Abstract
Plk1 has been essentially described as a critical regulator of many mitotic events. However, increasing evidence supports the notion that its molecular functions are not restricted to the cell cycle. In particular, recent reports suggest the existence of a molecular and functional link between Plk1 and the mammalian target of rapamycin (mTOR) pathway, which controls cell growth and proliferation via the raptor-mTOR (TORC1) and rictor-mTOR (TORC2) protein complexes. Herein, we have identified rapamycin-insensitive companion of mTOR (Rictor), a core component of mTORC2, as a new Plk1 substrate and have shown that Plk1 phosphorylates Rictor at Ser1162 in vitro and in vivo. Surprisingly, cells expressing the unphosphorylatable mutant (S1162A) of Rictor did not show any effect on well characterized canonical PI3K-mTOR pathway. However, we found that cells expressing the unphosphorylatable form of Rictor have an elevated level of mSin1 isoform (mSin1.5). Considering that mSin1.5-containing mTORC2 was reported to associate with stress signaling, we propose that phosphorylation of Rictor at Ser1162 by Plk1 might be involved in a novel signaling pathway by regulating the mSin1.5-defined mTORC2.
Abbreviations
| Plk1 | = | polo-like kinase 1 |
| mTOR | = | mammalian target of rapamycin |
| mTORC1 | = | mTOR complex 1 |
| mTORC2 | = | mTOR complex 2 |
| Rictor | = | rapamycin-insensitive companion of mTOR |
| Raptor | = | regulatory-associated protein of mTOR |
| PDK1 | = | 3-phophoinositide-dependent protein kinase 1 (PDK1) |
| S6K | = | S6 Kinase |
| 4E-BP1 | = | eIF4E-binding protein 1 |
| mSin1 | = | mammalian stress-activated map kinase-interacting protein 1 (mSin1) |
| GST | = | glutathione S-transferase |
| WT | = | wild type |
| aa | = | amino acids |
| IP | = | immunoprecipitation |
| IB | = | immunoblotting. |
Introduction
It has been well documented that expression of Polo-like kinase 1 (Plk1), a serine/threonine kinase, is cell cycle regulated, rising from a low level during S phase to a maximal level during G2/M phase.Citation1,2 Plk1 plays pivotal roles in mitotic progression, with established functions during G2/M transition and G2 DNA damage checkpoint recovery, and during various mitotic events, such as bipolar spindle assembly and cytokinesis.Citation2,3 However, accumulating evidence suggests that Plk1 is also involved in interphase events and other non-canonical cell cycle events. For instance, the implication of Plk1 in DNA replication during normal or stressful condition of S phase progression,Citation4-8 as well as the role of protection against apoptosis has been reported.Citation9,10 Plk1 was also described as a regulator of cancer cell invasiveness as cancer cells were apparently much more sensitive to Plk1 deletion than normal ones.Citation11,12 Intriguingly, Plk1 was also proposed to have a potential tumor suppressor function under certain conditions and to be essential for early embryonic development.Citation13 Very recently, the existence of molecular and functional link between Plk1 and the mammalian target-of-rapamycin (mTOR) pathway was discussed.Citation14
mTOR is the catalytic subunit of 2 distinct complexes called mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Unique accessory proteins distinguish these 2 complexes; regulatory-associated protein of mTOR (raptor) or rapamycin-insensitive companion of mTOR (rictor) defines mTORC1 or mTORC2, respectively.Citation15-17 The mTOR signaling pathway couples energy and nutrient abundance to the execution of cell growth and division. mTORC1 and mTORC2 exert their functions by regulating other important kinases, such as AKT and S6 Kinase (S6K).Citation18 The mTORC1 substrates S6 Kinase 1 (S6K1) and eIF4E-binding protein 1 (4E-BP1) associate with mRNAs and regulate mRNA translation initiation and progression, thus controlling the rate of protein synthesis.Citation19 mTORC2 was identified as a mediator of actin cytoskeletal organization and cell polarization.Citation17,20,21 The key finding of mTORC2 is that it can phosphorylate AKT at Ser473 located in the hydrophobic motif and, thus priming AKT for further phosphorylation at Thr308 in the catalytic domain by 3-phophoinositide-dependent protein kinase 1 (PDK1). These two phosphorylation events lead to full activation of AKT.Citation22-24
In this paper, we show that Plk1 interacts with and directly phosphorylates Rictor in vitro and in vivo at Ser1162. Moreover, inhibition of this phosphorylation event leads to an elevated expression of mSin1.5, suggesting that Plk1 phosphorylation of Rictor might be involved in stress signaling.
