Abstract
It had been known for decades that primordial follicles in mammalian ovaries are assembled with definite numbers and represent the ovarian reserve throughout the reproductive life. Intra-oocyte PI3K/mTOR pathways have been indicated to play a central role on the activation of primordial follicles. Genetic modified mouse models with chronic activation of PI3K/mTOR signals in primordial oocytes showed premature activation of all primordial follicles and eventually their exhaustion. On the other hand, this may suggest that, unlike chronic activation of PI3K/mTOR, its acute activation in infertility would activate primordial follicles, permitting fertility during the treatment. Previously, PI3K stimulators were reported as a temporary measure to accelerate primordial follicle activation and follicular development in both mouse and human, and were applied in the treatment of infertility in premature ovarian failure (POF) patients. To address whether mTOR stimulators could play similar role in the process, we transiently treated neonatal and aged mouse ovaries with mTOR stimulators-phosphatidic acid (PA) and propranolol. Our results demonstrated the stimulators increased activation of primordial follicles and the production of progeny. Human ovarian cortex cubes were also treated with mTOR or/and PI3K stimulators in vitro. When they were used separately, both of them showed similar promotive effects on primordial follicles. Surprisingly, after joint-treatment with the 2 kinds of stimulators together, synergistic effects on follicular development were observed. Based on increased efficiency of follicular activation in humans, here we propose in vitro transient treatment with mTOR and PI3K stimulators as an optimized protocol for the application in different clinical conditions with limited follicle reserve.
Abbreviations
| mTOR | = | mammalian target of rapamycin |
| PTEN | = | Phosphatase and Tensin Homolog deleted on chromosome 10 |
| TSC1 | = | tuberous sclerosis complex 1 or hamartin |
| TSC2 | = | tuberous sclerosis complex 2 or tuberin |
| POF | = | premature ovarian failure |
| PA | = | phosphatidic acid |
| PRO | = | propranolol. |
Introduction
In mammals, the functional oocytes that give rise to offspring are sequestered in primordial follicles before birth and remain quiescent in the ovary, sometimes for decades.Citation1 The recruitment of primordial follicles requires highly controlled mechanisms to ensure that only a limited number of primordial follicles could be activated at any given time, thus providing a steady supply of fertilizable oocytes during the reproductive lifespan.Citation2 In humans, natural menopause occurs around 50 y of age, when less than 1000 primordial follicles remain in the ovary.Citation3 Some women suffer premature menopause, also called premature ovarian failure (POF), due to cessation of ovarian function before the age of 40 years. It is a heterogeneous disorder affecting approximately 1% of women <40 years old and characterized by the disappearance of menstrual cycles associated with premature follicular depletion.Citation4 Many reasons can cause POF, such as genetic defects, autoimmunity and toxics.Citation5 Recently, with increasing survival rates following chemotherapy and radiotherapy for malignant diseases, their negative effects on ovarian reserve in young women have highlighted the need to preserve or restore patients’ fertility before or after oncologic therapy.Citation6 Because nearly no follicular development and ovulation were observed in POF patients, only IVF and embryo transfer using donor oocytes have demonstrated to be viable fertility treatments for them.Citation5 However, there is possibility that certain numbers of primordial follicles still remain in POF ovaries. The reason why these follicles cannot be used with current in vitro fertilization (IVF) techniques is that they do not express the receptor for follicle stimulating hormone (FSH) whereas FSH stimulation of the follicles is the first step of the procedure in obtaining fertilizable eggs.Citation2,7 Therefore, activating these primordial follicles and causing them to enter the growth stage is a new approach to helping POF patients reproduce their own genetic babies.
Currently, a series of studies using genetically modified mice revealed that the intra-oocyte PI3K signaling pathway plays a central role in regulating the activation of primordial follicles.Citation8,9 Targeted depletion of the key molecules of the pathway, such as Foxo3 (forkheadboxO3, a downstream inhibitory transcriptional factor), Pten (Phosphatase and Tensin Homolog deleted on chromosome 10, an upstream negative regulator), or Pdk1 (3-phosphoinositide dependent protein kinase-1) caused infertility through accelerated depletion of primordial follicles.Citation10-12 Double deletion of the oocyte Pten and global Foxo3 or double deletion of Pten and Pdk1 in oocytes revealed the linear upstream and downstream fashion in which the molecules regulated activation of primordial follicles.Citation13
Mammalian target of rapamycin (mTOR) is one of downstream signals that can be regulated by the activation of Akt. mTOR has also been shown to be involved in follicular activation. The mammalian target of rapamycin (mTOR) assembles a signaling network that regulates a myriad of cellular and developmental processes in response to nutrients.Citation14 As a protein Ser/Thr kinase, mTOR exists in 2 biochemically and functionally distinct complexes, mTORC1 and mTORC2, according to their sensitivity to rapamycin.Citation15 Heterodimeric complex of tuberous sclerosis complex 1 (TSC1 or hamartin) and TSC2 (or tuberin) is one of the most important sensors involved in the negative regulation of mTORC1 activity.Citation16 Mice lacking Tsc1 or Tsc2 genes in oocytes all showed an identical phenotype with premature activation of all primordial follicles.Citation12,17 Although Tsc/mTORC1 signaling has been proposed to be downstream of PTEN/PI3K signaling in some cell types, the functions of both PTEN and Tsc in maintaining the quiescence of primordial follicles do not seem to have an upstream and downstream arrangement in mouse oocytes.Citation18 Double deletion of Tsc1 and Pten in oocytes has been found to cause synergistic enhancement of oocyte growth relative to mice lacking either one but not both.Citation17 These findings suggest that the intracellular PTEN/PI3K pathway and mTOR pathway regulate follicular activation in a parallel and collaborative way.Citation13
Transient treatment of PI3K stimulators has been found to activate primordial follicles in neonatal mouse ovaries and in human ovarian cortical tissue.Citation19 However, though genetically modified mice can show premature activation of all primordial follicles, only some of the primordial follicles responded to PI3K stimulators and developed to the preovulatory stage. When this in vitro activation approach was used in human POF patients, one healthy baby was delivered out of 13 attempts.Citation20 The success rate is still very low. As it is a possible unique infertility therapy for POF patients who still contain residual follicles in their ovaries, it is important to optimize the approach by increasing the efficiency of follicular activation. Because the mTOR signaling pathway is also involved in the recruitment of primordial follicles and functions collaboratively with PTEN/PI3K signaling, it may be useful to determine whether or not mTOR stimulators have any effect on this in vitro activation approach. In the present study, the mTOR activators phosphatidic acid (PA) and propranolol were used to show the activation of primordial follicles in both neonatal and adult mice.Citation21-23 mTOR stimulators can also activate primordial follicles from human ovarian cortical tissues cultured in vitro. Synergistic enhancement of human follicular development was further observed with mTOR and PI3K joint treatment. It is proposed that increasing the efficiency of follicular activation in humans may be a good approach to treating POF patients.
