent pathways, as NFkB (yellow), AMPK/mTOR (gray) and the apoptosis pathways (blue). Aspirin also exerts an anti-cancer action in an indirect way through its inhibitory action on various actors of the tumor microenvironment, such as platelets or the anti-inflammatory immune system. Due to this wealth in the multiple inhibitory mechanisms involved, aspirin is capable of inhibiting many properties of neoplastic cells, such as their antiapoptotic capacity or their potential for migration, invasion, proliferation, metastatic dissemination, etc.
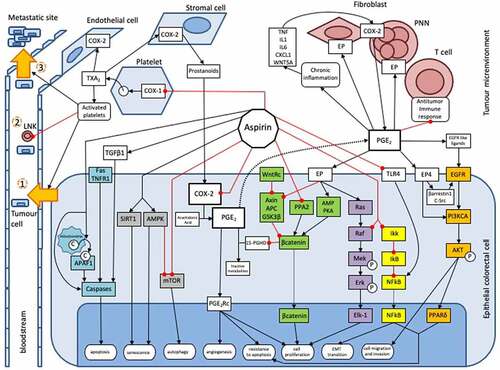
1a. Direct mechanisms on epithelial cancer cell
Prostaglandins-mediated actions
Prostaglandins (PG) are bioactive lipids from the prostanoid family involved in several biological processes, including tumourigenesis. Biosynthesis of prostanoids begins with arachidonic acid, which is transformed by cyclooxygenase enzyme (COX) to an unstable intermediate, prostaglandin H2. Next, tissue-specific isomerases generate five different prostanoids: thromboxane A2 (TXA2), prostaglandin D2, prostaglandin E2 (PGE2), prostaglandin F2α and prostacyclin.Citation4 Prostaglandins act through an autocrine or a paracrine effect by binding to specific transmembrane G protein-coupled receptors. PGE2 has four specific receptors, termed EP receptors 1, 2, 3 and 4. There are two different isoforms of COX enzyme: i) COX-1 exhibits ubiquitous expression and plays a role in platelet aggregation and gastric cytoprotection. Platelets express this isoform after maturation and produce TXA2. ii) COX-2 expression is only induced in certain tissues in response to inflammation, wound healing or neoplasia.Citation5 Finally, the ubiquitous 15 hydroperoxy prostaglandin dehydrogenase enzyme (15-hydroperoxyPGDH) metabolizes PG to both inactive metabolites, 13,14-dihydro-15-keto-PGE2 (PGEM) and 13,14-dihydro-15-keto-PGF2α.Citation6
Aspirin and others NSAIDs reduce PGE2 production through different mechanisms of COX enzyme inhibition.Citation7,Citation8 NSAIDs compete with arachidonic acid for reversible binding to a common docking site. Aspirin inhibits COX through irreversible selective acetylation of a critical serine residue. NSAIDs’ actions are thus reversible, whereas aspirin’s action is irreversible and requires new synthesis of COX enzymes.Citation5 Low dose aspirin inhibits COX-1 (see section below on platelet inhibition), whereas daily high-dose aspirin is required to inhibit COX-2.Citation4,Citation7 A recent study conducted on animal models has interestingly underlines interest of an intermittent dosing-regimen of a low-dose aspirin treatment to prevent the occurrence of CRC, without increasing gastro-intestinal side effects.Citation9
PGE2 is the prostanoid most involved in colorectal tumourigenesis.Citation10 Several studies have reported that PGE2 and/or COX-2 levels are significantly higher in adenoma and CRC than in healthy tissue.Citation11,Citation12 In contrast, 15-hydroperoxyPGDH acts as a tumor suppressor protein, and its expression is downregulated in CRC cells, and restoration of 15-hydroperoxyPGDH expression exerts an antitumour effect.Citation6,Citation13 Many mechanisms have been proposed to explain the protumour role of PGE2. PGE2 acts directly on tumor epithelial cells by stimulating cellular proliferation and survival, as well as promoting migration and invasion of CRC cells. PGE2 induces cytoskeletal reorganization that changes cellular shape and facilitates motility of CRC cells.Citation10,Citation14,Citation15
Action on intracellular pathways through PGE2 inhibition
PGE2 is implied in several intracellular pathways involved in colorectal tumorigenesis. The inhibitory action of aspirin on PGE2 will thus modulate colorectal tumorigenesis, through these pathways.
The WNT signaling pathway (in green on )
WNT signaling comprises an important pathway involved in colorectal tumourigenesis. Briefly, when the WNT pathway is inactive, a destruction complex, formed by Axin, adenomatous polyposis coli protein (APC) and glycogen synthase kinase 3β (GSK3β), phosphorylates specific N-terminal residues in β-catenin, which is then recognized as part of a ubiquitin ligase complex that undergoes polyubiquitination, leading to proteasomal degradation. Protein phosphatase 2A (PP2A) decreases levels of phosphorylated β-catenin, preventing its degradation. Activation of the WNT pathway leads to cytoplasmic accumulation of β-catenin, then to nuclear translocation and transcription of target genes involved in cell proliferation.Citation16 Several interaction loops exist between PGE2 and the WNT pathway. PGE2 stimulates the EP2 receptor, which binds to the Axin protein, inactivating the destruction complex.Citation17 PGE2 increases cytoplasmic levels of β-catenin through a signaling pathway involving cyclic adenosine monophosphate (AMP) and protein kinase A (PKA).Citation18 PGE2 also increases transcription of the peroxisome proliferator-activated receptor δ (PPARδ) gene.Citation19 This gene is involved in colorectal tumourigenesis by promoting resistance to apoptosis and is one of the genes targeted by the WNT pathway.Citation20,Citation21 Furthermore, β-catenin inhibits 15-hydroperoxyPGDH expression through direct binding to its promoter, increasing PGE2 levels.Citation22 Aspirin, in addition to its inhibitory role on PGE2 production, exerts an antitumour effect through another mechanism of negatively regulating the WNT pathway. Thus, aspirin causes inactivation of the phosphatase A2 (PPA2) protein through phosphorylation of its catalytic subunit and restores β-catenin degradation.Citation23–25
The EGFR/PIK3CA/AKT/PPARδ pathway (in orange on )
PGE2 stimulates the PIK3CA/AKT pathway primarily through EP4 receptor activation, which in turn either directly activates PIK3CA enzyme or EGFR transactivation through an intermediate β-arrestin-1–SRC complex or by stimulating secretion of EGF-like ligands.Citation19,Citation26,Citation27 Aspirin inhibits PIK3CA/AKT pathway by reducing PGE2 levels and has a greater inhibitory effect on tumor growth in CRC cells with activating PIK3CA mutations.Citation28,Citation29
The Ras/Raf/MAP kinase (MEK)/ERK pathway (in purple on )
The Ras/Raf/MEK/ERK cascade is an important pathway involved in colorectal tumourigenesis. PGE2 stimulates COX-2 expression through activation of the Ras/MEK/ERK pathway, forming a self-amplifying loop.Citation30 Aspirin inhibits this pathway by increasing inhibitory phosphorylation of Raf, avoiding its interaction with Ras and hence, its activation.Citation31
PGE2-independent actions
Several intracellular pathways involved in colorectal tumorigenesis are PGE2-independent. Aspirin also acts on these pathways, independently of PGE2 inhibition.
