Abstract
The phase of the cell cycle can determine whether a cancer cell can respond to a given drug. We previously reported monitoring of real-time cell cycle dynamics of cancer cells throughout a live tumor, intravitally in live mice, using a fluorescence ubiquitination-based cell-cycle indicator (FUCCI). Approximately 90% of cancer cells in the center and 80% of total cells of an established tumor are in G0/G1 phase. Longitudinal real-time imaging demonstrated that cytotoxic agents killed only proliferating cancer cells at the surface and, in contrast, had little effect on quiescent cancer cells, which are the vast majority of an established tumor. Moreover, resistant quiescent cancer cells restarted cycling after cessation of chemotherapy. These results suggested why most drugs currently in clinical use, which target cancer cells in S/G2/M, are mostly ineffective on solid tumors. In the present report, we used FUCCI imaging and Gelfoam® collagen-sponge-gel histoculture, to demonstrate in real time, that the cell-cycle phase distribution of cancer cells in Gelfoam® and in vivo tumors is highly similar, whereby only the surface cells proliferate and interior cells are quiescent in G0/G1. This is in contrast to 2D culture where most cancer cells cycle. Similarly, the cancer cells responded similarly to toxic chemotherapy in Gelfoam® culture as in vivo, and very differently than cancer cells in 2D culture which were much more chemosensitive. Gelfoam® culture of FUCCI-expressing cancer cells offers the opportunity to image the cell cycle of cancer cells continuously and to screen for novel effective therapies to target quiescent cells, which are the majority in a tumor and which would have a strong probability to be effective in vivo.
Introduction
The phase of the cell cycle can determine whether a cancer cell can respond to a given drug. Sakaue-Sawano et al. have demonstrated that the cell cycle phase in viable cells can be visualized using a fluorescent ubiquitination-based cell-cycle indicator (FUCCI) system.Citation1 We previously imaged real-time cell cycle dynamics of cancer cells throughout a live tumor intravitally using FUCCI. Approximately 90% of cancer cells in the center and 80% of total cells of an established tumor are in G0/G1 phase. FUCCI imaging demonstrated that cytotoxic agents killed only proliferating cancer cells at the surface and, in contrast, had little effect on quiescent cancer cells, which are the vast majority of an established tumor. Moreover, resistant quiescent cancer cells restarted cycling after the cessation of chemotherapy. However, in vivo systems are not amenable to continuous, long-term imaging, which can be critical for studying the cell cycle and its relationship to tumor behavior. In vivo-like culture systems can be of important use for long-term imaging of cancer-cell behavior.Citation2
Collagen-sponge-gel histoculture was developed by Leighton in the 1950s.Citation3 Sponge-gel histoculture enables cancer cells to form 3-dimensional structures. For example, Leighton observed that when C3HBA mouse mammary adenocarcinoma cells were grown on sponge-gel histoculture, the cells aggregated similar to the original in vivo tumor. Distinct structures were formed within the tumors such as lumina and stromal elements, with the glandular structures similar to the original tumor.Citation4
We have shown that in contrast to Gelfoam® histoculture, in Matrigel culture, cancer cells formed colonies but no other structures. The behavior of human 143B osteosarcoma cells on Gelfoam® in culture was remarkably different from those of these cells in monolayer culture or in Matrigel. Tissue-like structures were observed only in Gelfoam® culture. A flexible structural substrate such as Gelfoam® provides a more in vivo-like culture condition than monolayer culture or Matrigel.Citation5
We previously demonstrated, using FUCCI imaging, real-time visualization of the cell cycle kinetics of invading cancer cells in Gelfoam® histoculture, Cancer cells in G0/G1 phase in Gelfoam® histoculture migrated more rapidly and further than the cancer cells in S/ G2/M phase. After entry into S/G2/M phases, cancer cells ceased migrating and restarted migrating after division when the cells re-entered G0/G1. Migrating cancer cells were resistant to cytotoxic chemotherapy, since they were mostly in G0/G1, where cytotoxic chemotherapy is not effective.