Results
mTOR stimulators increased follicular development of newborn mice
Primordial follicles in newborn mice can be activated by transient incubation with PI3K stimulators.Citation19 Studies using mutant mice with oocyte-specific deletion of Tsc genes indicated that the mTOR signaling pathway regulates follicular activation.Citation12,17 For this reason, it was hypothesized transient incubation of ovaries with mTOR stimulators could also activate primordial follicles. Recent reports showed that mTOR signaling can be regulated by phosphatidic acid (PA).Citation24,25 Exogenous PA or propranolol (PRO), a PA phosphatase inhibitor that can prevent endogenous PA from being converted to diacylglycerol (DG), are widely used as mTOR activators in different cell types.Citation26,27
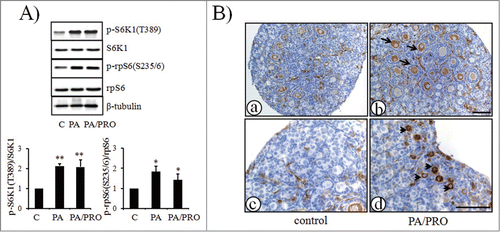
As shown in , when neonatal ovaries at day 3 of age were treated either with mTOR stimulators PA or PA plus PRO (PA/PRO) for 24 h, immunoblotting analyses indicated increased phosphorylation of S6K1 and rpS6, the downstream molecules of mTOR. In control ovaries, immunostaining of p-rpS6 was expressed mainly in the growing oocytes of secondary follicles (a). There was barely detectable staining in primordial or primary follicles (c). However, in treated ovaries, p-rpS6 was found to be expressed in some oocytes of primordial follicles (d, arrow heads). Results also showed more p-rpS6 positive secondary follicles compared with control ovaries (b, arrows) which demonstrated their better development potential after treatment with mTOR stimulators.
Figure 1. Activation of mTOR-S6K1-rpS6 signaling pathway in neonatal ovaries after treatment with mTOR stimulators, PA and PRO. Ovaries were collected after 24 h of treatment. (A) Western blot of ovarian proteins with specific antibody for p-S6K1(T389), S6K1, p-rpS6(S235/6), rpS6 and β-tubulin. S6K1, rpS6, and β-tubulin were used as internal controls. (A,lower), Densitometry of western blot was quantified and shown by p-S6K1(T389)to S6K1 ratios and p-rpS6(S235/6)to rpS6 ratios. The ratios of p-S6K1(T389)to S6K1 and p-rpS6(S235/6)to rpS6 in control were designated as 1. The data in the graphs were presented as mean±SD of three independent experiments. *, P < 0.05; **, P < 0.01, compared with controls. (B) Immunostaining of p-rpS6 in ovaries treated w/o PA/PRO. (a) and (c), control non-treated ovaries. (b) and (d), PA/PRO treated ovaries. (c), (d) is the higher magnification of (a) and (b), which showed primordial follicle pools in the ovary. Black arrow, representative p-rpS6 signals in oocytes of growing secondary follicles; black arrowhead, representative p-rpS6 signals in oocytes of primordial follicles. Bar = 100 μm.
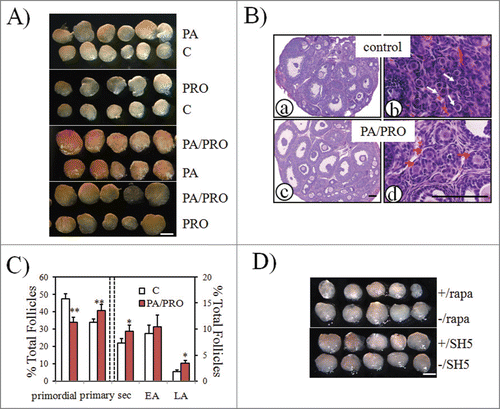
After 24 h of in vitro incubation, paired ovaries (treated and untreated) from the same donor were transplanted under each side of kidney capsules in the ovariectomized adult recipient. Fourteen days later, greater ovarian size was evident in groups treated with PA or PRO alone than in paired controls (). Furthermore, the most pronounced increase of ovarian size was found when ovaries were treated with PA plus PRO (). In order to see the best stimulatory effects of mTOR stimulators, co-treatment with PA and PRO was used in all the following studies. As shown by histological analysis, there were more large antral follicles in PA plus PRO treated ovaries (c) than in controls (a). Not like clusters of primordial follicles seen in control ovaries (b, red arrows), groups of primary follicles were more common in treated ovaries (d, white arrows). Follicle counting of serial ovarian sections also indicated a significant decrease in primordial follicles and increases of follicle growth at different stages between control and treated ovaries (). When samples were pretreated with mTOR inhibitor, rapamycin, or an Akt inhibitor such as SH5, only rapamycin was found to completely block the stimulatory effect of PA plus PRO (). This suggests that the stimulatory effects of PA and PRO on follicular development actually took place through activation of mTOR signaling in neonatal ovaries.
Figure 2. Increased ovarian development after PA and PRO stimulation. Paired neonatal ovaries were treated with or without mTOR stimulators, PA or PRO for 24h, followed by transplantation into kidney capsule of ovariectomized recipient mice. Transplanted ovarian grafts were collected 14 days later. (A) Isolated paired ovaries for PA (200 μM) vs. control; PRO (50 μM) vs. control; PA/PRO (200 μM/50 μM) vs. PA (200 μM); and PA/PRO (200 μM/50 μM) vs. PRO (50 μM). Bar = 1 mm. (B) ovarian histology by hematoxylin and eosin staining. (a) and (b), control ovaries; (c) and (d), PA/PRO treated ovaries. (b) and (d) are higher magnifications of (a) and (c), which showed clusters of primordial follicles (b, white arrows) in control ovaries and clusters of primary follicles (d, red arrows) in PA/PRO treated ovaries. Bar = 100 μm. (C) Distribution of follicles in ovaries treated with or without PA/PRO (n = 5). Data were shown with mean±SD. Sec, secondary follicle; EA, early antral follicle; LA, large antral follicle.*, P < 0.05; **, P < 0.01 vs. control. (D) Effects of PA plus PRO on ovarian development were blocked by mTOR inhibitor rapamycin (rapa) but not Akt inhibitor SH5. The symbol -/ indicated groups without the inhibitors. Bar = 1 mm.