The NFkB pathway (in yellow on )
NFkB is a transcription factor that stimulates expression of antiapoptotic genes and usually exists as a heterodimer complex bound in the cytoplasm by the inhibitor protein IKB. It has been reported that aspirin inhibits the NFkB pathway through binding to the IKB protein, belonging to the IKK complex. The IKB protein is normally phosphorylated by the IKK cellular kinase complex and then degraded by the proteasome machinery, enabling the release of sequestered NFkB in the cytoplasm and its translocation to the nucleus.Citation32 Aspirin may thus cause retention of NFkB protein in the cytoplasm and repression of antiapoptotic genes transcription. However, this action should require very high doses of aspirin and has not been proven by in vivo studies.Citation33 Furthermore, lipopolysaccharides from Gram-negative intestinal bacteria activate the epithelial-mesenchymal transition (EMT) of CRC cells through the stimulation of TLR-4 receptors with subsequent activation of the NFkB pathway. Aspirin has been shown to prevent EMT by inhibiting TLR-4 expression, which inhibits activation of the NFkB signaling pathway.Citation34
The AMPK/mTOR pathway (in gray on )
Sirtuin 1 (SIRT1) and AMP-activated protein kinase (AMPK) are two key regulators of cellular metabolism. Aspirin induces senescence of CRC cells by creating an energy imbalance through upregulation of SIRT1 and phospho-AMPK.Citation35 The mammalian target of rapamycine (mTOR) pathway is involved in protein synthesis and cell growth. Aspirin inhibits mTOR both through a direct action and the activation of AMPK. This inhibition in turn induces autophagy in CRC cells.Citation36
The apoptosis pathway (in blue on )
There are two major apoptosis pathways, the mitochondrial (or intrinsic) pathway and the extrinsic pathway. In the first, cytochrome c, released by the mitochondria into cytosol, binds to apoptotic protease activating factor 1 (APAF1) to induce consecutive activation of caspases, the effector enzymes of the apoptosis pathways. In the second one, stimulation of death receptors, such as Fas or TNFR1, induces recruitment of the death inducing signaling complex, which activates caspase 8 secondarily and subsequently, downstream caspases. Aspirin induces apoptosis by stimulating the release of cytochrome c from mitochondria to cytosol, and by stimulating caspase 8 and caspase 3.Citation37,Citation38 Aspirin additionally increases secretion of TGF-β1, a cytokine involved in cell growth that has a key role in apoptosis regulation. TGF-β1 induces CRC cell apoptosis through downregulation of antiapoptotic Bcl-2 family members, upregulation of the pro-apoptotic factor Bax, and activation of caspase proteases.Citation39
1b. Indirection mechanisms on tumor microenvironment
Action through platelet inhibition
As described above, the COX-2 enzyme plays an important role in colorectal carcinogenesis. COX-2 inhibition by aspirin is only temporary in nucleated cells because of de novo synthesis of COX-2; therefore, high-dose aspirin is required several times a day to directly inhibit COX-2 in epithelial cells. However, many observational studies have reported that low-dose aspirin given once daily decreases the incidence of CRC (see sections below). This finding could be due to the inhibitory effect of aspirin on platelets. COX-1 is the only isoform of the COX enzyme in mature platelets in which TXA2 is specifically produced. Due to lacking a nucleus, regeneration of COX-1 is not possible in platelets, and aspirin, despite its short half-life, exerts an irreversible action on COX-1. Platelets are activated in the tumor microenvironment after interaction with epithelial cancer cells.Citation40 Activated platelets generate soluble growth and angiogenic factors, such as TXA2 and sphingosine 1 phosphate. TXA2 and others released factors stimulate recruitment and aggregation of additional platelets, and enhance COX-2 expression in stromal and endothelial cells in the tumor microenvironment.Citation33 Then, stromal cells produce prostanoids and other growth factors that stimulate COX-2 expression in epithelial cancer cells. Thus, inhibition of platelet COX-1 by aspirin and COX-2 expression in return in epithelial cells is one of the inhibitory mechanisms of aspirin on platelet-mediated tumourigenesis.Citation33,Citation41,Citation42
Activated platelets intervene at multiple stages of carcinogenesis. Firstly, activated platelet promotes cell proliferation through an upregulation of c-MYC.Citation43 They also stimulate angiogenesis, and epithelial-mesenchymal transition of tumor cells. Thus, tumor cells become more invasive, and intravasate in blood vessels (step one in ).Citation44 Then, platelets promote tumor cell survival in blood circulation by protecting them from natural killer (NK) cells. Secretion of TGF-β by activated platelets inhibits both the activation and function of NK cells.Citation45 Transfer of major histocompatibility complex class I molecules from platelets to tumor cells might also allow them to evade lysis by NK cells (step two in ).Citation46 Finally, platelets make extravasation and metastatic spread possible by promoting interactions between tumor cells and the vascular endothelium. CRC cells roll on activated endothelium, and this anchoring process is due to binding between CD44 on CRC cells and P-selectin on activated endothelial cells. Activated platelets interact with CRC cells in the bloodstream to form heteroaggregates that support attachment to the endothelium.Citation47 Then, activated platelets release adenine nucleotides, which enhance endothelium permeability through their action on P2Y2 receptors of endothelial cells, stimulating extravasation of cancer cells (step three in ).Citation48 Therefore, aspirin also exerts an antitumour effect through the inhibition of platelet activation.Citation33,Citation49,Citation50
Action on inflammatory and on immune cells (in red in )
Chronic inflammation is an important hallmark of CRC since it contributes to its initiation, as well as its progression.Citation51 Inflammation-induced cells, such as polymorphonuclear neutrophils and fibroblasts, produce pro-inflammatory cytokines and chemokines (TNF-α, IL1, IL6, CXCL1, and WNT5A) in the tumor microenvironment. These cytokines stimulate the production of PGE2 through increased activity of COX-2 of neutrophils and fibroblasts.Citation52 Then, PGE2 activates EP2 receptors on fibroblasts and polymorphonuclear neutrophils in the tumor microenvironment, forming a positive feedback loop that exacerbates inflammation.Citation53 Thus, the EP2 receptor could be an attractive target to inhibit inflammation-induced tumourigenesis, and several studies are currently being conducted to evaluate potent selective antagonists of this receptor.Citation54 PGE2 induces massive recruitment of immune cells and modifies their functionality, particular in lymphocytes, by stimulation of Th1 differentiation and Th17 expansion.Citation55,Citation56 PGE2 also modifies local cytokine profiles through induction of IL-23 and inhibition of IL-12 expression to stimulate T helper 17 (Th17) cell expression.Citation57 PGE2 also enhances dendritic cell migration through upregulation of CCR7, as well as their capacity to activate T cells.Citation58
II. Aspirin use to prevent or treat colorectal adenoma or cancer
2a. Aspirin use to prevent recurrence of colorectal adenoma
Most CRCs develop from precancerous adenomatous lesions through an adenoma–carcinoma sequence.Citation59 Many observational studies and randomized controlled trials (RCTs) suggest a significant decrease in CRC incidence for regular aspirin users (see section below). Consequently, several studies have been conducted to assess whether aspirin prevents recurrence of adenoma () and whether this antineoplastic effect was due to a decreased risk of adenoma formation or whether it intervened on the transformation of adenoma into cancerous lesions. Most of these studies, including several well-designed RCTs, shows that chemoprevention by aspirin reduces colorectal adenoma recurrence rate and decreases the rate of advanced adenoma recurrence, the number of recurrent adenomas and delays the time to adenoma recurrence.Citation60–62,Citation64 A recent meta-analysis found similar findings with a significant 20% reduction in risk in low-dose aspirin users (80 to 160 mg per day) compared to control patients (RR 0.80, CI 95% 0.70–0.92).Citation67
Table 1. Primary studies concerning the prevention of colorectal adenoma recurrence by aspirin.
