ABSTRACT
Head and neck squamous cell carcinoma (HNSCC) is a heterogeneous and aggressive tumor with high mortality and unfavorable prognosis. Numerous long non-coding RNAs (lncRNAs) have been confirmed to exert pivotal parts in cancers. Nevertheless, the functions of most lncRNAs in HNSCC need deeper exploration. Our present research tried to clarify the biological role of TM4SF19 antisense RNA 1 (TM4SF19-AS1) and investigate its regulatory mechanism in HNSCC. RT-qPCR analysis was done to test TM4SF19-AS1 expression and identify the up-regulation of TM4SF19-AS1 in HNSCC cells. Loss-of-function assays were also involved, and the data implied that TM4SF19-AS1 knockdown hampered the proliferation, migration, invasion, along with epithelial-mesenchymal transition (EMT) of HNSCC cells. In vivo assays revealed TM4SF19-AS1 depletion restrained HNSCC tumor growth. Additionally, mechanism experiments were implemented to uncover the underlying regulatory mechanism of TM4SF19-AS1 in HNSCC cells. It turned out that TM4SF19-AS1 modulated laminin subunit gamma 1 (LAMC1) expression via sequestering microRNA-153-3p (miR-153-3p) and recruiting heterogeneous nuclear ribonucleoprotein C (HNRNPC) protein. Rescue assays confirmed that TM4SF19-AS1 contributed to HNSCC cell malignant behaviors via up-regulating LAMC1. To summarize, TM4SF19-AS1 played an oncogenic role in HNSCC cells, signifying TM4SF19-AS1 may have the potential to be used as a novel molecular target for HNSCC diagnosis.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is considered to be a heterogeneous malignancy responsible for high morbidity and fatalityCitation1. It encompasses various tumors that are derived from the squamous epithelium of the oral cavity, oropharynx, larynx, and hypopharynx.Citation2 It is commonly considered that tobacco and HPV infection are the main risk factors of HNSCC.Citation3 Currently, the main treatment options for HNSCC are surgery and radiotherapy. However, radiotherapy resistance and frequent relapse constantly influence the therapeutic effect of HNSCC.Citation4 As a result, the treatment of HNSCC remains tough and challenging. Targeted therapy is determined as a novel strategy in head and neck cancers.Citation5 Under this condition, the emergence of biomarkers is of great value to the diagnosis, treatment, and prognosis of HNSCC.Citation6
Long non-coding RNAs (lncRNAs) are classified as transcripts, which have over 200 nucleotides in length and lack coding potential. During the past years, growing evidence has supported that dysregulated lncRNAs can make crucial influence on malignant cell phenotypes in cancers.Citation7 Referring to precious research, lncRNA SNHG7 has been uncovered to facilitate lung cancer initiation and progression via up-regulating FAIM2 expression.Citation8 As reported by Qin et al., lncRNA MIR155 HG prompts pancreatic cancer process by serving as a sponge of miR-802.Citation9 Zhang et al. have pointed out that LINC01446 functions as an oncogene in glioblastoma through ceRNA mechanism.Citation10 LncRNAs widely participate in the biological processes of HNSCC through gene regulation.Citation11 According to published research work, lncRNA UCA1 affects the proliferation and cisplatin resistance of oral squamous cell carcinoma cells through absorbing miR-184.Citation12 Another study has uncovered the oncogenic effect of LINC00460/miR-612/AKT2 axis on HNSCC.Citation13 Aside from that, Yang et al. have illustrated lncRNA FKBP9P1 expedites HNSCC development via modulation of PI3K/AKT signaling pathway.Citation14
Zhang et al. have mentioned that TM4SF19-AS1 is one of the lncRNAs associated with overall survival and prognosis of HNSCC.Citation15 However, whether TM4SF19-AS1 influences malignant phenotypes of HNSCC cells has not been studied. In this study, we pay attention to discussing the role of TM4SF19-AS1 in HNSCC cells and investigating into the possible regulatory mechanisms behind TM4SF19-AS1.
Materials and methods
Cell culture
HNSCC cell lines (SCC-25, SCC-9, CAL-27, and FaDu) were all procured from American Type Culture Collection (ATCC; Manassas, VA, USA), and human normal squamous epithelial cell line (NOK) was procured from Huatuo Biotechnology Co., Ltd. (Shenzhen, China). As for the culture media, SCC-25 and SCC-9 cell lines were incubated in the mixture of Dulbecco’s modified Eagle’s medium (DMEM) and Ham’s F12 medium (1:1). FaDu cell line was left to grow in Eagle’s minimum essential medium (EMEM), while CAL-27 and NOK cell lines were cultivated in DMEM. The above mentioned culture media were all added with 10% FBS and kept under the condition of 37°C and 5% CO2.