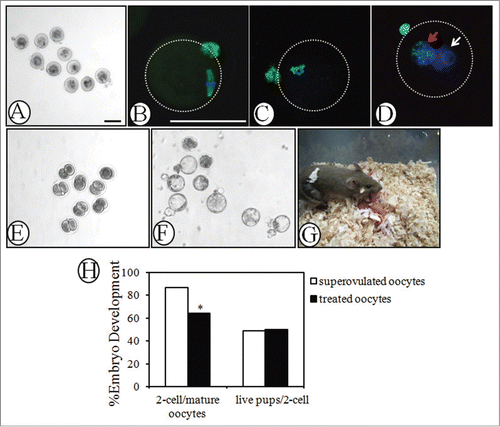
To determine whether mature oocytes can be obtained from activated follicles, neonatal ovaries treated with PA/PRO were transplanted under the kidney capsule of hosts for 18 days and a single i.p. injection of hCG was given at 12 h before oocyte collection. As shown in , mature MII oocytes were collected from PA/PRO-treated ovaries. Immunofluorescent staining of β-tubulin showed characteristic features of the metaphase II-stage meiotic spindles (). Methylation at the 5-position of cytosines is an important component of the epigenetic code.Citation28 Genome-wide erasure of DNA 5-methylcytosine (5mC) has been reported to occur along the paternal pronucleus in zygotes beginning as early as 6 h after fertilization.Citation29,30 As shown in , 5mC staining focused on the chromosomes of MII oocytes. After 10 h of in vitro fertilization, 5mC was expressed only in maternal pronucleus (, red arrow), but no staining signals were observed in the paternal pronucleus (, white arrow). These results suggest that oocytes collected from activated ovaries have normal epigenetic profiles during maturation and fertilization. To further evaluate the developmental potential of oocytes derived from activated follicles, collected MII oocytes were for IVF and early embryonic development was followed. After in vitro fertilization with donor sperm, the oocytes developed into 2-cell embryos (24 h, ) and eventually blastocysts (96 h, ). Some 2-cell embryos (total = 66) were also transferred into pseudo-pregnant females, leading to the delivery of 33 healthy pups capable of developing into adults with normal fertility. When compared the IVF rate and successful embryo-transferring rate with normal super-ovulated oocytes, although it's lower for the fraction of mature oocytes collected from transplanted ovaries developing into 2-cell embryos, similar percentage of live pups after embryo-transfer was obtained in both groups (). This suggests that the developmental potential of oocytes is not affected by PA/PRO transient incubation.
Figure 3. Evaluation of oocyte quality by immunofluorescence, in vitro fertilization and early embryonic development. Day 3 ovaries were treated with PA (200 μM) and PRO (50 μM) for 24 h and transplanted into the kidney capsules of recipient mice for 18 days. Mature MII oocytes were retrieved 12 h later, after a single injection of hCG into recipient mice. (A) MII oocytes punctured from the transplanted ovaries. (B) Immunofluorescence of β-tubulin on spindle and first polar body of MII oocytes. Green, β-tubulin; blue, DNA. (C) and (D) Immunofluorescence of 5mC on (C) chromosomes of mature MII oocyte and (D) pronuclear of zygote. Zygotes with 2 pronucli were obtained 10h after in vitro fertilization. Red arrow, maternal pronuclear; white arrow, paternal pronuclear. Green, 5mC; Blue, DNA. (E) and (F) Early embryonic development of retrieved oocytes after in vitro fertilization. Representative figures for embryos reaching (E) 2-cell (24 h) and (F) blastocyst (96 h) stages. (G) Healthy pups with a host mother following 2-cell embryonic transfer. Mature MII oocytes retrieved from activated follicles were in vitro fertilized with donor sperm of the same strain and obtained 2-cell embryos were transferred into pseudopregnant ICR mice to establish pregnancy. (H) Efficiency of embryonic development. IVF rate (2-cell/mature oocytes) and successful 2-cell embryo transfer rate (live pups/2-cell) of mature oocytes retrieved from transplanted grafts were compared with those of mature oocytes from super-ovulated mice. *, P < 0.05 vs. superovulated oocytes. All bars = 100 μm.
mTOR stimulators also increased follicular development in old mice
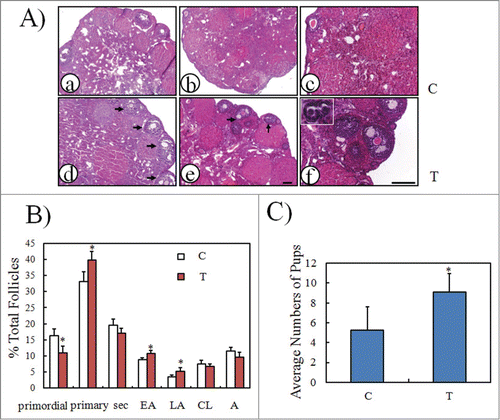
To determine whether mTOR stimulators could also increase follicular development in mice with minimal follicle reserve, 10-month old female mice were chosen for intra-bursal injection with PA/PRO in one lateral ovarian bursa. Saline was injected into the other lateral bursa as a control. Paired ovaries were collected at 14 days after injection. As shown in , there were more developmental follicles in ovaries treated with PA and PRO (d, e, f) when compared with the other lateral control ovaries (a, b, c). Follicle counting also showed significant increases in different stages of developmental follicles, ≈1.2, ≈1.2, and ≈1.5 fold increase of primary, early antral and large antral follicles in treated ovaries (). In another experiment, some mice were injected with PA/PRO or saline in both lateral ovarian bursas. Monitoring estrous cycle was started 18 days later and only mice in estrous were mated with fertile male mice. After mating, only 3 out of 6 (50%) control mice injected with saline were pregnant, but 8 out 11 (73%) mice subjected to intra-bursal injection of PA/PRO delivered live pups. There were more live pups delivered in the mice after PA plus PRO intra-bursal injection, with an average of 9 pups per mouse as compared with control 5 pups per mouse (). The results suggest that mTOR stimulators can stimulate follicular development even when the follicle reserve was very low.
Figure 4. Stimulation of follicular development in old mice by PA and PRO. PA/PRO (200 μM/50 μM) mixture was freshly prepared and injected directly into the ovarian bursa. For some animals, one lateral ovary was given PA/PRO and the other one received saline as control. Ovaries were collected 14 days later to evaluate follicular development. (A) Ovarian histology by hematoxylin and eosin staining. (a), (d) and (b), (e) show 2 pairs of representative ovaries. (a) and (b) are control ovaries with intra-bursal injection of saline and (d) and (e) are ovaries with intra-bursal injection of PA/PRO. Black arrows indicate developing antral follicles; (c) and (f) are higher magnifications of (b) and (e), respectively. The inset in (f) shows growing primary follicles. (B) Distribution of follicles in control and PA/PRO-treated ovaries (n = 6). Sec, secondary follicles; EA, early antral follicles; LA, large antral follicles; CL, corpus luteal; A, atresia. *, P < 0.05, as compared with controls. (C) Quantification of average pup numbers derived from old mice after bi-lateral intra-bursal injection of PA/PRO (T). Some animals served as controls and had only saline injection into bi-lateral ovarian bursa (C). Monitoring of the menstrual cycle began 18 days later and only the mice in estrous were mated, always with male mice of proven fertility. *, P < 0.05 vs. controls.