However, two recent RCTs found no effect of aspirin chemoprevention on colorectal adenoma recurrenceCitation65,Citation66 and one RCT showed that chemoprevention by aspirin reduced colorectal adenoma recurrence after 1 y, but not after 4 y, of follow-up.Citation63 Some characteristics of the patients included in these latter trials might explain the lack of a protective effect of aspirin.
It has previously been reported that chemoprevention with daily low-dose aspirin reduced colorectal adenoma recurrence primarily in nonsmoking patients, while it was useless in patients who smoked.Citation64 This could explain the negative results reported by Pomergaard et al due to the high proportion of smokers in this study.Citation65 In the seAFOod Polyp Prevention trial, included patients corresponded to a high-risk population.Citation66 The adenoma recurrence rate at 1 y was not significantly different between the experimental (300 mg aspirin per day) and control (placebo) groups (61% in both groups) and was substantially higher than those previously reported in similar trials. Secondary analysis showed that aspirin treatment reduced the total number of colorectal adenoma per participant.Citation66 Therefore, the number of recurrent adenomas might be a more appropriate endpoint for evaluating the interest of chemoprevention by aspirin in this specific population.Citation68
These findings suggest that daily low-dose aspirin decreases the risk of colorectal adenoma recurrence with a benefit that would appear to be reduced in smokers and high-risk patient subgroups. For this latter subgroup, further trials assessing specific experimental conditions (dosing and duration of treatment) and endpoints are warranted.
2b. Aspirin use to prevent CRC
Several observational studies have identified aspirin as a protective factor against CRC development (). For example, a Danish retrospective case-control study showed a significant reduction of CRC incidence in patients who were treated with daily low-dose aspirin for more than 5 y (odds ratio of 0.73 (CI 95% 0.54–0.99)).Citation69 Another recent similar study in the UK also found a 34% decrease in the risk of developing CRC in patients with daily low-dose aspirin (RR 0.66, CI 95% 0.60–0.74).Citation71 A meta-analysis of five RCTs showed a 24% decrease in the risk for developing CRC (HR 0.76, CI 95% 0.60–0.96). This risk was especially reduced for proximal colon cancers. Daily low-dose aspirin also showed a 40% reduction for the risk of CRC mortality (HR 0.60, CI 95% 0.42–0.86). However, the results of RCTs remain controversial. Recently, the ASPREE trial was conducted to assess the impact of low-dose aspirin on disability-free survival among older adults who did not have cardiovascular disease, dementia, or disability. Secondary analyses demonstrated that aspirin use was associated with a higher risk for cancer-related mortality (HR 1.31, CI 95% 1.10–1.56) and colorectal cancer-related mortality (HR = 1.77, CI 95% 1.02–3.06). The short median follow-up of 4.7 y and the inclusion of older patients could potentially explain this lack of benefit.Citation75
Table 2. Primary studies concerning primary prevention by aspirin of colorectal cancer.
However, the studies by Rothwell et al and McNeil et al were not initially designed to assess CRC prevention.Citation70,Citation75 Specific RCTs have been conducted to demonstrate a cause and effect relationship and have measured the occurrence of CRC as a primary endpoint. Two large RCTs, the Physicians’ Health Study (PHS) (n = 22,071) and the Women’s Health Study (WHS) (n = 39,876), failed to demonstrate a protective effect of low-dose aspirin on CRC development in men and women, respectively.Citation72,Citation73 Indeed, no difference in favor of the experimental arm was found for either trial at the planned end of these studies, despite a follow-up of 12 y in the PHS and 9 y in the WHS.Citation72,Citation73 However, in the WHS, an extended follow-up of 18 y after randomization showed a significant association between chemoprevention by aspirin and decreased incidence of CRC (HR = 0.80; 95% CI 0.67–0.97).Citation74 This long latent period to observe a benefit suggests that chemoprevention by aspirin act in the early stages of tumorigenesis.
All of these studies support that low-dose aspirin decreases long-term risk (more than 10 y) of CRC, as well as its related mortality, in populations over 50 y of age and who are at high cardiovascular risk. All these scientific studies have led to recent publication of the United States Preventive Service Task Force recommendations. Aspirin is currently recommended for primary prevention of cardiovascular events and CRC in patients aged 50 to 59 with a 10-y risk of cardiovascular event greater than 10%. This may also be the case for some patients aged 60 to 69 y old with a 10-y risk of cardiovascular event greater than 10% without significant risk of bleeding and with a life expectancy of at least 10 y. Aspirin is not yet recommended in primary prevention for only the risk of CRC.Citation76
2c. Aspirin use in patients with genetic predisposition to CRC
Patients with familial adenomatous polyposis (FAP) or hereditary non-polyposis colorectal cancer (HNPCC) are considered to have a very high risk for developing CRC. Interest in chemoprevention by aspirin has been studied in this specific population. The CAPP1 (Colorectal Adenoma/Carcinoma Prevention Programme 1) study, comparing 600 mg aspirin treatment to placebo in patients with FAP aged 10 to 21 did not find a significant difference in colorectal polyp burden (RR = 0.77, CI 95% [0.54–1.10]). However, this trial showed that aspirin treatment significantly reduced the size of the largest polyp.Citation77 The CAPP2 trial compared a 2-y treatment with aspirin at 600 mg to placebo in patients with Lynch syndrome.Citation78 Results from the 10-y follow-up of this cohort have shown that aspirin significantly decreased the risk of CRC (adjusted HR = 0.65, 95% CI [0.43–0.97]) without increasing risk of serious adverse events. However, no difference was seen concerning the risk of other cancers of the Lynch syndrome spectrum (adjusted HR = 0.94, 95% CI [0.59–1.50]).Citation79 Thus, daily chemoprevention with 600 mg of aspirin during at least 2 y must be considered in young patients with Lynch syndrome. Current trials in Lynch syndrome patient populations aim to identify the optimal duration of aspirin treatment, as well as the possibility of decreasing the dosage.Citation80,Citation81 Thus, the CAPP3 phase 3 trial (NCT02497820) compares daily dose of 600 mg versus 300 mg versus 100 mg aspirin treatment (primary outcome: number of new primary mismatch repair deficient cancers at 5 y), while the French AAS-Lynch trial (NCT02813824) compares a daily aspirin dose of 100 mg versus 300 mg versus placebo (primary outcome: number of patients with at least one colorectal adenoma at 4 y).
2d. Aspirin use to treat CRC
Evidence has also been reported concerning the interest of aspirin after CRC occurrence.Citation82 Two aspects should be considered, depending on whether the tumor burden has been removed or is still present. Patients treated with daily low-dose aspirin are less likely to develop advanced stage CRC.Citation71 This suggests that aspirin affects the progression of established CRC.Citation71 This treatment could also potentiate the effect of conventional therapies since a retrospective study reported that long-term use of 100 mg aspirin in combination with neoadjuvant chemoradiotherapy increased the rate of downstaging (67.6% vs 43.6%, p = .01) and the pathological response rate (46% vs 19%; p < .001) in patients with rectal cancer.Citation83 Daily low-dose aspirin treatment also decreases the risk of metastatic progression. A meta-analysis of five major studies comparing aspirin versus placebo in cardiovascular disease prevention showed that patients who develop adenocarcinoma are less likely to experience metastatic progression if they are treated with aspirin (HR 0.54, CI 95% 0.38–0.77, p = .0007). In patients with localized CRC, follow-up showed that the risk of metastatic progression was significantly lower in the aspirin group than in the control group (HR 0.26, CI 95% 0.11–0.57, p = .0008).Citation84 Daily low-dose aspirin reduces the number of circulating tumor cells and avoids the epithelial mesenchymal transition in patients with metastatic CRC.Citation85
In addition to this effect on tumor progression and downstaging, several studies have shown that aspirin use after CRC diagnosis was associated with reduced CRC-specificCitation82,Citation86–88 and overall mortality.Citation82,Citation88–90 However, two observational studies found no association between aspirin and either CRC-specific or overall survival in patients with CRC (). ,Citation91,Citation92
Table 3. Primary studies assessing aspirin as a treatment of colorectal cancer.