Cell transfection
Short hairpin RNAs (shRNAs) targeting TM4SF19-AS1 or HNRNPC, along with negative control shRNA (sh-NC) were offered by Genechem (Shanghai, China). In addition, pcDNA3.1 vectors targeting LAMC1 as well as the empty vector were purchased from Genechem. MiR-153-3p mimics/inhibitor and the negative control (Control mimics/NC inhibitor) were provided by RiboBio (Shanghai, China). Lipofectamine 3000 procured from Invitrogen company (Carlsbad, CA, USA) was applied to conduct cell transfection.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis
Above all, total RNA was collected via using TRIzol Reagent (Invitrogen). After that, cDNAs reversely transcribed from RNAs were obtained by using M-MLV reverse transcriptase (Promega, Madison, USA). SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was applied to examine RNA expression. The normalization of gene expression was dependent on GAPDH or U6 based on 2−ΔΔCt method. The independent assay was carried out in triplicate.
Cell Counting Kit-8 (CCK-8) assay
In the beginning, cells were seeded into 96-well plates (5 × 103 cells/well). Later, CCK8 solution was added. Eventually, cell viability was analyzed by a spectrophotometer (Thermo Fisher Scientific) following another 2-hour incubation. The independent assay was conducted in triplicate.
5-Ethynyl-2’-deoxyuridine (EdU)
To detect the changes of cell proliferation, EdU detection kit (RiboBio, Guangzhou, China) was employed following supplier’s instruction. Firstly, cells were incubated in 96-well plates, followed by the treatment of 50 µM EdU medium diluent for 2 h. Subsequently, cells were treated with 4% paraformaldehyde and 0.5% Triton X-100. Afterward, the cells were incubated with 100 µL of 1 × Apollo® 488 fluorescent staining reaction. At last, cells were stained with DAPI and the staining was observed with the use of a fluorescence microscope. The assay was independently implemented in triplicate.
Wound healing assay
HNSCC cells (3 × 103) were seeded in 6-well plates. Upon 80% cell confluence, the cells were moved to serum-free medium for incubation. Next, a straight wound was scratched on each well utilizing a pipette tip. After incubation for 24 more hours, scratches were observed and photographed. The experiment was independently performed in triplicate.
Transwell assay
To detect cell migration, transfected cells were suspended in serum-free culture media and plated into the top compartment of transwell plates. Then, the lower compartment containing 500 µL complete medium was prepared as well. After 24 h, cells that penetrated the interlayer to the other part accepted crystal violet staining. To observe cell invasion, the upper compartment was additionally coated with Matrigel (BD Biosciences San Diego, CA, USA), and the rest of procedures were the same as transwell migration assay. The assay was independently implemented three times.
Subcellular fractionation
By means of PARIS™ Kit (Ambion, Austin, TX), cytoplasmic and nuclear fragments of HNSCC cells were divided. GAPDH and U6 were deemed as cytoplasmic and nuclear reference, severally. The assay was independently implemented three times.
Fluorescent in situ hybridization (FISH)
After cell fixation in 4% formaldehyde, PBS with 0.5% Triton X-100 was utilized to permeabilize HNSCC cells. Then, cells were hybridized with fluorescence-conjugated TM4SF19-AS1 probe in buffer and subsequently counterstained with DAPI solution. The assay was independently implemented in triplicate.
Immunofluorescence (IF)
For IF analysis, HNRNPC primary antibody was first incubated with permeabilized HNSCC cells overnight at 4°C. Afterward, FITC-conjugated secondary antibody was co-cultivated as well. With the application of a confocal laser microscope (Olympus), images were captured. The independent assay was performed three times.
RNA pull down assay
Biotinylated (Bio) TM4SF19-AS1 probe was used to incubate with lysed cells. Next, magnetic beads were used to incubate with the obtained mixture. The complex precipitated with beads was purified for the subsequent RT-qPCR or western blot analysis. The experiment was independently implemented three times.
RNA binding protein immunoprecipitation (RIP)
RIP assay was performed by employing Z-Magna RIP™ RNA-binding Protein Immunoprecipitation kit (Millipore, Bedford, MA, USA). RIP lysis buffer was added to lyse HNSCC cells. Afterward, cell lysates were co-cultivated with anti-IgG (Abcam), anti-Ago2 (Abcam) or anti-HNRNPC (Abcam). Eventually, the enrichment of TM4SF19-AS1, miR-153-3p and LAMC1 was examined by RT-qPCR analysis. The independent assay was performed three times.
Luciferase reporter assay
The projected miR-153-3p binding regions on TM4SF19-AS1 or LAMC1 mRNA were cloned into psiCHECK-2 vector. Following the co-transfection of the former plasmids synthesized and miR-153-3p mimics/Control mimics into HNSCC cells, the Dual-luciferase reporter assay system (Promega) was finally adopted to test the luciferase activity. The independent experiment was performed three times.