Treatment with both mTOR and PI3K stimulators promoted the activation of primordial follicles of human ovarian cortex
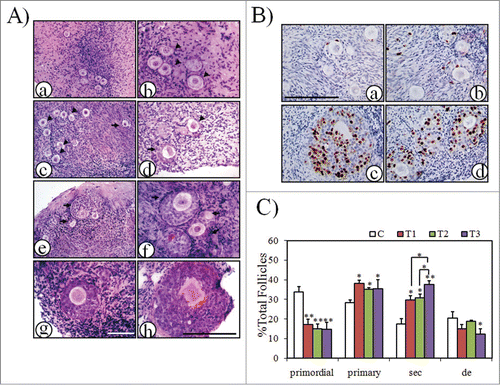
Human primordial follicles were found to develop to the secondary stage of pre-antral development when cultured for 6 days in a serum-free medium.Citation31 Because the stimulatory effects of mTOR activators were manifested in both neonatal and aged mouse ovaries, human ovarian cortex cubes were next divided into different groups and treated with mTOR and PI3K stimulators singly or jointly at the indicated times. After removal of the stimulators, ovarian cortex cubes were further cultured until 6 days of total cultural time. As shown in , most follicles in the ovarian cortex before culture were primordial or primary follicles (≈60% and ≈40%, respectively). After 6 days of in vitro culture, ovarian development was observed in all groups. In the control group, there were still a large proportion of follicles remains quiescent (, arrow heads). Secondary follicles were scattered in the cortex and most of them were at early stages (transitional follicles from primary to secondary stage or secondary follicles with only 2–3 layers of granulose cells, arrows). However, secondary follicles in treated groups were found to form clusters () and showed better development with more layers (>3) of granulose cells around the oocyte (). BrdU labeling indicated increased proliferation of granulosa cells in developing secondary follicles when ovarian cortex cubes were treated with mTOR and PI3K stimulators jointly (). Follicle counts were then performed in serial sections. As shown in , after ovarian cortical cubes were cultured in vitro for 6 days, there are significant increases of follicles recruited from the primordial pool and developed into growing follicles in all treated groups. Notably, more follicles developed into secondary follicles (≈2 fold increase over control values) and fewer follicles degenerated (≈1.6 fold decrease relative to control values) when mTOR and PI3K stimulators were added as indicated in the cultural media. These results suggest that human primordial follicles are activated by mTOR stimulators; mTOR and PI3K stimulators have a synergistic effect on human follicular development.
Figure 5. Activation of human primordial follicles by mTOR and/or PI3K stimulators. Human ovarian cortex cubes were divided into 4 groups and treated as the following: C, control; T1, mTOR stimulators, PA/PRO (200 μM/50 μM) incubation for 24 h; T2, PI3K stimulators, bpV/740Y-P (100 mM/250 μg/ml) for 24 h, 740Y-P(250 μg/ml) only for another 24 h; T3, mTOR and PI3K simulators, PA/PRO (200 μM/50 μM) for 24 h; bpV/740Y-P (100 μM/250 μg/ml) for another 24 h. After treatment, the tissues were continuously cultured in normal control media for another 4-5 days until a total 6 days of culture time. (A) Representative histology of human ovarian cortex cubes after 6 days of in vitro culture. Black arrow head, primordial follicles; black arrow, secondary follicles. (a) and (b), Ovarian cortex with primordial follicles before culture: (b) higher magnification of (a). (c) and (d), Follicles in control group after 6 days of culture: (c) primordial follicle cluster; (d) early secondary follicle. (e) and (f), Two representative clusters of growing secondary follicles in the group treated with mTOR stimulators (T1 group): (e) lower magnification; (f) higher magnification. (g) Representative secondary follicle with more than 3 layers of granulosa cells in PI3K stimulators treated group (T2 group). (h) Large secondary follicle after combined application of mTOR and PI3K stimulators (T3 group). (A, left), the same bar; (A, right), the same bar. Bars = 100 μm. (B) BrdU incorporation into granulosa cells of growing follicles. BrdU was added to culture media during the last 24h before collecting samples for fixation. (a) and (b), control group; (c) and (d), mTOR and PI3K stimulators joint-treated group (T3 group). Bar = 100 μm. (C) Follicle dynamics after treatment with or without mTOR and/or PI3K stimulators. Sec, secondary follicles; de, degenerated follicles. *, P < 0.05; **, P < 0.01 vs. control or as indicated.
Discussion
Although it still remains unknown why only a part of follicles are selected to grow during initial recruitment, recent genetic animal models revealed pivotal roles of intracellular oocyte PI3K signaling pathway in controlling primordial follicle activation.Citation8,13 mTORC1 is an indirect target of survival signals generated by PI3K.Citation18 Oocyte-specific deletion of mTORC1 negative regulators TSC1 and TSC2 showed identical phenotypes with premature activation of all primordial follicles.Citation17,32 However, in double knockout mice lacking both Tsc1 and Pten, a negative regulator of PI3K in oocytes, rapid oocyte enlargement, and accelerated follicular activation indicated AKT-independent activation of mTOR during primordial follicle activation.Citation17 In addition to PI3K/AKT, other intracellular signals, such as PKC, MEK1, Erk, and RSK1 (p90 ribosomal S6 kinase), also contribute to mTOR activation by phosphorylating different residues of TSC1/TSC2.Citation13 Phosphatidic acid (PA), an intracellular lipid second messenger, was shown to activate mTOR signaling through displacement of the endogenous inhibitor FKBP38 from mTOR and allosteric activation of the kinase.Citation33 PA is a central node for lipid signaling and the most important source of PA in cells is PLD-mediated hydrolysis of phosphatidylcholine.Citation34,35 PA can also be generated from lysophosphatidic acid (LPA) and diacylglycerol (DG) and converted to both DG and LPA by PA phosphatase (PAP′ tase) and a type 2 phospholipase (PLA2).Citation36 It has been reported that extracellular PA can stimulate intracellular PLD and generate intracellular PA, thus amplifying the original signal.Citation22 In this study, treatment with PA or PA/PRO activated phosphorylation of mTOR downstream regulators S6K1 and rpS6 in cultured neonatal ovaries. After ovarian allo-transplantation, experimentation further testified to the stimulatory effects of PA and PRO on follicular development. When PA and PRO were used jointly, a synergistic effect was observed. The stimulatory effect of PA plus PRO was found to be blocked by mTOR inhibitor rapamycin but not by Akt inhibitor SH5. These results suggest that, like PI3K stimulators, PA and PRO can be used as mTOR stimulators to activate primordial follicles.