In the present report, we compared spatial-temporal cell-cycle dynamics and chemosensitivity of cancer cells forming tumors on Gelfoam® with cancer cells growing in tumor spheres and on monolayers on plastic, as well as in vivo.
Results and Discussion
Gelfoam® histoculture of cancer cells
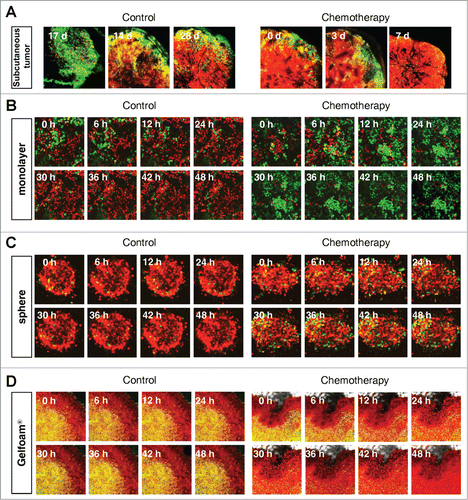
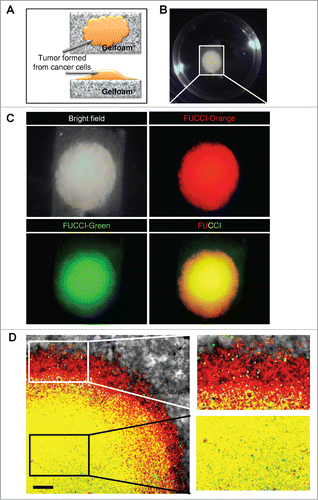
FUCCI-expressing MKN45 cells formed tumors after seeding in Gelfoam® histoculture. The cancer cells forming tumors on Gelfoam® brightly expressed either mK02-hCdt1 (green fluorescence) or mAG-hGem (orange-red fluorescence), which report the phases of the cell cycle, S/G2/M and G0/G1, respectively ().
Figure 1. Gelfoam® histoculture of FUCCI-expressing cancer cells. (A) Schema of FUCCI-expressing MKN45 stomach cancer cells forming a tumor on Gelfoam®. (B) Macroscopic appearance of the tumor formed on Gelfoam® histoculture. (C) Macro images of a tumor formed on Gelfoam® demonstrating FUCCI fluorescence. (D) FUCCI-expressing cancer cells in the tumor formed on Gelfoam®. Images at the single-cell level were acquired by confocal laser-scanning microscopy. High magnification images (×10) of an invading area of the tumor (upper right) and a non-invading area (lower right) of the tumor on Gelfoam®.
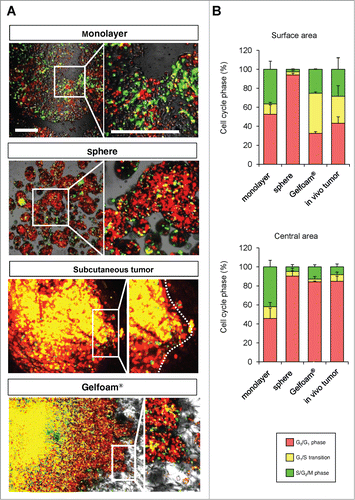
Comparison of cell-cycle-phase distribution of FUCCI-expressing MKN45 cells cultured in monolayer, sphere, Gelfoam®, and in vivo
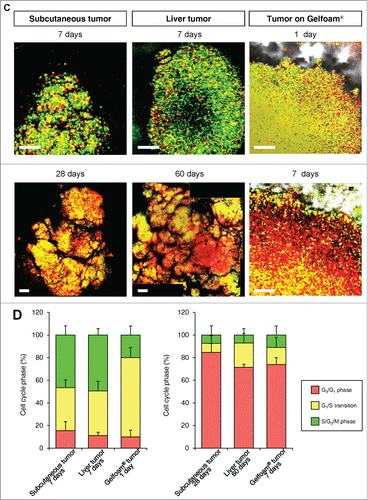
In monolayer culture, in both the central and edge areas, approximately 50% of the cells were in S/G2/M. In tumor spheres, most of the cells were in G0/G1 at both the surface and center. In both in vivo and in Gelfoam® histoculture, the majority of the surface cells of the tumor were in S/G2/M. In contrast, in the central area of the tumor, only approximately 10% of the cells were in S/G2/M (). A comparison was made of the cell-cycle phase distribution in a subcutaneous tumor, liver tumor and Gelfoam®, all formed from FUCCI-expressing MKN45 stomach cancer cells. At the early stages of each tumor, whether subcutaneous or in the liver, or on Gelfoam®, approximately 90% of the cells were in S/G2/M. In contrast as each tumor matured, approximately 80% of the cells were in G0/G1. The early-stages and mature-stage cell-cycle-phase distribution was very similar for each tumor, subcutaneous, liver and on Gelfoam® ().