Materials and Methods
Cell culture and synchronization. HeLa, HEK293T, PC3, DU145 and PANC1 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 100 units ml−1 penicillin, and 100 units ml−1 streptomycin at 37°C in 6% CO2. Cells were treated with 0.3 mM mimosine for 20 h, 4 mM hydroxyurea for 24 h, or 200 ng ml−1 nocodazole for 12h to arrest at G1, S, or M phase, respectively.
Antibodies. The phospho-specific antibody against S1162 of Rictor was generated by Proteintech (Chicago, IL). Briefly, a peptide containing phospho-S1162 (QKTLQLETSFMGNKHIEC) was synthesized and used to immunize rabbits. After the antibody was affinity purified, a series of control experiments were performed to confirm the specificity of the antibody. The following antibodies were also used: anti-Plk1 (Santa Cruz Biotechnology), anti-Myc, anti-FLAG and anti-β-actin (Sigma), anti-Sin1 (Millipore), anti-Rictor, anti-phospho-AKT, anti-phospho-S6 (Cell Signaling).
Recombinant protein purification and DNA transfection. Various domains of Rictor were amplified by PCR, subcloned into pGEX-KG vector, expressed in Escherichia coli, and purified. Point mutations were generated with the QuickChange site-directed matagenesis kit (Stratagene). Plasmid DNA was transfected with MegaTran (Origene) according to the manufacturer's protocols.
Kinase assay. In vitro kinase assays were performed in TBMD buffer (50 mM Tris [PH 7.5], 10 mM MgCl2, 5 mM dithiothreitol, 2 mM EGTA, 0.5 mM sodium vanadate, 20 mM p-nitrophenyl phosphate) supplemented with 125 μM ATP and 10 μCi of [γ-32P]ATP at 30°C for 30 min in the presence of GST-Rictor proteins and purified Plk1. After the reaction mixtures were resolved by SDS-PAGE, the gels were stained with Coomassie brilliant blue, dried and subjected to autoradiography.
Immunoprecipitation (IP) and immunoblotting (IB). Cell lysates were incubated with anti-Myc or anti-phospho-S1162-Rictor antibodies overnight at 4°C, followed by 2h of incubation with protein A/G Plus-Agarose beads (Santa Cruz, sc-2003). Immunocomplexes were resolved by SDS-PAGE, and coimmunoprecipitated proteins were detected by IB with different antibodies, as indicated in specific experiments.
Vector construction and RNAi. To specifically deplete Plk1, plasmid pLKO.1-Plk1 was constructed with the targeting sequence AGATCACCCTCCTTAAATATT and lentivirus was generated. Lentivirus depletion was performed in the presence of Polybrene (10 μg/ml) and HEPES (10 mM).
Results
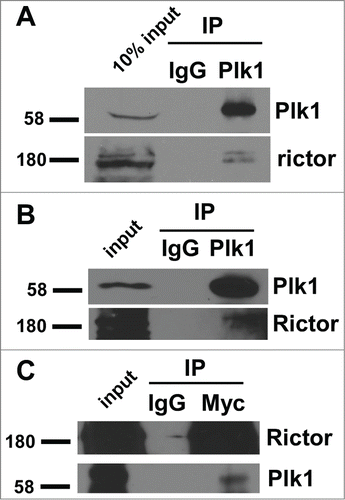
Rictor interacts with Plk1 in cells. Proteomic studies suggest that Rictor is a potential Plk1 target.Citation25 To ask whether Rictor interacts with Plk1, we treated HEK293T cells with nocodazole for 12 h to arrest cells at prometaphase. The interaction between endogenous Rictor and Plk1 was detected in the co-immunoprecipitation experiment (), suggesting that Rictor associates with Plk1 in cells. Interaction between endogenous Plk1 and Rictor can also be detected in asynchronous cells (), suggesting that the Plk1/Rictor interaction is cell-cycle independent. Moreover, reciprocal IP was performed to confirm the physical interaction between Plk1 and Rictor ().