Regarding this evidence, several RCTs assessing aspirin as an adjuvant therapy in CRC are currently being conducted. The ASCOLT trial (NCT00565708) aims to evaluate whether adjuvant treatment with 200 mg aspirin improves survival in patients with stage III and high-risk stage II CRC compared to placebo.Citation93 ASPIK is a French trial assessing 100 mg aspirin versus placebo in a specific population of patients with PIK3CA-mutated stage III or high-risk stage II tumors (See predictive biomarkers section).Citation94 The APREMEC trial (NCT02607072) is testing an adjuvant treatment with 100 mg or 200 mg aspirin compared to placebo in patients who underwent CRC resection.Citation95 The ADD-ASPIRIN trial (ISRCTN74358648) is assessing whether daily 100 mg or 300 mg aspirin use after standard cancer therapy prevents recurrence and prolongs survival in patients with early-stage solid tumors, including those of CRC.Citation96
III. Predictive biomarkers indicating the efficacy of aspirin
The benefits of aspirin require long-term administration whose side effects are rare but may sometimes be life threatening. This highlights the need to develop a precision medicine strategy to identify patients who are most likely to benefit from prophylactic low-dose aspirin treatment.
3a. Genetic biomarkers
Constitutional biomarkers are present physiologically throughout the body, as opposed to the somatic markers present or produced specifically after the development of cancer. Several studies have shown that chemoprevention by aspirin differed according to certain genetic variations. Indeed, specific single nucleotide polymorphisms (SNPs) have been associated with a lower risk of CRC in response to regular aspirin use (). ,Citation97,Citation98,Citation99,Citation100,Citation101 These genetic markers have so far been primarily described in situations of primary prevention. Validation of such genetic markers could be helpful for selecting responder patients to long-term aspirin use and could represent the first step of a chemoprevention strategy adapted to individual genetic characteristics.
Table 4. Studies on germline genetic biomarkers associated with efficacy of aspirin chemoprevention on colorectal adenoma or cancer occurrence.
3b. Somatic biomarkers
We previously stated that long-term aspirin use reduced the risk of tumor progression, as well as the recurrence rate, and improved overall survival after resection. This benefit seems to differ depending on the molecular tumor characteristics and the pathways involved in carcinogenesis.
Prostanoids pathway
Aspirin exerts its biological effects primarily through binding to the COX enzyme with a consequent reduction in the functionality of the enzyme and in turn, the resultant production of downstream mediators, such as PGE2. It is therefore natural to imagine that a preexisting imbalance in the concentration of mediators involved in this pathway could modify the effects of aspirin. A recent meta-analysis reported that COX-2 expression highlighted in the tumor tissue was significantly associated with a decreased risk of death in postdiagnosis aspirin users (HR 0.65, CI 95% 0.50–0.85).Citation86 Gray et al also confirmed these findings, showing that COX-2 expression was associated with improved overall survival in aspirin users (HR = 0.64, 95% CI 0.42–0.98) compared to non-users (HR = 1.28, 95% CI 0.80–2.03).Citation102 Prostaglandin metabolite (PGE-M) could also be an interesting biomarker since several studies have found that advanced and/or multiple colorectal adenomas were significantly associated with higher levels of PGE-M.Citation103,Citation104 A prospective case–control study nested within the Nurses’ Health Study found that regular aspirin use reduced the risk for adenoma only in women with high levels of PGE-M compared to those with low levels (multivariate OR = 0.61; 95% CI, 0.43–0.87).Citation104 Finally, the 15-hydroperoxyPGDH enzyme, which catalyses prostaglandin degradation, could also be a major factor in determining aspirin efficacy. Another case-control study conducted within the Nurses’ Health Study and the Health Professionals Follow-Up Study showed that long-term aspirin users, a lower risk of colorectal cancer was found only in patients with high levels of 15-hydroperoxyPGDH (multivariable HR = 0.49; 95% CI, 0.34 to 0.71).Citation105 This biomarker could also be interesting, as its levels appear stable over time and seem not to be altered by aspirin exposition.Citation106
Ras/Raf/MAP and EGFR/PI3KCA/AKT pathways
RAS and BRAF mutations are mutually exclusive and correspond to common molecular alterations in colorectal carcinogenesis. Aspirin’s effects seem more pronounced in wild-type tumors for both genes. A retrospective study found that regular aspirin use was associated with reduced risk of BRAF wild-type CRC but not with BRAF-mutated CRC.Citation107 Another study showed that postdiagnostic NSAID use, including aspirin, improved overall survival only in patients with KRAS wild-type tumors (HR = 0.60, 95% CI [0.46–0.80]) compared to KRAS-mutated tumors (HR = 1.24, 95% CI [0.78–1.96]).Citation108 In this study, BRAF mutation status was not predictive of postdiagnostic aspirin efficacy. PIK3CA mutations occur in 11–15% of CRCs and could be a predictive biomarker of aspirin efficacy in patients with CRC.Citation86 Subgroup analysis suggested that aspirin use may reduce CRC recurrence rate after resection and improve CRC-specific survival in patients with PIK3CA-mutated CRCs.Citation109,Citation110 However, the results from four additional recent retrospective studies were inconsistent with this observation ().Citation102,Citation109,Citation110,Citation111,Citation112,Citation113 A meta-analysis of the first three studiesCitation109,Citation110,Citation111 showed an association between aspirin and a reduced risk of death in patients with PIK3CA-mutated CRC (HR = 0.58, 95% CI [0.37–0.90]).Citation86 Given these discordant results, a French RCT (PRODIGE 50 – ASPIK) is currently being conducted. This trial compares adjuvant treatment with aspirin (100 mg per day) to placebo in patients with PIK3CA-mutated tumors who underwent surgery for stage III or high-risk stage II CRC. The primary endpoint corresponds to the 3 y disease-free survival rate.Citation94
Table 5. Retrospective studies assessing the efficacy of aspirin in patients with PIK3CA mutation who underwent colorectal cancer resection.