Western blot analysis
In this assay, proteins were first extracted from HNSCC cells, and then transferred to PVDF membranes (Millipore). After that, specific primary antibodies were added to the membranes sealed up with nonfat milk. β-actin worked as internal reference. Next, the incubation of secondary antibody was carried out. In the end, Chemiluminescence system (GE Healthcare, Chicago, USA) was employed to quantify protein expression. The experiment was independently implemented in triplicate.
Xenograft assay
Balb/c nude mice were provided by Model animal Institute of Nanjing University. SCC-25 cells were transfected with sh-TM4SF19-AS1#1 or sh-NC. Subsequently, mice were divided into different groups by random selection, and were injected with the stably transfected cells. The tumor volume was monitored every 3 days starting from the fourth day. Twenty-eight days later, xenograft tumors were resected from the mice and weighed. Animal study was approved by First Hospital of Jilin University.
Statistical analysis
The data were processed with the application of SPSS 22.0 statistical software package. The gathered quantitative data were demonstrated in the form of mean ± standard deviation (SD). The data difference between two groups or among multiple groups was evaluated using Student’s t-test or analysis of variance (ANOVA). All independent experiments were operated three times. Statistics difference had significance only when P value was smaller than 0.05.
Results
LncRNA TM4SF19-AS1 exhibits high expression in HNSCC
To assess the level of TM4SF19-AS1 in HNSCC, we initially analyzed data from GEPIA 2 database (http://gepia2.cancer-pku.cn/#index) and the boxplot implied that TM4SF19-AS1 expression was remarkably higher in HNSCC tissues relative to normal tissues (). The data obtained from starBase (http://starbase.sysu.edu.cn/index.php) also revealed that in comparison with 44 normal tissues, TM4SF19-AS1 was remarkably up-regulated in 502 HNSCC tissues (). In addition, RT-qPCR examined TM4SF19-AS1 expression in HNSCC cell lines (SCC-25, SCC-9, CAL-27, and FaDu) and normal cell line (NOK). We noticed that TM4SF19-AS1 was significantly up-regulated in HNSCC cells (), especially in SCC-25 and SCC-9 cells, for which the two cell lines were involved in the following investigation. The above results suggested that TM4SF19-AS1 exhibited high expression in HNSCC.
Figure 1. LncRNA TM4SF19-AS1 exhibits high expression in HNSCC. (a) The level of TM4SF19-AS1 in 519 HNSCC tissues and 44 normal tissues was obtained from GEPIA 2 database. (b) StarBase demonstrated TM4SF19-AS1 expression in HNSCC tissues. (c) RT-qPCR analyzed TM4SF19-AS1 expression in HNSCC cells and NOK cells. * P < .05, ** P < .01.

Deficiency of TM4SF19-AS1 suppresses HNSCC cell phenotype and tumor growth
To better understand the function of TM4SF19-AS1 in HNSCC cells, loss-of-function assays were implemented. Above all, sh-TM4SF19-AS1#1/2/3 was utilized to cut down TM4SF19-AS1 expression, and sh-TM4SF19-AS1#1 and sh-TM4SF19-AS1#2 exhibited the best interference efficiency (). As demonstrated in CCK-8 assay, the viability of HNSCC cells was restricted when TM4SF19-AS1 was silenced (). Likewise, the experimental results of EdU assay also indicated that TM4SF19-AS1 down-regulation dramatically hindered cell proliferation (). Furthermore, wound healing assay clarified that after TM4SF19-AS1 was decreased, the migratory capacity of HNSCC cells was attenuated (). Subsequently, cell migratory and invasive processes were monitored in transwell assays, and the collected data reflected that depletion of TM4SF19-AS1 led to the suppression of the migration and invasion of HNSCC cells (). The following western blot examined the expression of markers linked to EMT. It turned out that TM4SF19-AS1 knockdown elevated E-cadherin expression while decreasing N-cadherin and Vimentin levels (). Furthermore, we also conducted in vivo assays. It turned out TM4SF19-AS1 depletion led to inhibited tumor growth (Figure S1A-C). To be summarized, TM4SF19-AS1 contributed to the proliferation, migration, invasion, along with EMT of HNSCC cells, and it also drove HNSCC tumor growth.
Figure 2. Deficiency of TM4SF19-AS1 suppresses the proliferation, migration, invasion and EMT of HNSCC cells. (a) The efficiency of TM4SF19-AS1 knockout was tested by RT-qPCR in HNSCC cells. (b-c) CCK-8 along with EdU assays jointly detected proliferative ability of HNSCC cells upon TM4SF19-AS1 depletion. (d) The migration of TM4SF19-AS1-depleted HNSCC cells was assessed by wound healing assay based on the wound closure rate. (e-f) Transwell assays evaluated the migratory and invasive processes of HNSCC cells upon TM4SF19-AS1 deficiency. (g) Western blots reflected the changing expression of EMT markers after TM4SF19-AS1 was silenced. ** P < .01.