Figure 1. Rictor interacts with Plk1. HEK 293T cells were treated with (A) or not (B) nocodazole for 12 h and harvested for anti-Plk1 immunoprecipitation (IP), followed by immunoblotting (IB). IgG IP was used as a non-specific binding control. (C) 293T cells were co-transfected with Flag-Plk1 and Myc-Rictor and harvested for anti-myc IP, followed by anti-Plk1 IB.
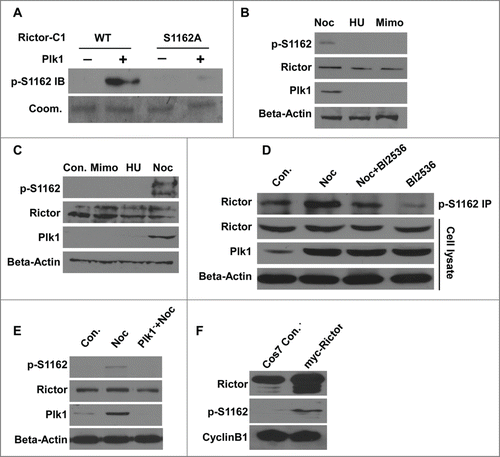
Plk1 phosphorylates rictor at Ser1162. We next asked whether Rictor is a substrate of Plk1. In the in vitro kinase assay, Rictor-amino acids 901–1200 (C1) was indeed phosphorylated by Plk1 (). After virtually every single serine or threonine within Rictor-C1 was mutated into alanine by site-directed mutagenesis, we mapped Ser1162 as the Plk1 phosphorylation site in vitro (). Rictor-S1162 is apparently conserved between murine and human sequences (). To test whether Ser1162 of Rictor is phosphorylated by Plk1 in cells, a polyclonal antibody directed against a peptide containing phospho-Ser1162 (pS1162) was generated. Upon incubation with Plk1, only wild-type Rictor (Rictor-C1), but not Rictor-S1162A, was recognized by the pS1162-Rictor antibody, suggesting that the antibody specifically recognizes phosphorylated Rictor at S1162 caused by Plk1-associated activity (). Next, PC3 prostate cancer cells were treated with mimosine, hydroxyurea or nocodazole to block at G1, S, or M phase, respectively. The phosphorylation of Rictor at Ser1162 could be detected by direct Western blotting when most cells were in M phase and Plk1 was maximally expressed (). The similar result also could be obtained in Panc1 pancreatic cancer cells (). Thus, phosphorylation of Rictor at Ser1162 was specific in M phase and correlated with cell cycle-dependent expression of Plk1. Moreover, DU145 prostate cancer cells were treated with nocodazole, BI2536 or their combination for 12h, respectively. Cell extracts were subjected to anti-pS1162-Rictor IP, followed by anti-Rictor Western blot analysis. As shown in , the phosphorylation level of Rictor at Ser1162 was decreased after Plk1 activity was inhibited with BI2536, indicating that Ser1162 of Rictor is phosphorylated directly by Plk1-associated kinase activity in cells. Moreover, anti-pS1162-Rictor identified the phosphorylated Rictor in the cell lysates from HeLa cervical cancer cells treated with nocodazole, but not from Plk1-deleted cells (), confirming that endogenous Rictor is phosphorylated at Ser1162 in a Plk1-dependent manner. The enriched Rictor phosphorylation upon nocodazole treatment is unlikely due to a possible function of this phosphorylation event during the mitotic stage as simple overexpression of Myc-Rictor in Cos7 cells also leads to increased phospho-S1162 signal and the levels of Cyclin B1 suggest the same stage of cell cycle ().