In mammals, the reproductive lifespan of the ovary is limited by oocyte quantity and quality.Citation37 At the end of reproductive life, the decline in fertility with increasing age is related to the concomitant decline of the primordial follicle pool.Citation38 Although the mechanism underlying age-associated decreases in the ovarian follicle pool remains unclear, direct depletion of resting follicles during initial recruitment may be part of the reason.Citation39,40 In the present study, although not many primordial follicles remained in the ovaries of older mice (≈15% of total follicles), after injection of PA and PRO into one lateral ovarian bursa, more growing follicles were observed than in control lateral ovaries. Follicle counting results showed these growing follicles were come from accelerated recruitment of primordial follicles after the treatment of PA plus PRO. These results suggest that, in old adult ovaries, mTOR stimulators also activated primordial follicles as in neonatal ovaries. Once activated, primordial follicles developed through primary and secondary stages until they became preovulatory follicles.Citation19 After females mated with fertile males, mice whose bi-lateral ovarian bursa had been injected with mTOR stimulators produced more live pups than other mice. In today's society, many women choose to delay motherhood beyond age 30 because of the requirements of their education, career, or other situation. Advancing female age is an important factor to the increasing incidence of reported infertility, which is usually associated with a noteworthy decrease in ovulated oocyte quality.Citation41 Although assisted reproduction (AR) can increase many couples’ means of conceiving, the outcome of AR is also adversely affected by advancing maternal age.Citation42,43 Older prospective mothers display a reduced number of retrieved oocytes after ovarian stimulation for IVF.Citation44 In this way, although it is still unknown whether mTOR stimulators can overcome age-related decreases in oocyte quality, an approach to recruitment of more primordial follicles is here proposed: It may be helpful for women suffering aging-related infertility to obtain more mature oocytes for IVF treatment.
Complete in vitro folliculogenesis starting from primordial follicles has been achieved only in a multistep culture system in mouse.Citation45,46 Although a 2-step serum-free culture system supports development of human oocytes from primordial follicles, current culture conditions do not consistently produce follicles from the primordial stage up to the preovulatory stage.Citation31 However, this in vitro culture system with human ovarian cortex is enough for us to investigate the early steps of follicle recruitment after treatment with mTOR and/or PI3K stimulators. In our study, after 6 days of culture, follicular development was observed in all groups as in previous studies. When mTOR and PI3K stimulators were added separately for culture, both showed a nearly 2-fold decrease in the numbers of primordial follicles and obvious increases of secondary follicle development by histological analysis and follicle counting. Thus, as for the stimulatory effects on primordial follicles, there is no obvious difference between mTOR and PI3K stimulators.
Because studies in mice with oocyte-specific double knockout of Pten and Tsc1 showed accelerated follicular activation, mTOR and PI3K stimulators were next used in combination to assess any synergistic effects that they might have on follicular activation.Citation17 Although there was only a subtle decrease in percentage of primordial follicles as compared with that in mTOR or PI3K separate treated groups, the development of primordial follicles to secondary follicles increased significantly after co-treatments with mTOR and PI3K stimulators. Follicle counting further demonstrated that the reason for such kind of increase was mainly because of the decrease of degenerated follicles. By using the same in vitro culture model, McLaughlin et al. demonstrated the stimulatory effects of the PTEN inhibitor, bpV(HOpic), on human primordial follicles. However, isolated and cultured follicles that had been exposed to bpV(HOpic) showed limited growth and reduced survival.Citation47 In mice, the other physiological function of intra-oocyte PI3K signaling is the regulation of primordial follicle survival.Citation9 In human ovarian cortex strips cultured in vitro, treatment with rapamycin, the mTOR inhibitor, resulted in oocyte loss characterized by empty follicles.Citation48 The results suggest PI3K or mTOR signaling participates in the regulation of the survival of primordial follicles. However, it is still unknown whether the 2 pathways regulate the survival of follicles in human in a coordinated fashion. Over the last decade, fertility preservation with ovarian transplantation and cryopreservation has been successfully reported worldwide, resulting in around 18 healthy babies by frozen cortical ovarian tissue transplantations.Citation49 Although the large number of primordial follicles could survive the freezing/thawing procedure, grafting of frozen ovarian tissues often means the limited developmental potential of those follicles.Citation50,51 In a case study in which multiple (>20) follicles were grown after autografting of the cryopreserved tissue, many of the follicles were found to contain immature or atretic oocytes.Citation52 In this way, the current protocol, which has joint incubation with mTOR and PI3K stimulators, represents a better approach to increasing follicular survival for patients undergoing auto-transplantation with cryo-thawed ovarian tissues.
Previous studies showed that elevated activity of mTORC1 in oocytes resulted in follicular depletion in early adulthood and premature ovarian failure.Citation17,32 Whereas rapamycin, caloric restriction (CR) or metformin, that function mainly by the inhibition of mTORC1, can slow down age-related infertility in rodents.Citation53-55 The results testified the opinion that ovarian aging is quasi-programmed.Citation56 It can be delayed pharmacologically by preserving primordial follicle pool in the ovary. Therefore, mTOR signaling pathway likes a double-blade sword to regulate the balance between quiescence and senescence of oocytes. On one hand, mTOR stimulators can be used as a transient measure to activate primordial follicles under special conditions as mentioned. On the other hand, mTOR inhibitors serve as a long-term measure to preserve primordial follicles in the ovary.
In summary, the present study showed primordial follicles in both mouse and human ovarian tissues could be activated by mTOR stimulators, PA and PRO. When mTOR and PI3K stimulators were added jointly on cultured human ovarian cortex cubes, synergistic effects were observed with much better follicular development and significant decrease of follicular degeneration. In the years since the in vitro activation (IVA) method was established, it has been successfully applied in clinic for POF patients with a live birth reported last year.Citation20 Here, mTOR and PI3K stimulators together were found to provide an optimized protocol that can be used to treat infertility in middle-aged women and in POF patients with very limited residual follicles and in cancer patients with variable follicle density and distribution.
Materials and Experimental Methods
Ethical approval
All experiments requiring the use of animals were approved by Committee on the Ethics of Animal Experiments of Nanjng Medical University. The study about human ovarian tissues was approved by the Institutional Review Boards of Nanjing Medical University and informed consent was obtained from each patient at recruitment.
Experimental animals
B6CBAF1 hybrid mice are the offspring of crosses between C57BL/6J females (B6) and CBA/J males (CBA). Both strains were obtained from Model Animal Research Center of Nanjing University (Nanjing, Jiangsu, China) and housed in the animal facility at Nanjing Medical University. Mice were maintained under controlled lighting conditions (12L:12D) with free access to food and water. Ovaries of 3-day-old B6CBAF1 females were used for short-term incubation and transplantation into kidney capsules of adult female mice of the same strain at 8–10 weeks of age. For IVF and embryo transfers, 25-day-old B6CBAF1 female mice were used to collect ovulated oocytes as positive controls. Pseudo-pregnant ICR mice, 8-weeks old, were used as recipients for 2-cell embryo transfer. 10-month old female ICR mice were chosen for intra-bursal injection and mating experiments.