IV. Aspirin and CRC screening
CRC chemoprevention by long-term use of aspirin raises the question of its association with CRC screening. Fecal immunochemical testing (FIT) is the most often used method for CRC screening. This test is based on the identification of human hemoglobin in a stool sample. It has been proven to reduce CRC mortality.Citation114 Aspirin treatment may affect FIT results in inconsistent ways. On the one hand, aspirin could increase bleeding from colorectal adenoma or carcinoma, enhancing FIT sensitivity; but on the other hand, aspirin could increase physiological blood loss from colonic mucosa or from non-tumor lesions, decreasing FIT specificity and predictive positive value. Concerning FIT sensibility, Brenner et al showed that a single aspirin dose given before the FIT did not increase its sensitivity in patients not taking antithrombotic drugs.Citation115 Concerning FIT predictive positive values, a large Norwegian cohort study found that regular aspirin use was associated with lower positive predictive value of FIT for detection of CRC and advanced adenomas.Citation116 However, a recent meta-analysis showed that aspirin treatment did not affect FIT accuracy.Citation117 According to these studies, current guidelines recommend to continue aspirin treatment during the FIT.Citation118
V. Risks of long-term aspirin treatment
Long-term aspirin use is associated with the occurrence of adverse effects, primarily including an increased risk of bleeding events.Citation119 A meta-analysis involving 35 RCTs (low-dose aspirin alone) and 87,581 individuals confirmed this increase of any bleeds, any major gastrointestinal bleeds (GI) and any GI bleeds with hazard ratios of 1.54 (95% CI 1.36–1.74), 1.55 (95% CI 1.27–1.90) and 1.31 (95% CI 1.21–1.42), respectively.Citation120 However, there was no meaningful difference concerning the risk for fatal bleeds (OR = 1.22, 95% CI [0.78–1.89]). Furthermore, there is a relationship between the dose of aspirin or increasing age and the risk of bleeding events.Citation121 Prophylaxis for bleeding may be associated with co‐administration of a proton pump inhibitor, especially in cases of aspirin use combined with another platelet aggregation inhibitor or anticoagulant, since it has been reported to reduce the risk of major GI bleeding (OR = 0.34, 95% CI [0.21–0.57]).Citation120
Conclusions
Daily low-dose aspirin treatment is a protective factor against the occurrence of CRC. Regarding the scientific evidence, US authorities published recommendations concerning chemoprevention of CRC using aspirin in patients aged 50 to 59 with a 10-y risk of cardiovascular events greater than 10%. In patients with CRC, aspirin use seems to reduce the risk of tumor progression and the recurrence rate after resection and may also increase overall survival. The knowledge of inhibitory mechanisms induced by aspirin on colorectal carcinogenesis has made it possible to identify predictive biomarkers for CRC chemoprevention. This effect seems to be particularly interesting in tumors with PIK3CA mutations and/or involvement of the prostanoid pathway.
Ethical approval
As this work was a review of the literature, no ethics committee approval was required.
Supplemental Material
Download PDF (137.8 KB)Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Disclosure statement
None
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384047.2022.2104561
Additional information
Funding
Notes on contributors
Adrien Grancher
Dr Grancher is a MD of hepatogastroenterology and digestive oncology. His areas of research focuses on screening and chemoprevention of colorectal cancer, as well as the interest of circulating tumor DNA in both localized or metastatic colorectal cancer.
Pierre Michel
Pr Michel is a MD PhD of hepatogastroenterology and digestive oncology. His areas of research focuses on therapeutics strategies in digestive cancers, and in particular the value of aspirin treatment in PI3KCA-mutated patients with an advanced colorectal cancer.
Frederic Di Fiore
Pr Di Fiore is a MD PhD of hepatogastroenterology and digestive oncology. His areas of research focuses on the interest of circulating tumor DNA for diagnosis and prognosis in both colorectal and liver cancers.
David Sefrioui
Dr Sefrioui is a MD of hepatogastroenterology and digestive oncology. His areas of research focuses on the interest of circulating tumor DNA for diagnosis and prognosis in both colorectal and liver cancers.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
- Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. 2020;158(2):368–388. doi:10.1053/j.gastro.2019.06.047.
- Qiao Y, Yang T, Gan Y, Li W, Wang C, Gong Y, Lu Z. Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies. BMC Cancer. 2018;18 doi:10.1186/s12885-018-4156-5.
- Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–2383. doi:10.1056/NEJMra052717.
- Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–267. doi:10.1038/nrclinonc.2011.199.
- Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, Lawrence E, Lutterbaugh J, Lu S, Willson JKV, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-β-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004;101(50):17468–17473. doi:10.1073/pnas.0406142101.
- Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. doi:10.1056/NEJMoa067208.
- Kuo C-N, Pan -J-J, Huang Y-W, Tsai H-J, Chang W-C. Association between nonsteroidal anti-inflammatory drugs and colorectal cancer: a population-based case–control study. Cancer Epidemiol Biomarkers Prev. 2018;27(7):737–745. doi:10.1158/1055-9965.EPI-17-0876.
- Mohammed A, Janakiram NB, Madka V, Zhang Y, Singh A, Biddick L, Li Q, Lightfoot S, Steele VE, Lubet RA, et al. Intermittent dosing regimens of aspirin and naproxen inhibit azoxymethane-induced colon adenoma progression to adenocarcinoma and invasive carcinoma. Cancer Prevention Research (Philadelphia, Pa. 2019;12(11):751. doi:10.1158/1940-6207.CAPR-19-0312.
- Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi:10.1038/nrc2809.
- Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107(4):1183–1188. doi:10.1016/0016-5085(94)90246-1.
- Zhang H, Sun X-F. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97(4):1037–1041. doi:10.1111/j.1572-0241.2002.05625.x.
- Myung S-J, Rerko RM, Yan M, Platzer P, Guda K, Dotson A, Lawrence E, Dannenberg AJ, Lovgren AK, Luo G, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103(32):12098–12102. doi:10.1073/pnas.0603235103.
- Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276(21):18075–18081. doi:10.1074/jbc.M009689200.
- Wang D, DuBois RN. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013;19(6):502–510. doi:10.1097/PPO.0000000000000003.
- Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6(1):479–507. doi:10.1146/annurev-pathol-011110-130235.
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a G s -Axin-ß-catenin signaling axis. Science. 2005;310(5753):1504–1510. doi:10.1126/science.1116221.
- Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, Weidinger G, Puder M, Daley GQ, Moon RT, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–1147. doi:10.1016/j.cell.2009.01.015.
- Wang D, Wang H, Shi Q, Katkuri S, Walhi W, Desvergne B, Das SK, Dey SK, DuBois RN. Prostaglandin E2 promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor δ. Cancer Cell. 2004;6(3):285–295. doi:10.1016/j.ccr.2004.08.011.
- Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator–activated receptor-δ accelerates intestinal adenoma growth. Nat Med. 2004;10(3):245–247. doi:10.1038/nm993.
- He T-C, Chan TA, Vogelstein B, Kinzler KW. PPARδ is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99(3):335–345. doi:10.1016/S0092-8674(00)81664-5.
- Smartt HJM, Greenhough A, Ordóñez-Morán P, Talero E, Cherry CA, Wallam CA, Parry L, Al Kharusi M, Roberts HR, Mariadason JM, et al. β-catenin represses expression of the tumour suppressor 15-prostaglandin dehydrogenase in the normal intestinal epithelium and colorectal tumour cells. Gut. 2012;61(9):1306–1314. doi:10.1136/gutjnl-2011-300817.
- Gala MK, Chan AT. Molecular pathways: aspirin and wnt signaling—a molecularly targeted approach to cancer prevention and treatment. Clin Cancer Res. 2015;21(7):1543–1548. doi:10.1158/1078-0432.CCR-14-0877.
- Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16(3):173–186. doi:10.1038/nrc.2016.4.
- Bos CL, Kodach LL, van den Brink GR, Diks SH, van Santen MM, Richel DJ, Peppelenbosch MP, Hardwick JCH. Effect of aspirin on the Wnt/β-catenin pathway is mediated via protein phosphatase 2A. Oncogene. 2006;25(49):6447–6456. doi:10.1038/sj.onc.1209658.
- Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of β-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci U S A. 2006;103(5):1492–1497. doi:10.1073/pnas.0510562103.
- Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278(37):35451–35457. doi:10.1074/jbc.M302474200.
- Zumwalt TJ, Wodarz D, Komarova NL, Toden S, Turner J, Cardenas J, Burn J, Chan AT, Boland CR, Goel A. Aspirin-induced chemoprevention and response kinetics are enhanced by PIK3CA mutations in colorectal cancer cells. Cancer Prev Res (Phila). 2017;10(3):208–218. doi:10.1158/1940-6207.CAPR-16-0175.