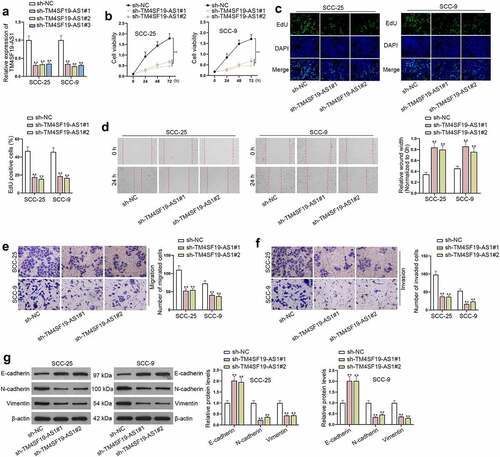
TM4SF19-AS1 binds to miR-153-3p
To unveil the possible regulatory mechanism underlying TM4SF19-AS1 in HNSCC cells, subcellular fractionation assay together with FISH assay was implemented. It turned out TM4SF19-AS1 had a large proportion in cytoplasm (). Additionally, RIP assay displayed an interaction between TM4SF19-AS1 and Ago2 protein (). Hence, we presumed that TM4SF19-AS1 might act as miRNA sponges in HNSCC cells. Through starBase, potential miRNAs sponged by TM4SF19-AS1 were predicted. Further, we discovered that miR-153-3p had the highest enrichment in the biotin-labeled TM4SF19-AS1 probe (). MiR-153-3p level was also uncovered to be lower in HNSCC cells compared with NOK cells (). The potential binding regions of TM4SF19-AS1 and miR-153-3p were obtained from starBase (). To stably overexpress miR-153-3p, HNSCC cells were subject to transfection with miR-153-3p mimics (). The outcomes of luciferase reporter assay manifested that miR-153-3p augment diminished the luciferase activity of TM4SF19-AS1-wild type (Wt) rather than TM4SF19-AS1-mutant type (Mut) (). Taken together, TM4SF19-AS1 interacted with miR-153-3p in HNSCC cells.
Figure 3. TM4SF19-AS1 sequesters miR-153-3p in HNSCC cells. (a) Nuclear and cytoplasmic TM4SF19-AS1 distribution in HNSCC cells was detected through subcellular fractionation assay. (b) The main distribution of TM4SF19-AS1 in HNSCC cells was manifested via FISH assay. (c) TM4SF19-AS1 enrichment in Ago2 protein was presented in RIP assay results. (d) The enrichment of predicted miRNAs in the biotinylated TM4SF19-AS1 probe was ascertained through RNA pull down assay. (e) MiR-153-3p level in HNSCC cells and NOK cells was quantified through RT-qPCR analysis. (f) The sequence alignment of miR-153-3p and its projected binding sites in TM4SF19-AS1 were displayed. (g) The overexpression efficacy of miR-153-3p mimics was tested with the use of RT-qPCR. (h) The luciferase activities of TM4SF19-AS1-Wt and TM4SF19-AS1-Mut were evaluated by luciferase reporter assay before or after miR-153-3p up-regulation. * P < .05, ** P < .01.
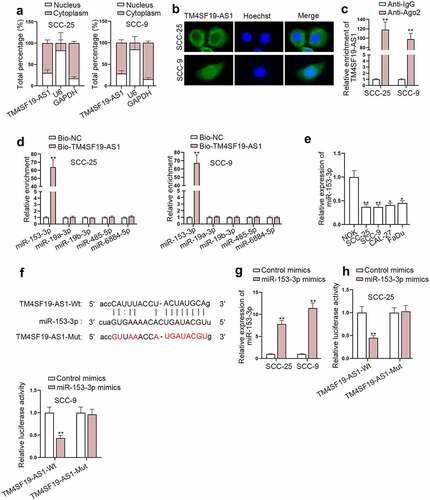
LAMC1 is targeted by miR-153-3p
Online bioinformatics tools, including miRmap, PITA, and PicTar, were utilized to select miR-153-3p downstream targets, and GEPIA 2 was employed to project mRNAs, which were up-regulated in HNSCC. As shown in , 9 possible mRNAs (ADAM19, RAI14, LAMC1, EXT1, DDIT4, LRP12, WIPF1, MFHAS1, and APP) shared by the two databases were obtained. Next, RT-qPCR was employed to determine mRNA levels of nine potential target genes in HNSCC cells upon miR-153-3p overexpression, and we observed LAMC1 expression was significantly lessened when miR-153-3p was up-regulated (). The projected binding sequence of LAMC1 (Wt/Mut) on miR-153-3p was presented (). RIP assay unveiled TM4SF19-AS1, miR-153-3p, and LAMC1 were substantially accumulated in Ago2-containing complex (). Data from luciferase reporter assay reflected the binding affinity of miR-153-3p and LAMC1 3’-UTR (). Moreover, based on the analyses of RT-qPCR and western blot, reduced LAMC1 expression caused by TM4SF19-AS1 depletion was partly restored by miR-153-3p inhibition (). To verify the participation of TM4SF19-AS1 in malignant processes of HNSCC cells through sequestering miR-153-3p and elevating LAMC1, rescue assays were implemented. Firstly, CCK-8 and EdU assays validated that miR-153-3p