Short term in vitro incubation of mouse ovaries and ovarian transplantation
Paired ovaries were collected and treated as reported previously.Citation19 In brief, one ovary served as a control and the other one was treated with 200 μM phosphatidic acid (PA, 840875, Avanti Polar Lipids) and 50 μM propranolol (PRO, BML-ST405-0005, Enzo Life Sciences) for 24 h. The culture media was MEMalpha supplemented with 0.23 mM pyruvic acid, 50 mg/l streptomycin sulfate, 75 mg/l penicillin G, 3 mg/ml BSA, and 0.03 IU/ml FSH (Puregon, Merck). In some experiments, one ovary from the pair was cultured with PA plus PRO and the contra-lateral one was pretreated with the Akt inhibitor SH-5 (50 μM, 270-349-MC05, Enzo Life sciences) or mTOR inhibitor rapamycin (2.5 μM, R8781, Sigma) 1 h before PA and PRO were added to the culture medium.
After the treatment, paired ovaries from the same donor were randomly inserted under each kidney capsule of the same host. The host was ovariectomized to increase endogenous gonadotropin levels. One day after transplantation, hosts were treated daily with 1 IU FSH. Some animals were sacrificed at 14 days after transplantation to assess follicular development and some animals were given a single i.p. injection of hCG (10 IU, 110251282, Ningbo) at 18 days after transplantation to collect oocytes from grafted ovarian tissues.
Intra-bursal injection and mating protocol
After animals were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (12 mg/kg) (K113, Sigma), 2 small incisions were made on the peritoneum to externalize the ovaries. PA (200 μM) and PRO (50 μM) in 50 μl saline were freshly prepared and injected through the fat pad into the ovarian bursa using a 31-gauge insulin needle. For some animals, one lateral ovarian bursa was given PA plus PRO and the other lateral bursa received saline and served as a control. Ovaries were collected 14 days later to evaluate follicular development. For some animals, PA/PRO and saline were injected into bursas of both ovaries. Monitoring of the estrous cycle was started 18 days later and only mice in estrous were mated, always with males of proven fertility. After confirming mating with the formation of the fertilization plug (within 3–5 days), females were separated from males and pregnancy was allowed to progress until delivery. The mating rates and numbers of live pups were determined.
Follicle counting
Grafted ovaries and ovaries subjected to intra-bursal injection were collected and fixed in 10% buffered formalin for 12 h, embedded in paraffin, serially sectioned at a thickness of 7 μm, and then stained with hematoxylin and eosin. Ovarian follicles were counted using the fractionator and nucleator principles.Citation57 Simply, all follicles with a visible nucleus were counted every fifth section. The follicle classification system was based on Pederson's system:Citation58 oocytes surrounded by a flat layer of granulose cell were named as primordial follicles; Pederson class 3 follicles were named as primary follicles; Pederson class 4–5 follicles were named as secondary follicles; Pederson class 6 follicles were named as early antral follicles and Pederson 7-8 follicles were named as large antral follicles. Follicles were considered atretic if they contained either a degenerating oocyte, disorganized granulosa cells, pyknotic nuclei, shrunken granulosa cells, or apoptotic bodies.Citation57 Finally, to estimate the total number of follicles per ovary, all follicle numbers present in the marked sections was multiplied by 5.Citation59-61
Immunoblotting analysis
Proteins were extracted by RIPA lysis buffer (P0013B, Beyotime Institute of Biotechnology) with protease inhibitor cocktails (M221, Amresco). After separation by electrophoresis, proteins were electronically transferred to polyvinylidene fluoride membranes. After blocking in 5% skimmed milk-TBST (TBS containing 0.1% Tween 20) for 2 h, the membranes were incubated overnight at 4°C with specific antibodies. Antibodies against S6K1 (2708), p-S6K1 (T389) (9234), rpS6 (2217), p-rpS6 (S235/6) (4858), and β-tubulin (2128) were all rabbit antibodies and were purchased from Cell Signaling Technology. Horseradish peroxidase-conjugated goat anti-rabbit IgG (SC2004, Santa Cruz) were then used to detect proteins through enhanced chemiluminescence (RPN2232, Amersham).
Immunohistochemistry
Mouse ovaries were fixed in 10% buffered formalin for paraffin embedding and sectioning. After deparaffinization and rehydration, sections were processed for blocking of endogenous peroxidase activity and antigen retrieval pretreatment. Immunohistochemical analyses were performed using a Histostain Kit (856743, Invitrogen) with antibodies against p-rpS6 (S235/236) overnight at 4°C. For some sections, primary antibodies were replaced with non-immune rabbit IgG as negative controls.
Immunofluorescence
Mature MII (metaphase II) oocytes collected from superovulated mice and grafted ovaries were for β-tubulin staining, and zygotes with 2 pronuclei collected after 10 h of IVF were used for anti-5-methylcytosine (5mC) staining (ab73938, Abcam). Collected oocytes and zygotes were first fixed with 4% paraformaldehyde (PFA) for 1 h at room temperature and then permeabilized for 15 min in PBS containing 0.1% Triton X-100 (T8787, Sigma). For 5mC staining, permeabilized zygotes were incubated in 4N HCl solution at room temperature for 10 min followed by neutralization in Tris-Cl, pH 8.0, for 10 min. After blocking non-specific binding of the antibodies with 5% BSA for 1 h at room temperature, samples were incubated with antibodies against β-tubulin and 5mC in PBS-1% BSA overnight at 4°C. After three washes in PBS-1% BSA, they were incubated with Alexa Fluor 488 goat anti-rabbit (CA11008, Invitrogen) and Alexa Fluor 488 goat anti-mouse secondary antibodies (CA11005, Invitrogen) at RT for 1 h. Then the nuclei were stained with 0.01 mg/ml Hoechst 33342 (H1399, Invitrogen) for 20 min. Oocytes and zygotes were viewed under a laser scanning confocal microscope (Zeiss LSM 510 META, Zeiss). For some oocytes and zygotes, non-immune IgGs were used as controls.
IVF and embryo transfer
At 18 days after transplantation, hosts were treated with 10 IU hCG. Twelve hours later, grafted ovaries were collected into M2 medium (MR-015P-5D, Millipore). Oocytes were collected in media containing 0.3 mg/ml hyaluronidase (H3506, Sigma) by mechanically puncturing the grafted ovaries with fine needles. Only MII oocytes were used for in vitro fertilization. MII oocytes flushed from superovulated B6CBAF1 mice served as controls. Donor sperm were collected from B6CBAF1 mice (10 to 14 weeks old) into HTF media (2001, InVitroCare, incubated under oil for 1 h at 37°C in a 5% CO2 and 95% air for capacitation. Oocytes were then placed in 250 μl media with sperm (2–3 × 105/ml) for 6 h and inseminated oocytes were removed into 20 μl droplets of KSOM media (MR-020P-5F, Millipore) under mineral oil and incubated at 37°C. Embryonic development was then scored and monitored until blastocyst stage.Citation19,62 For embryo transfer, 2-cell embryos were transferred into the oviducts of pseudopregnant ICR mice mated with vasectomized males of the same strain.