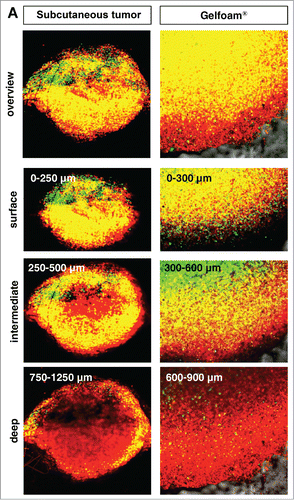
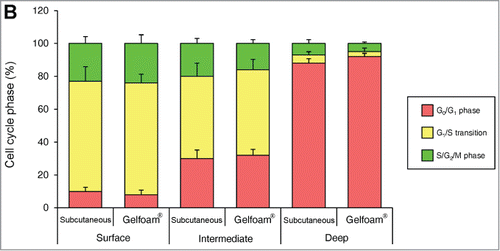
Cancer cells in Gelfoam® histoculture and in vivo tumors have similar 3-dimensional-spatial cell-cycle phase distribution
In both tumors in vivo and in Gelfoam® culture, cancer cells were proliferating only near the surface of the tumor. The majority of cancer cells were in S/G2/M both subcutaneous tumors and in Gelfoam®, as deep as 500–600 μm from the surface. At deeper levels, the vast majority of the cells were in G0/G1 in both tumors and on Gelfoam®. At greater depths, approximately 20% of the cells in the in vivo liver tumor were in S/G2/M and in Gelfoam® histoculture, approximately 10% of the cells were in S/G2/M, with the other cells in G0/G1 in both the subcutaneous tumor and on Gelform® ().
Cancer cells on Gelfoam®, but not 2D culture, have the same cell cycle response to cytotoxic agents as in vivo tumors
Mostly chemotherapy agents targets only proliferating cancer cells and have little effect on quiescent cancer cells. In monolayer culture, chemotherapy blocked cancer cells in G2/M phase. In sphere culture, chemotherapy had little effect since most cancer cells were in G0/G1, where they remained after chemotherapy. In Gelfoam® histoculture and the subcutaneous tumor, chemotherapy targeted only proliferating cancer cells and had little effect on quiescent cancer cells, which were the majority of the cells. In both the subcutaneous tumors and the tumors in Gelfoam® histoculture, chemotherapy killed the surface proliferating cells, but the remaining cells were blocked in G0/G1 and resistant to chemotherapy ( and Video S1).