inhibition partly restored the suppressed proliferation of HNSCC cells caused by TM4SF19-AS1 knockdown (Figure S2A-B). Additionally, the experimental data of wound healing assay and transwell assay implied that TM4SF19-AS1 depletion restricted cell migration and invasion, which was partially recovered upon miR-153-3p inhibition (Figure S2C-E). Western blot analysis also suggested that the suppressed EMT process of HNSCC cells imposed by TM4SF19-AS1 depletion was partly restored after miR-153-3p inhibition (Figure S2F). Subsequently, LAMC1 expression was enhanced in SCC-25 cells (Figure S3A). Then, cell proliferation assays confirmed that the impeded proliferation of SCC-25 cells due to TM4SF19-AS1 deficiency was completely countervailed by LAMC1 up-regulation (Figure S3B-C). Apart from that, wound healing and transwell assays revealed LAMC1 overexpression completely countervailed the inhibiting impact of TM4SF19-AS1 inhibition on cell migration and invasion (Figure S3D-F). Similarly, knockdown of TM4SF19-AS1 hampered the EMT ability of SCC-25 cells, while this effect was completely rescued by LAMC1 up-regulation (Figure S3G). To conclude, TM4SF19-AS1 regulated LAMC1 expression by sponging miR-153-3p to affect HNSCC cell malignant behaviors.
Figure 4. LAMC1 is targeted by miR-153-3p. (a) Venn diagram displayed possible target genes of miR-153-3p. (b) The expression of potential downstream targets of miR-153-3p was measured in miR-153-3p-up-regulated HNSCC cells through RT-qPCR. (c) The projected binding region of miR-153-3p and LAMC1 was displayed. (d) RIP assay displayed the level of TM4SF19-AS1, miR-153-3p and LAMC1 precipitated with Ago2 protein. (e) The binding affinity of miR-153-3p and LAMC1 was examined through luciferase reporter assay. (f-g) RT-qPCR and western blot examined LAMC1 expression in HNSCC cells under different conditions. * P < .05, ** P < .01.
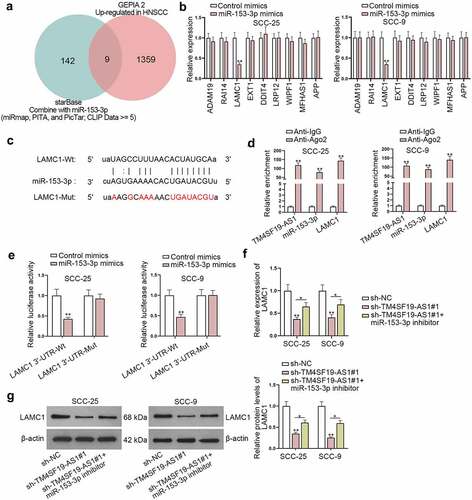
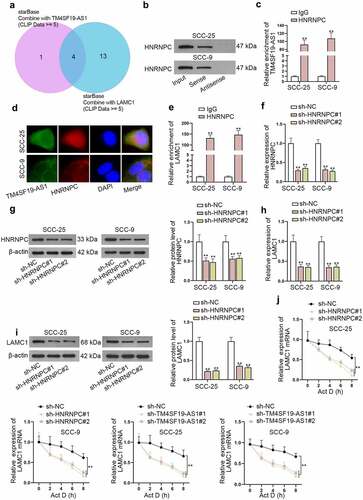
LAMC1 mRNA is stabilized by HNRNPC protein
As miR-153-3p inhibition could only partly counteract the impact of TM4SF19-AS1 depletion on LAMC1 expression, we made a conjecture that TM4SF19-AS1 might also recruit certain proteins to modulate LAMC1 expression. Under the condition of CLIP Data ≥ 5, 4 possible RNA binding proteins (RBPs) (U2AF2, HNRNPA1, SRSF1, and HNRNPC) shared by TM4SF19-AS1 and LAMC1 were predicted (). Among the four RBPs, HNRNPC has been identified to be capable of stabilizing mRNAs.Citation16,Citation17 Hence, HNRNPC was selected for subsequent study. The strong affinity of TM4SF19-AS1 with HNRNPC was verified after RNA pull down assay (). Moreover, RIP assay revealed that TM4SF19-AS1 was largely enriched in HNRNPC antibody (). FISH and IF analyses also reflected the cytoplasmic accumulation of TM4SF19-AS1 and HNRNPC in HNSCC cells (). The interaction of HNRNPC and LAMC1 was further testified after the performance of RIP assay (). To knock down HNRNPC, sh-HNRNPC#1/2 plasmids were transfected into HNSCC cells, and the significant interference efficacy was ensured based on the detection of RT-qPCR and western blot (). Meanwhile, we noticed that LAMC1 were both lessened at mRNA and protein levels in response to HNRNPC depletion (). With the treatment of actinomycin D (Act D), LAMC1 mRNA stability was weakened when HNRNPC or TM4SF19-AS1 was down-regulated (). Collectively, TM4SF19-AS1 interacted with HNRNPC protein to stabilize LAMC1 mRNA.