- Gu M, Nishihara R, Chen Y, Li W, Shi Y, Masugi Y, Hamada T, Kosumi K, Liu L, da Silva A, et al. Aspirin exerts high anti-cancer activity in PIK3CA -mutant colon cancer cells. Oncotarget. 2017;8(50):87379–87389. doi:10.18632/oncotarget.20972.
- Wang D, Buchanan FG, Wang H, Dey SK, DuBois RN. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 2005;65(5):1822–1829. doi:10.1158/0008-5472.CAN-04-3671.
- Pan M-R, Chang H-C, Hung W-C. Non-steroidal anti-inflammatory drugs suppress the ERK signaling pathway via block of Ras/c-Raf interaction and activation of MAP kinase phosphatases. Cell Signal. 2008;20(6):1134–1141. doi:10.1016/j.cellsig.2008.02.004.
- Yin M-J, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature. 1998;396(6706):77–80. doi:10.1038/23948.
- Dovizio M, Bruno A, Tacconelli S, Patrignani P. Mode of action of aspirin as a chemopreventive agent. Recent Results Cancer Res. 2013;191:39–65.
- Ying J, Zhou H, Liu P, You Q, Kuang F, Shen Y, Hu Z. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci. 2018;8(1). doi:10.1186/s13578-017-0198-7.
- Jung YR, Kim EJ, Choi HJ, Park -J-J, Kim H-S, Lee Y-J, Park M-J, Lee M. Aspirin targets SIRT1 and AMPK to induce senescence of colorectal carcinoma cells. Mol Pharmacol. 2015;88(4):708–719. doi:10.1124/mol.115.098616.
- Din FVN, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142(7):1504–15.e3. doi:10.1053/j.gastro.2012.02.050.
- Zimmermann KC, Waterhouse NJ, Goldstein JC, Schuler M, Green DR. Aspirin induces apoptosis through release of cytochrome c from mitochondria. Neoplasia. 2000;2(6):505–513. doi:10.1038/sj.neo.7900120.
- Gu Q, Wang JD, Xia HHX, Lin MCM, He H, Zou B, Tu SP, Yang Y, Liu XG, Lam SK, et al. Activation of the caspase-8/Bid and Bax pathways in aspirin-induced apoptosis in gastric cancer. Carcinogenesis. 2005;26(3):541–546. doi:10.1093/carcin/bgh345.
- Wang Y, Du C, Zhang N, Li M, Liu Y, Zhao M, Wang F, Luo F. TGF-β1 mediates the effects of aspirin on colonic tumor cell proliferation and apoptosis. Oncol Lett. 2018;15(4):5903–5909. doi:10.3892/ol.2018.8047.
- Dovizio M, Maier TJ, Alberti S, Di Francesco L, Marcantoni E, Münch G, John CM, Suess B, Sgambato A, Steinhilber D, et al. Pharmacological inhibition of platelet-tumor cell cross-talk prevents platelet-induced overexpression of cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol Pharmacol. 2013;84(1):25–40. doi:10.1124/mol.113.084988.
- Patrono C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J Am Coll Cardiol. 2015;66(1):74–85. doi:10.1016/j.jacc.2015.05.012.
- Boutaud O, Sosa IR, Amin T, Oram D, Adler D, Hwang HS, Crews BC, Milne G, Harris BK, Hoeksema M, et al. Inhibition of the biosynthesis of prostaglandin E2 by low-dose aspirin: implications for adenocarcinoma metastasis. Cancer Prev Res (Phila). 2016;9(11):855–865. doi:10.1158/1940-6207.CAPR-16-0094.
- Mitrugno A, Sylman JL, Ngo ATP, Pang J, Sears RC, Williams CD, McCarty OJT. Aspirin therapy reduces the ability of platelets to promote colon and pancreatic cancer cell proliferation: implications for the oncoprotein c-MYC. Am J Physiol Cell Physiol. 2017;312(2):C176–89. doi:10.1152/ajpcell.00196.2016.
- Xu XR, Yousef GM, Ni H. Cancer and platelet crosstalk: opportunities and challenges for aspirin and other antiplatelet agents. Blood. 2018;131(16):1777–1789. doi:10.1182/blood-2017-05-743187.
- Kopp H-G, Placke T, Salih HR. Platelet-derived transforming growth factor-β down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009;69(19):7775–7783. doi:10.1158/0008-5472.CAN-09-2123.
- Placke T, Örgel M, Schaller M, Jung G, Rammensee H-G, Kopp H-G, Salih HR. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. 2012;72(2):440–448. doi:10.1158/0008-5472.CAN-11-1872.
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–134. doi:10.1038/nrc3004.
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–137. doi:10.1016/j.ccr.2013.05.008.
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi:10.1016/j.ccr.2011.09.009.
- Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A, Grande R, Alberti S, Arena V, Cirillo M, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7(22):32462–32477. doi:10.18632/oncotarget.8655.
- Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56(2):69–83. doi:10.3322/canjclin.56.2.69.
- Aoki T, Narumiya S. Prostaglandin E2-EP2 signaling as a node of chronic inflammation in the colon tumor microenvironment. Inflammation and Regeneration. 2017;37(1). doi:10.1186/s41232-017-0036-7.
- Ma X, Aoki T, Tsuruyama T, Narumiya S. Definition of prostaglandin E2–EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 2015;75(14):2822–2832. doi:10.1158/0008-5472.CAN-15-0125.
- Mizuno R, Kawada K, Sakai Y. Prostaglandin E2/EP signaling in the tumor microenvironment of colorectal cancer. Int J Mol Sci. 2019;20(24):6254. doi:10.3390/ijms20246254.
- Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–788. doi:10.1038/onc.2009.421.
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, Sugimoto Y, Narumiya S. Prostaglandin E2–EP4 signaling promotes immune inflammation through TH1 cell differentiation and TH17 cell expansion. Nat Med. 2009;15(6):633–640. doi:10.1038/nm.1968.
- Sheibanie AF, Yen J-H, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→IL-17 axis. J Immunol. 2007;178(12):8138–8147. doi:10.4049/jimmunol.178.12.8138.
- Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100(4):1354–1361. doi:10.1182/blood-2001-11-0017.
- Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–1013. doi:10.1016/0016-5085(87)90563-4.
- Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348(10):883–890. doi:10.1056/NEJMoa021633.
- Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348(10):891–899. doi:10.1056/NEJMoa021735.
- Logan RFA, Grainge MJ, Shepherd VC, Armitage NC, Muir KR, ukCAP Trial Group. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134(1):29–38. 10.1053/j.gastro.2007.10.014
- Benamouzig R, Uzzan B, Deyra J, Martin A, Girard B, Little J, Chaussade S, Association pour la Prévention par l’Aspirine du Cancer Colorectal Study Group (APACC). Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. 2012;61(2):255–261. 10.1136/gutjnl-2011-300113
- Ishikawa H, Mutoh M, Suzuki S, Tokudome S, Saida Y, Abe T, Okamura S, Tajika M, Joh T, Tanaka S, et al. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut. 2014;63(11):1755–1759. doi:10.1136/gutjnl-2013-305827.
- Pommergaard H-C, Burcharth J, Rosenberg J, Raskov H. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. 2016;150(1):114–122.e4. doi:10.1053/j.gastro.2015.09.010.