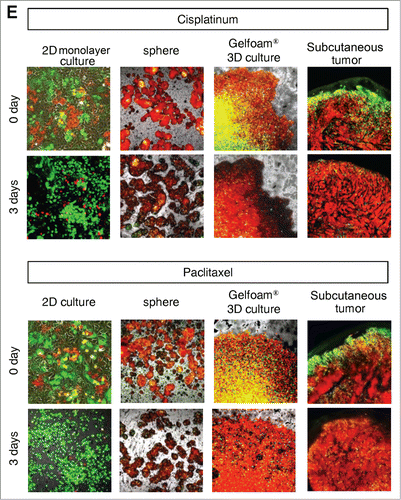
Figure 4. FUCCI-expressing cancer cells on Gelfoam® have the same cell cycle response to cytotoxic agents as subcutaneous tumors. Time-course imaging of FUCCI-expressing cancer cells (A) subcutaneous, (B) in monolayer culture, (C) in tumor spheres on agar and (D) on Gelfoam®, before and after chemotherapy. (E) Representative images of FUCCI-expressing cancer cells in monolayer culture, spheres on agar, on Gelfoam®, and subcutaneous tumors, before and after chemotherapy with cisplatinum or paxlitaxel. In monolayer culture, chemotherapy blocked cancer cells in S/G2/M phase. Chemotherapy had little effect on quiescent tumor spheres. In contrast, tumors on Gelfoam® histoculture and subcutaneous tumors had a similar initial response to chemotherapy with cells becoming blocked in G0/G1. (F) Histograms of cell-cycle phase distribution before and after chemotherapy of 2D monolayer, sphere and Gelfoam® cultures and subcutaneous tumors. (Also see Video S1).
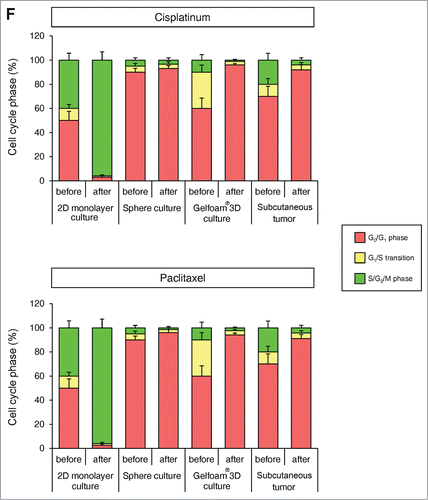
Cancer cells on Gelfoam® have similar spatial-temporal recovery from cisplatinum and paclitaxel treatment as in vivo tumors, in contrast to 2D monolayer and sphere culture
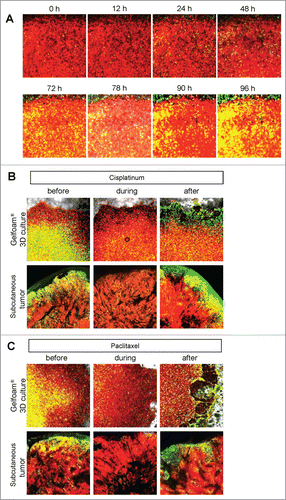
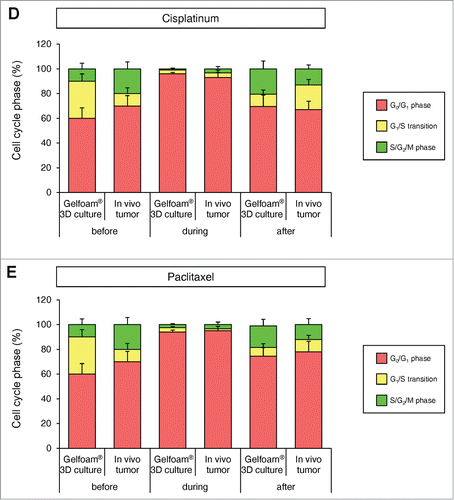
In 2D monolayer culture, approximately 50% of the cells were in S/G2/M before chemotherapy and after chemotherapy, approximately 90% of the cells were in S/G2/M. In sphere culture, approximately 10% of the cells were in S/G1/M before chemotherapy, and after chemotherapy, almost 100% of the cells were in G0/G1. In Gelfoam® histoculture, approximately 40% of the cells were in S/G0/M before chemotherapy and during chemotherapy, almost 100% of the cancer cells were in G0/G1 and after termination of chemotherapy approximately 20% of the cancer cells re-entered S/G2/M, mostly at the surface, for both cisplatinum and palitaxel. In the subcutaneous tumor, before chemotherapy approximately 30% of the cells were in S/G2/M. During chemotherapy, almost 100% of the cancer cells were in G0/G1. After termination of chemotherapy, approximately 20% of the cancer cells re-entered S/G2/M for both cisplatinum and paclitaxel, mostly at the surface and very similar to tumors on Gelfoam® (). After the cessation of chemotherapy, the cancer cells at the surface of the tumor resumed cycling in both the liver and on Gelfoam®.