Figure 5. LAMC1 mRNA is stabilized by HNRNPC protein. (a) Potential RBPs which had a binding with both TM4SF19-AS1 and LAMC1 were predicted. (b-c) RNA pull down assay together with RIP assays jointly examined the binding affinity of TM4SF19-AS1 and HNRNPC protein. (d) FISH and IF assays explored the accumulation of TM4SF19-AS1 and HNRNPC in HNSCC cells. (e) RIP assay revealed LAMC1 enrichment in HNRNPC antibody. (f-g) HNRNPC expression was detected in HNSCC cells upon HNRNPC knockout. (h-i) LAMC1 expression was tested via RT-qPCR after HNRNPC was depleted. (j) The stability of LAMC1 mRNA was analyzed when HNRNPC or TM4SF19-AS1 was down-regulated. ** P < .01.
Discussion
Although the treatment of HNSCC has improved, the molecular mechanism involved in the development of HNSCC remains elusive and needs to be explored.Citation18 Recent studies have suggested that dysregulation of lncRNAs may exert great influence on the process of HNSCC.Citation11,Citation19 LncRNA TM4SF19-AS1 has been rarely investigated in human diseases except that Zhang et al. have mentioned that TM4SF19-AS1 is involved in the ceRNA regulatory network in HNSCC.Citation15 In our research, we confirmed that TM4SF19-AS1 expression was notably heightened in HNSCC tissues and cells. Loss-of-function experiments demonstrated the inhibition of TM4SF19-AS1 hindered the proliferative, migratory and invasive capacities, as well as EMT of HNSCC cells. Moreover, in vivo assays uncovered that TM4SF19-AS1 knockdown impeded HNSCC tumor growth.
It is commonly considered that lncRNA-mediated ceRNA mechanism occupies an important position in post-transcriptional events.Citation20 Through our investigation, we firstly clarified that chiefly cytoplasmic accumulation of TM4SF19-AS1 in cytoplasm HNSCC cells, representing that TM4SF19-AS1 might function as a miRNA sponge in HNSCC cells. After starBase prediction and mechanism analyses, miR-153-3p was testified to combine with TM4SF19-AS1. MiR-153-3p has been widely identified to play a tumor inhibiting part in melanoma,Citation21 breast cancer,Citation22 oral cancer,Citation23 and etc. Additionally, Jiang et al. have manifested that miR-153-3p exerts the suppressive effect on HNSCC.Citation24 Consistent with these published studies, we observed that miR-153-3p was deficient in HNSCC cells. Moreover, rescue experiments uncovered that miR-153-3p inhibition partially restored the restricted malignant behaviors of HNSCC cells, which was responsible for TM4SF19-AS1 silencing. LAMC family has been validated to participate in the progression of HNSCC.Citation25 As one of the family members of LAMC family, LAMC1 has been identified to make the promoting impact on multiple malignancies, endometrial cancer,Citation26 glioma,Citation27 and hepatocellular carcinomaCitation28 are included. Via informatics tools, LAMC1 was found to be highly expressed in HNSCC. From the perspective of mechanism, LAMC1 was certified to be the target gene of miR-153-3p and was adversely regulated by miR-153-3p. In addition, rescue experiments attested that LAMC1 augment completely abrogated the suppressive impact of TM4SF19-AS1 depletion in the malignant phenotype of HNSCC cells.
RBPs are also associated with the prognosis of HNSCC.Citation29 Based on the previous experimental results, we speculated that TM4SF19-AS1 might interact with certain RBPs. HNRNPC has been pointed out to demonstrate high expression in HNSCC tissues.Citation30 Moreover, Zhang et al. have illustrated that HNRNPC is able to maintain the stability of mRNAs.Citation16 Likewise, we also verified that TM4SF19-AS1 interacted with HNRNPC protein to stabilize LAMC1 mRNA. To sum up, our study discloses the oncogenic role of TM4SF19-AS1/miR-153-3p/HNRNPC/LAMC1 axis in HNSCC, which might offer a novel insight for HNSCC diagnosis. Nevertheless, some limitations remain in this research. Specifically, the regulatory mechanism about up-regulation of TM4SF19-AS1 in HNSCC was not discussed in depth. Other possible signaling pathways engaging in the development of HNSCC also need to be further explored. In brief, more follow-up studies are required to be carried out to thoroughly elucidate the relevant molecular cross-talk.