- Hull MA, Sprange K, Hepburn T, Tan W, Shafayat A, Rees CJ, Clifford G, Logan RF, Loadman PM, Williams EA, et al. Eicosapentaenoic acid and aspirin, alone and in combination, for the prevention of colorectal adenomas (seAFOod Polyp Prevention trial): a multicentre, randomised, double-blind, placebo-controlled, 2 × 2 factorial trial. Lancet. 2018;392(10164):2583–2594. doi:10.1016/S0140-6736(18)31775-6.
- Veettil SK, Lim KG, Ching SM, Saokaew S, Phisalprapa P, Chaiyakunapruk N. Effects of aspirin and non-aspirin nonsteroidal anti-inflammatory drugs on the incidence of recurrent colorectal adenomas: a systematic review with meta-analysis and trial sequential analysis of randomized clinical trials. BMC Cancer. 2017;17(1). doi:10.1186/s12885-017-3757-8.
- Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, Uchiyama T, Taniguchi L, Hata Y, Uchiyama S, et al. Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. Lancet Oncol. 2016;17(4):475–483. doi:10.1016/S1470-2045(15)00565-3.
- Friis S, Riis AH, Erichsen R, Baron JA, Sørensen HT. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med. 2015;163(5):347–355. doi:10.7326/M15-0039.
- Rothwell PM, Wilson M, Elwin C-E, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi:10.1016/S0140-6736(10)61543-7.
- García Rodríguez LA, Soriano-Gabarró M, Bromley S, Lanas A, Cea Soriano L. New use of low-dose aspirin and risk of colorectal cancer by stage at diagnosis: a nested case–control study in UK general practice. BMC Cancer. 2017;17(1). doi:10.1186/s12885-017-3594-9.
- Stürmer T, Glynn RJ, Lee IM, Manson JE, Buring JE, Hennekens CH. Aspirin use and colorectal cancer: post-trial follow-up data from the Physicians’ Health Study. Ann Intern Med. 1998;128(9):713–720. doi:10.7326/0003-4819-128-9-199805010-00003.
- Cook NR, Lee I-M, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi:10.1001/jama.294.1.47.
- Cook NR, Lee I-M, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;159(2):77–85. doi:10.7326/0003-4819-159-2-201307160-00002.
- McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, Kirpach B, Shah RC, Ives DG, Storey E, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–1528. doi:10.1056/NEJMoa1803955.
- Chan AT, Ladabaum U. Where do we stand with aspirin for the prevention of colorectal cancer? The USPSTF recommendations. Gastroenterology. 2016;150(1):14–18. doi:10.1053/j.gastro.2015.11.018.
- Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, Eccles D, Ellis A, Evans DG, Fodde R, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila). 2011;4(5):655–665. doi:10.1158/1940-6207.CAPR-11-0106.
- Burn J, Gerdes A-M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081–2087. doi:10.1016/S0140-6736(11)61049-0.
- Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin J-P, Möslein G, McRonald FE, Bertario L, Evans DG, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. 2020;395(10240):1855–1863. doi:10.1016/S0140-6736(20)30366-4.
- Burn J, Mathers JC, Bishop DT. Chemoprevention in Lynch syndrome. Fam Cancer. 2013;12(4):707–718. doi:10.1007/s10689-013-9650-y.
- Effect of chemoprevention by low-dose aspirin of new or recurrent colorectal adenomas in patients with lynch syndrome - full text view - ClinicalTrials.gov; 2020. [accedded 2020 Apr 30 https://clinicaltrials.gov/ct2/show/NCT02813824.
- Chan AT. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302(6):649–658. doi:10.1001/jama.2009.1112.
- Restivo A, Cocco IMF, Casula G, Scintu F, Cabras F, Scartozzi M, Zorcolo L. Aspirin as a neoadjuvant agent during preoperative chemoradiation for rectal cancer. Br J Cancer. 2015;113(8):1133–1139. doi:10.1038/bjc.2015.336.
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. The Lancet. 2012;379(9826):1591–1601. doi:10.1016/S0140-6736(12)60209-8.
- Yang L, Lv Z, Xia W, Zhang W, Xin Y, Yuan H, Chen Y, Hu X, Lv Y, Xu Q, et al. The effect of aspirin on circulating tumor cells in metastatic colorectal and breast cancer patients: a phase II trial study. Clin Transl Oncol. 2018;20(7):912–921. doi:10.1007/s12094-017-1806-z.
- Li P, Wu H, Zhang H, Shi Y, Xu J, Ye Y, Xia D, Yang J, Cai J, Wu Y. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64(9):1419–1425. doi:10.1136/gutjnl-2014-308260.
- Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJM, van Herk-sukel MPP, Lemmens V, van den Broek CBM, Coebergh JW, Herings RMC, van de Velde CJH, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106(9):1564–1570. doi:10.1038/bjc.2012.101.
- McCowan C, Munro AJ, Donnan PT, Steele RJC. Use of aspirin post-diagnosis in a cohort of patients with colorectal cancer and its association with all-cause and colorectal cancer specific mortality. Eur J Cancer. 2013;49(5):1049–1057. doi:10.1016/j.ejca.2012.10.024.
- Goh CH, Goh HH, Leong WQ, Chew MH, Pan YS, Tony LKH, Chew L, Tan IBH, Toh HC, Tang CL, et al. Post-operative aspirin use and colorectal cancer-specific survival in patients with stage I-III colorectal cancer. Anticancer Res. 2014;34(12):7407–7414.
- Bains SJ, Mahic M, Myklebust TÅ, Småstuen MC, Yaqub S, Dørum LM, Bjørnbeth BA, Møller B, Brudvik KW, Taskén K. Aspirin as secondary prevention in patients with colorectal cancer: an unselected population-based study. J Clin Oncol. 2016;34(21):2501–2508. doi:10.1200/JCO.2015.65.3519.
- Cardwell CR, Kunzmann AT, Cantwell MM, Hughes C, Baron JA, Powe DG, Murray LJ. Low-dose aspirin use after diagnosis of colorectal cancer does not increase survival: a case–control analysis of a population-based cohort. Gastroenterology. 2014;146(3):700–708.e2. doi:10.1053/j.gastro.2013.11.005.
- Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Low-dose aspirin use and survival in colorectal cancer: results from a population-based cohort study. BMC Cancer. 2018;18(1):228. doi:10.1186/s12885-018-4142-y.
- Ali R, Toh H-C, Chia W-K. The utility of Aspirin in dukes C and high risk dukes B colorectal cancer - The ASCOLT study: study protocol for a randomized controlled trial. Trials. 2011;12(1):261. doi:10.1186/1745-6215-12-261.
- Michel P, Boige V, Andre T, Aparicio T, Bachet JB, Dahan L, Guimbaud R, Lepage C, Manfredi S, Tougeron D, et al. Aspirin versus placebo in stage III or high-risk stage II colon cancer with PIK3CA mutation: a French randomised double-blind phase III trial (PRODIGE 50-ASPIK). Dig Liver Dis. 2018;50(3):305–307. doi:10.1016/j.dld.2017.12.023.
- Aspirin for prevention of postsurgical recurrence and metastasis in asian colorectal cancer patients: a multi-center randomized trial - full text view - ClinicalTrials.gov; 2020. [cited 2020 Apr 30]. https://clinicaltrials.gov/ct2/show/NCT02607072.
- Coyle C, Cafferty FH, Rowley S, MacKenzie M, Berkman L, Gupta S, Pramesh CS, Gilbert D, Kynaston H, Cameron D, et al. ADD-ASPIRIN: a phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp Clin Trials. 2016;51:56–64. doi:10.1016/j.cct.2016.10.004.