Human ovarian cortex culture and BrdU labeling
Ovarian fragments were obtained from patients with cervical cancer who had undergone risk-reducing bilateral salpingo-oophorectomy. Only patients who exhibited normal menstrual cycles were chosen for tissue collection. Except for one small piece of cortex that was fixed for histological examination, the left cortex from each patient was cut into small cubes (≈1 × 1 × 1 mm) and cultured on the membrane insert (10 pieces/insert, PICM01250, Millopore). The culture media was MEMalpha media containing 10% SSS (serum substitute supplement, 99193, Irvine Scientific), Penicillin G (75 μg/ml), streptomycin (50 μg/ml), ascorbic acid (50 μg/ml), sodium pyruvate (2 mM), and FSH (0.3 IU/ml). The media was added below the membrane insert to allow covering of the cubes with a thin layer of media. The cortical cubes were divided into 4 groups for treatment: (1) control; (2) PA (200 μM)/PRO (50 μM) incubation for 24 h; (3) bpV (100 μM) (1794-5, BioVision)/740Y-P (250 μg/ml)(1983, Tocris) for 24 h, 740Y-P (250 μg/ml) only for another 24 h; (4) PA (200 μM)/PRO (50 μM) for 24 h; bpV (100 μM)/740Y-P (250 μg/ml) for another 24 h. After treatment, culturing was continued in normal media for a total of 6 days of incubation with medium changing every 2 days. To evaluate cell proliferation, BrdU (00-0103, Invitrogen) at 0.01 mg/ml was added to the media and cultured cubes were collected 24 h later. Cubes were fixed immediately in 10% buffered formalin and serially sectioned for H&E or BrdU staining (XM-0013, ZSGB-BIO). During the period of in vitro culture, ovarian cortex samples from each patient were analyzed for follicle reserve by continuous sections followed by follicle counting. Only ovarian cortex containing 10–15 follicles/mm3 was chosen for the final statistical analysis.
Statistical analysis
Chi-square test or one-way ANOVA and Mann-Whitney U test were used to evaluate differences between groups. Data are mean ± SEM. A value of P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
XS, YS and J Li were responsible for the study design; XS, YH, JZ, WL and J Li were responsible for the animal experiments, data collection and analysis; YS, HZ, ZH and J Liu were responsible for collection and culture of human ovarian tissues and data analysis; YS and J Li were responsible for drafting the manuscript.
Acknowledgment
We would like to thank Prof. Youqiang Su (State Key Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing, China) for critical review of this paper.
Funding
This study was funded by the National Basic Research Program of China (973 program, 2013CB945502), the National Natural Science Foundation of China (31371522), Science Fund of Jiangsu Province (BK2012836), Fund of State Key Laboratory of Reproductive Medicine, Nanjing Medical University (SKLRM-KF-1206) and Key project of Jiangsu provincial health office, maternal and child health (F201211).
References
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction 2009; 137:1-11; PMID:18827065; http://dx.doi.org/10.1530/REP-08-0118
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev 2000; 21:200-14; PMID:10782364
- Macklon NS, Fauser BC. Aspects of ovarian follicle development throughout life. Horm Res 1999; 52:161-70; PMID:10725781; http://dx.doi.org/10.1159/000023456
- Okeke T, Anyaehie U, Ezenyeaku C. Premature menopause. Ann Med Health Sci Res 2013; 3:90-5; PMID:23634337; http://dx.doi.org/10.4103/2141-9248.109458
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005; 11:391-410; PMID:15919682; http://dx.doi.org/10.1093/humupd/dmi012
- Linkeviciute A, Boniolo G, Chiavari L, Peccatori FA. Fertility preservation in cancer patients: The global framework. Cancer Treat Rev 2014; 40(8):1019-27; PMID:24953980; http://dx.doi.org/10.1016/j.ctrv.2014.06.001
- Buratini J, Price CA. Follicular somatic cell factors and follicle development. Reprod Fertil Dev 2011; 23:32-9; PMID:21366978; http://dx.doi.org/10.1071/RD10224
- Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev 2009; 30:438-64; PMID:19589950; http://dx.doi.org/10.1210/er.2008-0048
- Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol 2012; 356:24-30; PMID:21684319; http://dx.doi.org/10.1016/j.mce.2011.05.027
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science 2003; 301:215-8; PMID:12855809; http://dx.doi.org/10.1126/science.1086336
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008; 319:611-3; PMID:18239123; http://dx.doi.org/10.1126/science.1152257
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet 2009; 18:2813-24; PMID:19423553; http://dx.doi.org/10.1093/hmg/ddp217
- Reddy P, Zheng W, Liu K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol Metab 2010; 21:96-103; PMID:19913438; http://dx.doi.org/10.1016/j.tem.2009.10.001
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010; 40:310-22; PMID:20965424; http://dx.doi.org/10.1016/j.molcel.2010.09.026
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12:21-35; PMID:21157483; http://dx.doi.org/10.1038/nrm3025
- Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 2012; 18:524-33; PMID:22749019; http://dx.doi.org/10.1016/j.molmed.2012.05.007
- Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 2010; 19:397-410; PMID:19843540; http://dx.doi.org/10.1093/hmg/ddp483
- Yang Q, Guan KL. Expanding mTOR signaling. Cell Res 2007; 17:666-81; PMID:17680028; http://dx.doi.org/10.1038/cr.2007.64
- Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan EK, Hsueh AJ. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci U S A 2010; 107:10280-4; PMID:20479243; http://dx.doi.org/10.1073/pnas.1001198107
- Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, Ho CH, Kawamura N, Tamura M, Hashimoto S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A 2013; 110:17474-9; PMID:24082083; http://dx.doi.org/10.1073/pnas.1312830110
- Taga M, Mouton-Liger F, Paquet C, Hugon J. Modulation of oxidative stress and tau phosphorylation by the mTOR activator phosphatidic acid in SH-SY5Y cells. FEBS Lett 2011; 585:1801-6; PMID:21510936; http://dx.doi.org/10.1016/j.febslet.2011.04.022
- Frondorf K, Henkels KM, Frohman MA, Gomez-Cambronero J. Phosphatidic acid is a leukocyte chemoattractant that acts through S6 kinase signaling. J Biol Chem 2010; 285:15837-47; PMID:20304930; http://dx.doi.org/10.1074/jbc.M109.070524
- Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A 2006; 103:4741-6; PMID:16537399; http://dx.doi.org/10.1073/pnas.0600678103
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 2001; 294:1942-5; PMID:11729323; http://dx.doi.org/10.1126/science.1066015
- O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 2009; 587:3691-701; PMID:19470781; http://dx.doi.org/10.1113/jphysiol.2009.173609