Authors’ contributions
Jian Wang, Yiming Zhu, Song Ni and Shaoyan Liu contributed to this work in the first draft. But In the revision process, they four decided to give up the further study because of their different research perspective. At the same time, Zhi Yu, Xin Wang, Kai Niu, Le Sun, Dongjie Li and Siyuan He took part in the revision and helped to finish the rest part. The new first author is responsible for all the experiments of revision and contributed to study design significantly in the revision. Our authors have made a lot of supports in quipment, experiment guidance during the revision. We thought that the authors list should be modified based on the actual contributions of authors. Therefore, all authors have approved the final authorships. We are sorry for the authorship changes and thanks for your query.
Supplemental Material
Download Zip (4 MB)Acknowledgments
We sincerely thank you for the help provided by all lab personnel in this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15384047.2022.2116923
Additional information
Funding
Notes on contributors
Zhi Yu
Zhi YU, MM, Department of otolaryngology, first hospital of Jilin university. Interest of study: pathology and pathophysiology of Head and neck tumor. Surgical and medical treatment of nose tumor.
Xin Wang
Xin WANG, MD, professor, Department of otolaryngology, first hospital of Jilin university. Interest of study: pathology and pathophysiology of Head and neck cancer. Surgical and medical treatment of head and neck cancer.
Kai Niu
Kai NIU, MD, associate professor Department of otolaryngology, first hospital of Jilin university. Interest of study: pathology and pathophysiology of Head and neck cancer. Surgical and medical treatment of head and neck cancer.
Le Sun
Le Sun, MD , Department of otolaryngology ,first hospital of Jilin university. Interest of study: pathology and pathophysiology of Head and neck tumor. Surgical and medical treatment of nose tumor.
Dongjie Li
Dongjie LI, MD, Department of otolaryngology, first hospital of Jilin university. Interest of study: pathology and pathophysiology of Head and neck cancer. Surgical and medical treatment of head and neck cancer.
References
- Jin Y, Yang Y. Identification and analysis of genes associated with head and neck squamous cell carcinoma by integrated bioinformatics methods. Mol Genet Genomic Med. 2019;7(8):e857. doi:10.1002/mgg3.857.
- Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol. 2018;52:228–240. doi:10.1016/j.semcancer.2018.01.008.
- Saada-Bouzid E, Peyrade F, Guigay J. Molecular genetics of head and neck squamous cell carcinoma. Curr Opin Oncol. 2019;31(3):131–137. doi:10.1097/CCO.0000000000000536.
- Alsahafi E, Begg K, Amelio I, Raulf N, Lucarelli P, Sauter T, Tavassoli M. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10(8):540. doi:10.1038/s41419-019-1769-9.
- Christy AW, Bojan A. Targeted therapy: a novel approach in head and neck cancer. Indian J Dent Res. 2013;24(2):261–266. doi:10.4103/0970-9290.116692.
- Hsieh JC, Wang HM, Wu MH, Chang KP, Chang PH, Liao CT, Liau CT. Review of emerging biomarkers in head and neck squamous cell carcinoma in the era of immunotherapy and targeted therapy. Head Neck. 2019;41 Suppl 1(S1):19–45. doi:10.1002/hed.25932.
- Bach DH, Lee SK. Long noncoding RNAs in cancer cells. Cancer Lett. 2018;419:152–166. doi:10.1016/j.canlet.2018.01.053.
- She K, Huang J, Zhou H, Huang T, Chen G, He J. lncRNA-SNHG7 promotes the proliferation, migration and invasion and inhibits apoptosis of lung cancer cells by enhancing the FAIM2 expression. Oncol Rep. 2016;36(5):2673–2680. doi:10.3892/or.2016.5105.
- Qin Y, Liu X, Pan L, Zhou R, Zhang X. Long noncoding RNA MIR155HG facilitates pancreatic cancer progression through negative regulation of miR-802. J Cell Biochem. 2019;120(10):17926–17934. doi:10.1002/jcb.29060.
- Zhang L, Wang Q, Wang F, Zhang X, Zhang L, Tang Y, Wang S. LncRNA LINC01446 promotes glioblastoma progression by modulating miR-489-3p/TPT1 axis. Biochem Biophys Res Commun. 2018;503:1484–1490. doi:10.1016/j.bbrc.2018.07.067.
- Ghafouri-Fard S, Mohammad-Rahimi H, Jazaeri M, Taheri M. Expression and function of long non-coding RNAs in head and neck squamous cell carcinoma. Exp Mol Pathol. 2020;112:104353. doi:10.1016/j.yexmp.2019.104353.
- Fang Z, Zhao J, Xie W, Sun Q, Wang H, Qiao B. LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression. Cancer Med. 2017;6(12):2897–2908. doi:10.1002/cam4.1253.