Figure 5. FUCCI-expressing cancer cells on Gelfoam® have a similar recovery pattern from cisplatinum and paclitaxel as subcutaneous tumors. (A) Timecourse imaging (96 hours) of FUCCI-expressing MKN45 cells histocultured on Gelfoam® after cisplatinum chemotherapy. (B) Representative images of FUCCI-expressing cancer cells on Gelfoam® and subcutaneous tumors before and after recovery from chemotherapy with (B, D) cisplatinum or (C, E) paclitaxel. G0/G1 quiescent cancer cells on Gelfoam® and in subcutaneous tumors are resistant to chemotherapy and can restart proliferation after treatment is terminated. Scale bars; 500 μm.
FUCCI imaging demonstrated that cancer cells in monolayer culture (2D culture) and spheres on agar behave very differently than cancer cells in vivo or on Gelfoam®. Gelfoam® tumor histoculture provides an in vivo-like microenvironment for cancer cells enabling the cancer cells to mimic the spatial-temporal cell-cycle phase distribution of tumors in vivo.Citation5
FUCCI imaging demonstrated that cancer cells on monolayer culture and spheres on agar have a different response to chemotherapy from cancer cells in a tumor in vivo. Gelfoam®-histocultured tumors, however, had a similar response to chemotherapy as in vivo tumors, suggesting that the combination of FUCCI imaging and Gelfoam® provides a new platform for developing and evaluating anticancer agents, as well as studying basic cancer biology, such as the cell cycle.Citation8
An important feature of Gelfoam® histoculture is that it is a very convenient system for imaging long-term experiments. The possibility of long-term imaging, provided by Gelfoam® histoculture of FUCCI-expressing cancer cells, described in the present report, is especially important when studying the relationship of cell cycle phase to cancer-cell migration and invasion.Citation6,7
Previously-developed concepts and strategies of highly-selective tumor-targetingCitation9-16 can take advantage of the long-term Gelfoam® histoculture of FUCCI-expressing cancer cells described in the present report.
Materials and Methods
Cells
MKN45 is a poorly-differentiated stomach adenocarcinoma-derived from a liver metastasis of a patient. The cells were grown in RPMI 1640 medium with 10% fetal bovine serum and penicillin/streptomycin.Citation2,17
Establishment of MKN45 cells stably transfected with FUCCI-vector plasmids
For cell cycle-phase visualization, the FUCCI (fluorescent ubiquitination-based cell cycle indicator) expression system was used.Citation1 Plasmids expressing mKO2-hCdt1 (green fluorescent protein) or mAG-hGem (orange-red fluorescent protein) were obtained from the Medical & Biological Laboratory (Nagoya, Japan). Plasmids expressing mKO2-hCdt1 were transfected into MKN45 cells using Lipofectamine™ LTX (Invitrogen, Carlsbad, CA). The cells were incubated for 48 h after transfection and were then trypsinized and seeded in 96-well plates at a density of 10 cells/well. In the first step, cells were sorted into green (S, G2, and M phase) cells using a FACSAria cell sorter (Becton Dickinson, Franklin Lakes, NJ). The first-step-sorted green-fluorescent cells were then re-transfected with mAG-hGem and then sorted by orange fluorescence.Citation1,2,6
Gelfoam® histoculture
Sterile Gelfoam® sponges (Pharmacia & Upjohn, Kalamazoo, MI), prepared from porcine skin, were cut into 1 cm cubes. The Gelfoam® cubes were placed in 6-well tissue-culture plates. RPMI 1640 medium was added and Gelfoam® was incubated at 37°C in order that the Gelfoam® absorbed the medium. Cancer cells (1×106 ) expressing FUCCI were then seeded on top of the hydrated Gelfoam® and incubated for 1 h. Medium was carefully added up to the top of the Gelfoam®. Cells were incubated at 3°C in a humidified incubator with 5% CO2.Citation5,6,18-21
Animal experiments
Athymic nu/nu nude mice (AntiCancer, Inc., San Diego, CA) were maintained in a barrier facility under HEPA filtration and fed with autoclaved laboratory rodent diet (Teklad LM-485; Harlan Labs, Hayward, CA). All animal studies were conducted in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873–1.