- Liu Y, Kach A, Ziegler U, Ong AC, Wallace DP, Arcaro A, Serra AL. The role of phospholipase D in modulating the MTOR signaling pathway in polycystic kidney disease. PLoS One 2013; 8:e73173; PMID:24009738; http://dx.doi.org/10.1371/journal.pone.0073173
- Jaafar R, De Larichaudy J, Chanon S, Euthine V, Durand C, Naro F, Bertolino P, Vidal H, Lefai E, Nemoz G. Phospholipase D regulates the size of skeletal muscle cells through the activation of mTOR signaling. Cell Commun Signal 2013; 11:55; PMID:23915343; http://dx.doi.org/10.1186/1478-811X-11-55
- Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci U S A 2011; 108:3642-7; PMID:21321204; http://dx.doi.org/10.1073/pnas.1014033108
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature 2000; 403:501-2; PMID:10676950; http://dx.doi.org/10.1038/35000656
- Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol 2002; 241:172-82; PMID:11784103; http://dx.doi.org/10.1006/dbio.2001.0501
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008; 23:1151-8; PMID:18326514; http://dx.doi.org/10.1093/humrep/den070
- Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod 2009; 15:765-70; PMID:19843635; http://dx.doi.org/10.1093/molehr/gap092
- Yoon MS, Sun Y, Arauz E, Jiang Y, Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J Biol Chem 2011; 286:29568-74; PMID:21737445; http://dx.doi.org/10.1074/jbc.M111.262816
- Foster DA. Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim Biophys Acta 2009; 1791:949-55; PMID:19264150; http://dx.doi.org/10.1016/j.bbalip.2009.02.009
- Sun Y, Chen J. mTOR signaling: PLD takes center stage. Cell Cycle 2008; 7:3118-23; PMID:18927511; http://dx.doi.org/10.4161/cc.7.20.6881
- Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci 2005; 62:2305-16; PMID:16143829; http://dx.doi.org/10.1007/s00018-005-5195-z
- Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev 2009; 30:465-93; PMID:19589949; http://dx.doi.org/10.1210/er.2009-0006
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BM, de Jong FH, Groome NP, Themmen AP, Visser JA. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 2006; 147:3228-34; PMID:16556768; http://dx.doi.org/10.1210/en.2005-1588
- de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, te Velde ER. Ultrastructure of the resting ovarian follicle pool in healthy young women. Biol Reprod 2002; 66:1151-60; PMID:11906936; http://dx.doi.org/10.1095/biolreprod66.4.1151
- de Bruin JP, Dorland M, Spek ER, Posthuma G, van Haaften M, Looman CW, te Velde ER. Age-related changes in the ultrastructure of the resting follicle pool in human ovaries. Biol Reprod 2004; 70:419-24; PMID:14561658; http://dx.doi.org/10.1095/biolreprod.103.015784
- Lambalk CB, van Disseldorp J, de Koning CH, Broekmans FJ. Testing ovarian reserve to predict age at menopause. Maturitas 2009; 63:280-91; PMID:19631481; http://dx.doi.org/10.1016/j.maturitas.2009.06.007
- Armstrong S, Akande V. What is the best treatment option for infertile women aged 40 and over? J Assist Reprod Genet 2013; 30:667-71; PMID:23536151; http://dx.doi.org/10.1007/s10815-013-9980-6
- Cobo A, Garcia-Velasco JA, Domingo J, Remohi J, Pellicer A. Is vitrification of oocytes useful for fertility preservation for age-related fertility decline and in cancer patients? Fertil Steril 2013; 99:1485-95; PMID:23541405; http://dx.doi.org/10.1016/j.fertnstert.2013.02.050
- Mellembakken JR, Berga SL, Kilen M, Tanbo TG, Abyholm T, Fedorcsak P. Sustained fertility from 22 to 41 years of age in women with polycystic ovarian syndrome. Hum Reprod 2011; 26:2499-504; PMID:21724569; http://dx.doi.org/10.1093/humrep/der214
- Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54:197-207; PMID:8838017; http://dx.doi.org/10.1095/biolreprod54.1.197
- O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68:1682-6; PMID:12606400; http://dx.doi.org/10.1095/biolreprod.102.013029
- McLaughlin M, Kinnell HL, Anderson RA, Telfer EE. Inhibition of phosphatase and tensin homologue (PTEN) in human ovary in vitro results in increased activation of primordial follicles but compromises development of growing follicles. Mol Hum Reprod 2014; 20(8):736-44; PMID:24830779; http://dx.doi.org/10.1093/molehr/gau037
- McLaughlin M, Patrizio P, Kayisli U, Luk J, Thomson TC, Anderson RA, Telfer EE, Johnson J. mTOR kinase inhibition results in oocyte loss characterized by empty follicles in human ovarian cortical strips cultured in vitro. Fertil Steril 2011; 96:1154-9.e1; PMID:22036052; http://dx.doi.org/10.1016/j.fertnstert.2011.08.040
- Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril 2012; 97:1260-8; PMID:22656306; http://dx.doi.org/10.1016/j.fertnstert.2012.04.042
- Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod Biomed Online 2009; 18:568-77; PMID:19401001; http://dx.doi.org/10.1016/S1472-6483(10)60136-8
- Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril 2010; 94:2191-6; PMID:20171622; http://dx.doi.org/10.1016/j.fertnstert.2009.12.073
- Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, Opsahl M, Rosenwaks Z. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 363:837-40; PMID:15031026; http://dx.doi.org/10.1016/S0140-6736(04)15728-0
- Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 2013; 523:82-7; PMID:23566837; http://dx.doi.org/10.1016/j.gene.2013.03.039
- Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell 2008; 7:622-9; PMID:18549458; http://dx.doi.org/10.1111/j.1474-9726.2008.00409.x
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011; 3:148-57; PMID:21386129
- Blagosklonny MV. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging (Albany NY) 2010; 2:265-73; PMID:20519781
- Flaws JA, Abbud R, Mann RJ, Nilson JH, Hirshfield AN. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol Reprod 1997; 57:1233-7; PMID:9369192; http://dx.doi.org/10.1095/biolreprod57.5.1233
- Pedersen T. Determination of follicle growth rate in the ovary of the immature mouse. J Reprod Fertil 1970; 21:81-93; PMID:5461007; http://dx.doi.org/10.1530/jrf.0.0210081
- Vitt UA, McGee EA, Hayashi M, Hsueh AJ. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology 2000; 141:3814-20; PMID:11014238
- Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res 2004; 59:287-304; PMID:14749507; http://dx.doi.org/10.1210/rp.59.1.287
- Panwar S, Herrid M, Kauter KG, McFarlane JR. Effect of passive immunization against leptin on ovarian follicular development in prepubertal mice. J Reprod Immunol 2012; 96:19-24; PMID:22989496; http://dx.doi.org/10.1016/j.jri.2012.07.004
- Li J, Klein C, Liang C, Rauch R, Kawamura K, Hsueh AJ. Autocrine regulation of early embryonic development by the artemin-GFRA3 (GDNF family receptor-alpha 3) signaling system in mice. FEBS Lett 2009; 583:2479-85; PMID:19580811; http://dx.doi.org/10.1016/j.febslet.2009.06.050