- Xie X, Xiong G, Wang Q, Ge Y, Cui X. Long non-coding RNA LINC00460 promotes head and neck squamous cell carcinoma cell progression by sponging miR-612 to up-regulate AKT2. Am J Transl Res. 2019;11(10):6326–6340.
- Yang YF, Feng L, Shi Q, Ma HZ, He SZ, Hou LZ, Wang R, Fang JG. Silencing novel long non-coding RNA FKBP9P1 represses malignant progression and inhibits PI3K/AKT signaling of head and neck squamous cell carcinoma in vitro. Chin Med J (Engl). 2020;133(17):2037–2043. doi:10.1097/CM9.0000000000000933.
- Zhang C, Cao W, Wang J, Liu J, Liu J, Wu H, Li S, Zhang C. A prognostic long non-coding RNA-associated competing endogenous RNA network in head and neck squamous cell carcinoma. PeerJ. 2020;8:e9701. doi:10.7717/peerj.9701.
- Zhang Y, Chen W, Pan T, Wang H, Zhang Y, Li C. LBX2-AS1 is activated by ZEB1 and promotes the development of esophageal squamous cell carcinoma by interacting with HNRNPC to enhance the stability of ZEB1 and ZEB2 mRNAs. Biochem Biophys Res Commun. 2019;511(3):566–572. doi:10.1016/j.bbrc.2019.02.079.
- Shetty S. Regulation of urokinase receptor mRNA stability by hnRNP C in lung epithelial cells. Mol Cell Biochem. 2005;272(1–2):107–118. doi:10.1007/s11010-005-7644-2.
- Deshpande AM, Wong DT. Molecular mechanisms of head and neck cancer. Expert Rev Anticancer Ther. 2008;8(5):799–809. doi:10.1586/14737140.8.5.799.
- Denaro N, Merlano MC, Russi EG, Lo Nigro C. Non coding RNAs in head and neck squamous cell carcinoma (HNSCC): a clinical perspective. Anticancer Res. 2014;34(12):6887–6896.
- Pan Y, Liu G, Wang D, Li Y. Analysis of lncRNA-Mediated ceRNA crosstalk and identification of prognostic signature in head and neck squamous cell Carcinoma. Front Pharmacol. 2019;10:150. doi:10.3389/fphar.2019.00150.
- Luan W, Shi Y, Zhou Z, Xia Y, Wang J. circRNA_0084043 promote malignant melanoma progression via miR-153-3p/Snail axis. Biochem Biophys Res Commun. 2018;502(1):22–29. doi:10.1016/j.bbrc.2018.05.114.
- Sun L, Wang H, Jiang J, Bi X. miR-153-3p inhibits proliferation and migration of breast cancer cells via down-regulating ROCK1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36(2):138–144.
- Ge C, Dong J, Chu Y, Cao S, Zhang J, Wei J. LncRNA FGD5-AS1 promotes tumor growth by regulating MCL1 via sponging miR-153-3p in oral cancer. Aging (Albany NY). 2020;12(14):14355–14364. doi:10.18632/aging.103476.
- Jiang Y, Wu K, Cao W, Xu Q, Wang X, Qin X, Wang X, Li Y, Zhang J, Chen W. Long noncoding RNA KTN1-AS1 promotes head and neck squamous cell carcinoma cell epithelial–mesenchymal transition by targeting miR-153-3p. Epigenomics. 2020;12(6):487–505. doi:10.2217/epi-2019-0173.
- Jiang P, He S, Li Y, Xu Z. Identification of therapeutic and prognostic biomarkers of lamin C (LAMC) family members in head and neck squamous cell Carcinoma. Med Sci Monit. 2020;26:e925735. doi:10.12659/MSM.925735.
- Kunitomi H, Kobayashi Y, Wu RC, Takeda T, Tominaga E, Banno K, Aoki D. LAMC1 is a prognostic factor and a potential therapeutic target in endometrial cancer. J Gynecol Oncol. 2020;31(2):e11. doi:10.3802/jgo.2020.31.e11.
- Liu J, Liu D, Yang Z, Yang Z. High LAMC1 expression in glioma is associated with poor prognosis. Onco Targets Ther. 2019;12:4253–4260. doi:10.2147/OTT.S205333.
- Yang ZP, Ma HS, Wang SS, Wang L, Liu T. LAMC1 mRNA promotes malignancy of hepatocellular carcinoma cells by competing for MicroRNA-124 binding with CD151. IUBMB Life. 2017;69(8):595–605. doi:10.1002/iub.1642.
- Jin Y, Qin X. Comprehensive analysis of the roles and prognostic value of RNA-binding proteins in head and neck squamous cell Carcinoma. DNA Cell Biol. 2020;39(10):1789–1798. doi:10.1089/dna.2020.5641.
- Zhao X, Cui L. Development and validation of a m(6)A RNA methylation regulators-based signature for predicting the prognosis of head and neck squamous cell carcinoma. Am J Cancer Res. 2019;9(10):2156–2169.