- Nan H, Hutter CM, Lin Y, Jacobs EJ, Ulrich CM, White E, Baron JA, Berndt SI, Brenner H, Butterbach K, et al. Association of Aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA. 2015;313(11):1133–1142. doi:10.1001/jama.2015.1815.
- Nan H, Morikawa T, Suuriniemi M, Imamura Y, Werner L, Kuchiba A, Yamauchi M, Hunter DJ, Kraft P, Giovannucci EL, et al. Aspirin use, 8q24 single nucleotide polymorphism rs6983267, and colorectal cancer according to CTNNB1 alterations. J Natl Cancer Inst. 2013;105(24):1852–1861. doi:10.1093/jnci/djt331.
- Resler AJ, Makar KW, Heath L, Whitton J, Potter JD, Poole EM, Habermann N, Scherer D, Duggan D, Wang H, et al. Genetic variation in prostaglandin synthesis and related pathways, NSAID use and colorectal cancer risk in the Colon Cancer Family Registry. Carcinogenesis. 2014;35(9):2121–2126. doi:10.1093/carcin/bgu119.
- Hubner RA, Muir KR, Liu J-F, Logan RFA, Grainge MJ, Houlston RS. Members of the UKCAP Consortium. Ornithine decarboxylase G316A genotype is prognostic for colorectal adenoma recurrence and predicts efficacy of aspirin chemoprevention. Clin Cancer Res. 2008;14(8):2303–2309. doi:10.1158/1078-0432.CCR-07-4599.
- Barry EL, Mott LA, Sandler RS, Ahnen DJ, Baron JA. Variants downstream of the ornithine decarboxylase gene influence risk of colorectal adenoma and Aspirin Chemoprevention. Cancer Prev Res (Phila). 2011;4(12):2072–2082. doi:10.1158/1940-6207.CAPR-11-0300.
- Gray RT, Cantwell MM, Coleman HG, Loughrey MB, Bankhead P, McQuaid S, O’Neill RF, Arthur K, Bingham V, McGready C, et al. Evaluation of PTGS2 expression, PIK3CA mutation, aspirin use and colon cancer survival in a population-based cohort study. Clin Transl Gastroenterol. 2017;8(4):e91. doi:10.1038/ctg.2017.18.
- Shrubsole MJ, Cai Q, Wen W, Milne G, Smalley WE, Chen Z, Ness RM, Zheng W. Urinary prostaglandin E2 metabolite and risk for colorectal adenoma. Cancer Prev Res (Phila). 2012;5(2):336–342. doi:10.1158/1940-6207.CAPR-11-0426.
- Bezawada N, Song M, Wu K, Mehta RS, Milne GL, Ogino S, Fuchs CS, Giovannucci EL, Chan AT. Urinary PGE-M levels are associated with risk of colorectal adenomas and chemopreventive response to anti-inflammatory drugs. Cancer Prev Res (Phila). 2014;7(7):758–765. doi:10.1158/1940-6207.CAPR-14-0120.
- Fink SP, Yamauchi M, Nishihara R, Jung S, Kuchiba A, Wu K, Cho E, Giovannucci E, Fuchs CS, Ogino S, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD). Sci Transl Med. 2014;6(233):233re2. doi:10.1126/scitranslmed.3008481.
- Fink SP, Yang D-H, Barnholtz-Sloan JS, Ryu Y-M, Mikkola D, Potter JD, Lampe JW, Markowitz SD, Myung S-J. Colonic 15-PGDH levels are stable across distance and time and are not perturbed by aspirin intervention. Dig Dis Sci. 2013;58(9):2615–2622. doi:10.1007/s10620-013-2670-5.
- Nishihara R, Lochhead P, Kuchiba A, Jung S, Yamauchi M, Liao X, Imamura Y, Qian ZR, Morikawa T, Wang M, et al. Aspirin use and risk of colorectal cancer according to braf mutation status. JAMA. 2013;309(24):2563–2571. doi:10.1001/jama.2013.6599.
- Hua X, Phipps AI, Burnett-Hartman AN, Adams SV, Hardikar S, Cohen SA, Kocarnik JM, Ahnen DJ, Lindor NM, Baron JA, et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol. 2017;35(24):2806–2813. doi:10.1200/JCO.2017.72.3569.
- Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):1596–1606. doi:10.1056/NEJMoa1207756.
- Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, Davidson B, Kerr DJ, Tomlinson IPM, Midgley R. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31(34):4297–4305. doi:10.1200/JCO.2013.50.0322.
- Reimers MS, Bastiaannet E, Langley RE, van Eijk R, van Vlierberghe RLP, Lemmens VEP, van Herk-sukel MPP, van Wezel T, Fodde R, Kuppen PJK, et al. Expression of HLA class I antigen, aspirin use, and survival after a diagnosis of colon cancer. JAMA Intern Med. 2014;174(5):732–739. doi:10.1001/jamainternmed.2014.511.
- Kothari N, Kim R, Jorissen RN, Desai J, Tie J, Wong H-L, Farragher I, Jones I, Day FL, LI S, et al. Impact of regular aspirin use on overall and cancer-specific survival in patients with colorectal cancer harboring a PIK3CA mutation. Acta Oncol. 2015;54(4):487–492. doi:10.3109/0284186X.2014.990158.
- Murphy C, Turner N, Wong H-L, Sinnathamby M, Tie J, Lee B, Desai J, Skinner I, Christie M, Hutchinson R, et al. Examining the impact of regular aspirin use and PIK3CA mutations on survival in stage 2 colon cancer. Intern Med J. 2017;47(1):88–98. doi:10.1111/imj.13312.
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology. 2004;126(7):1674–1680. doi:10.1053/j.gastro.2004.02.018.
- Brenner H, Calderazzo S, Seufferlein T, Ludwig L, Dikopoulos N, Mangold J, Böck W, Stolz T, Eisenbach T, Block T, et al. Effect of a single aspirin dose prior to fecal immunochemical testing on test sensitivity for detecting advanced colorectal neoplasms. JAMA. 2019;321(17):1686–1692. doi:10.1001/jama.2019.4755.
- Randel KR, Botteri E, Romstad KMK, Frigstad SO, Bretthauer M, Hoff G, de Lange T, Holme Ø. Effects of oral anticoagulants and aspirin on performance of fecal immunochemical tests in colorectal cancer screening. Gastroenterology. 2019;156(6):1642–1649.e1. doi:10.1053/j.gastro.2019.01.040.
- Nieuwenburg SAV, Vuik FER, Kruip MJHA, Kuipers EJ, Spaander MCW. Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: a systematic review and meta-analysis. Gut. 2019;68(5):866–872. doi:10.1136/gutjnl-2018-316344.
- Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Rex DK. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US multi-society task force on colorectal cancer. Gastroenterology. 2017;152(5):1217–1237.e3. doi:10.1053/j.gastro.2016.08.053.
- Tsoi KK, Chan FC, Hirai HW, Sung JJ. Risk of gastrointestinal bleeding and benefit from colorectal cancer reduction from long-term use of low-dose aspirin: a retrospective study of 612 509 patients. J Gastroenterol Hepatol. 2018;33(10):1728–1736. doi:10.1111/jgh.14261.
- Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic acid increase risk of gastrointestinal bleeding in a meta-analysis. Clinical Gastroenterology and Hepatology. 2011;9(9):762–768.e6. doi:10.1016/j.cgh.2011.05.020.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Baggish JS, Bhatt DL, Topol EJ. Analysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trials. Am J Cardiol. 2005;95(10):1218–1222. doi:10.1016/j.amjcard.2005.01.049.