Tumor models
All animal procedures were performed under anesthesia using s.c. administration of a ketamine mixture (10 μl ketamine HCl, 7.6 μl xylazine, 2.4 μl acepromazine maleate, and 10 μl PBS) (Henry-Schein, Melville, NY). FUCCI-expressing MKN45 cells were harvested by brief trypsinization. Single-cell suspensions were prepared at a final concentration of 2 × 105 cells/5 μl Matrigel (Becton Dickinson). After laparotomy of 5-week-old female nude mice, the mouse liver was exteriorized and the cancer cells subserosally injected directly into the left lobe of the liver using a 31-gauge needle. After cancer cell implantation, the abdominal wall of mice was closed with 6–0 sutures.Citation2 In order to obtain subcutaneous tumors, FUCCI-expressing MKN45 cells (5 × 106/mouse) were injected in the flank of 5-week-old female nude mice (under the ketamine-mixrure anesthesia previously described).
Confocal laser microscopy
The liver, with a FUCCI-expressing tumor, was exteriorized and a cover glass was gently put on the liver, which inhibited vibration caused by heartbeat and respiratory movement. Subcutaneous tumors, and spheres, 2D monolayer and Gelfoam® cultures with FUCCI-expressing cancer cells were directly imaged by confocal microscopy. Confocal laser scanning microscopy (CLSM) was performed using the FV-1000 (Olympus Corp., Tokyo, Japan) with 2-laser diodes (473 nm and 559 nm). A 4 × (0.20 numerical aperture immersion) objective lens and 20 × (0.95 numerical aperture immersion) objective lens (Olympus) were used. 800 × 800 pixels and 1.0-μm z steps were scanned, which took 1–2 s per section, with 6–8 min per full 3D scan. Scanning and image acquisition were controlled by Fluoview software (Olympus).Citation2
3D image analysis
The tracing data were imported to Volocity 6.0 version (Perkin Elmer, Waltham, MA), where all further analysis was performed.Citation2
Statistical analysis
Data are shown as means ± SD.
Author Contribution
SY and RMH conceived the idea for this project. SY and RMH designed all experiments and wrote the manuscript. SY, SM, SM, YH and FU performed all experiments. HK, HT, MZ, MB, and TF provided crucial ideas and helped with data interpretation. HT provided special technical assistance.
Disclosure of Potential Conflicts of Interest
MZ is an employee of AntiCancer Inc. SY, SM, SM, YH, FU, HK and RMH are or were unsalaried associates of Anti Cancer Inc. There are no other competing financial interests.
Dedication
This paper is dedicated to the memory of A.R. Moossa, MD.
1000685_Supplementary_Materials.zip
Download Zip (3.3 MB)Funding
This study was supported in part by National Cancer Institute grant CA132971.
References
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. Visualizing spatiotemporal dynamics of multicellular cell cycle progression. Cell 2008; 132:487-98; PMID:18267078; http://dx.doi.org/10.1016/j.cell.2007.12.033
- Yano S, Zhang Y, Miwa S, Tome Y, Hiroshima Y, Uehara F, Yamamoto M, Suetsugu A, Kishimoto H, Tazawa H, et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle 2014; 13:2110-9; PMID:24811200; http://dx.doi.org/10.4161/cc.29156
- Leighton J. A sponge matrix method for tissue culture; formation of organized aggregates of cells in vitro. J Natl Cancer Inst 1951; 12:545-61; PMID:14889259
- Leighton J, Justh G, Esper M, Kronenthal RL. Collagen coated cellulose sponge: Three-dimensional matrix for tissue culture of Walker tumor 256. Science 1967; 155:1259-61; PMID:6018647; http://dx.doi.org/10.1126/science.155.3767.1259-a
- Tome Y, Uehara F, Mii S, Yano S, Zhang L, Sugimoto N, Maehara H, Bouvet M, Tsuchiya H, Kanaya F, et al. 3-dimensional tissue is formed from cancer cells in vitro on Gelfoam®, but not on MatrigelTM. J Cell Biochem 2014; 115:1362-7; PMID:24497277; http://dx.doi.org/10.1002/jcb.24780
- Yano S, Miwa S, Mii S, Hiroshima Y, Uehara F, Yamamoto M, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, et al. Invading cancer cells are predominantly in G0/G1 resulting in chemoresistance demonstrated by real-time FUCCI imaging. Cell Cycle 2014; 13:953-60; PMID:24552821; http://dx.doi.org/10.4161/cc.27818
- Kagawa Y, Matsumoto S, Kamioka Y, Mimori K, Naito Y, Ishii T, Okuzaki D, Nishida N, Maeda S, Naito A, et al. Cell cycle-dependent Rho GTPase activity dynamically regulates cancer cell motility and invasion in vivo. PLoS One 2013; 8:e83629; PMID:24386239; http://dx.doi.org/10.1371/journal.pone.0083629
- Yano S, Li S, Han Q, Tan Y, Bouvet M, Fujiwara T, Hoffman RM. Selective methioninase-induced trap of cancer cells in S/G2 phase visualized by FUCCI imaging confers chemosensivity. Oncotarget 2014; 5:8729-36.
- Blagosklonny MV. How cancer could be cured by 2015. Cell Cycle 2005; 4:269-78; PMID:15655345
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003; 89:1147-51; PMID:14520435; http://dx.doi.org/10.1038/sj.bjc.6601256
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-7; PMID:14678733; http://dx.doi.org/10.1016/S1359-6446(03)02806-X
- Blagosklonny MV. “Targeting the absence” and therapeutic engineering for cancer therapy. Cell Cycle 2008; 7:1307-12; PMID:18487952; http://dx.doi.org/10.4161/cc.7.10.6250
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005; 4:1518-21; PMID:16258270; http://dx.doi.org/10.4161/cc.4.11.2208
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID:11417480; http://dx.doi.org/10.1038/sj.leu.2402127
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia 2006; 20:385-91; PMID:16357832; http://dx.doi.org/10.1038/sj.leu.2404075
- Blagosklonny MV. Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biol Ther 2007; 6:1684-90; PMID:18344680; http://dx.doi.org/10.4161/cbt.6.11.5167
- Yano S, Tazawa H, Hashimoto Y, Shirakawa Y, Kuroda S, Nishizaki M, Kishimoto H, Uno F, Nagasaka T, Urata Y, et al. A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M phases. Clin Cancer Res 2013; 19:6495-505; PMID:24081978; http://dx.doi.org/10.1158/1078-0432.CCR-13-0742
- Freeman A, Hoffman RM. 1986. In vivo-like growth of human tumors in vitro. Proc Natl Acad Sci USA 1986; 83:2694-8; PMID:3458228; http://dx.doi.org/10.1073/pnas.83.8.2694
- Hoffman RM. Histocultures and their use. In: Encyclopedia of life sciences. Chichester: John Wiley and Sons Ltd. Published Online. 2010; DOI: 10.1002/9780470015902. a0002573. pub2
- Hoffman RM. Tissue culture. In: Brenner's Encyclopedia of Genetics. 2nd edition. Elsevier, 2013; 73-76.
- Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y, Saito N, Katsuoka K, Hoffman RM. The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3-D culture. J Cell Biochem 2013; 114:1674-84; PMID:23444061; http://dx.doi.org/10.1002/jcb.24509