Figure 2. Plk1 phosphorylates Rictor-S1162 in vitro. (A) Recombinant Plk1 was incubated with 6 purified GST-Rictor regions [amino acidsCitation32 1–300, 301–600, 601–900, 901–1200, 1201–1500, 1501–1709] in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS-PAGE, stained with Coomassie brilliant blue (Coom.), and detected by autoradiography. (B) Plk1 was incubated with the indicated forms of GST-Rictor fragment (aa 901–1200, C1). (C) Alignment of Rictor protein sequences containing Ser1162 in different species.
![Figure 2. Plk1 phosphorylates Rictor-S1162 in vitro. (A) Recombinant Plk1 was incubated with 6 purified GST-Rictor regions [amino acidsCitation32 1–300, 301–600, 601–900, 901–1200, 1201–1500, 1501–1709] in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS-PAGE, stained with Coomassie brilliant blue (Coom.), and detected by autoradiography. (B) Plk1 was incubated with the indicated forms of GST-Rictor fragment (aa 901–1200, C1). (C) Alignment of Rictor protein sequences containing Ser1162 in different species.](/cms/asset/a276aabf-55a6-46a8-a5af-00f1fbcf1bf8/kccy_a_998050_f0002_b.gif)
Figure 3. Plk1 phosphorylates Rictor-S1162 in variety of human cancer cell lines. (A) Plk1 was incubated with GST-Rictor-aa 901–1200 (WT or S1162A) in the presence of unlabelled ATP, followed by anti-pS1162-Rictor IB. (B and C) PC3 (B) and Panc1 (C) cells were treated with mimosine (Mimo), hydroxyurea,Citation26 or nocodazole (Noc) to arrest cells at G1, S or M phase, respectively, and harvested for IB. (D) DU145 cells were treated with nocodazole, BI2536 or combination of these 2 drugs and harvested for anti-pS1162-Rictor IP, followed by anti-Rictor IB. (E) HeLa cells were depleted of Plk1 with dsRNA, treated with nocodazole and immunoblotted. (F) Cos7 cells were transfected with Myc-Rictor and harvested for IB.
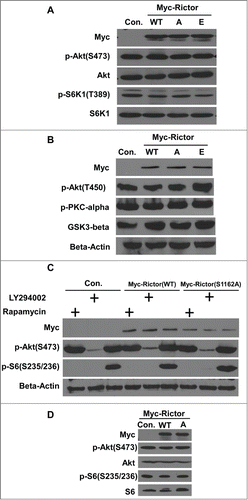
Phosphorylation of Rictor-S1162 by Plk1 does not affect the PI3K/AKT/mTOR pathway. Because mTORC2 phosphorylates the hydrophobic motif site S473 of AKT and because Rictor is one of the core components of mTORC2, we asked whether Plk1 phosphorylation of Rictor-S1162 is involved in AKT-S473 phosphorylation. Accordingly, HEK 293T cells were transfected with various forms of Rictor (WT, S1162A, S1162E) constructs and harvested for IB. As indicated in , cells expressing different forms of Rictor show similar levels of pS473-AKT and pT389-S6K1, a major downstream target of mTOR, indicating that phosphorylation of Rictor-S1162 by Plk1 does not affect the activity of mTORC2. We next examined several effectors regulated by mTORC2, including pT450-AKT, p-PKCα and GSK3β. Again, Plk1 phosphorylation of Rictor-S1162 does not affect the status of pT450-AKT, p-PKCα and GSK3β (). Considering that 2 complexes of mTOR affect each other, we speculated that phosphorylation of Rictor-S1162 might contribute to phosphorylation of AKT-S473 upon inhibition of mTORC1. For that purpose, cells expressing different forms of Rictor constructs were treated with LY294002 and rapamycin, respectively. Here, rapamycin can only disrupt Raptor-mTOR as LY294002 serves as an mTOR kinase inhibitor. We analyzed the levels of pS473-AKT and pS235/6-S6 under this condition. (Ser235/246) were then analyzed by Western Blotting. As indicated in , LY294002 blocked the phosphorylation of AKT-S473 in cells expressing both WT and S1162A-Rictor. Upon rapamycin-induced inhibition of Raptor-mTOR indicated by pS235/6-S6 (Ser235/246), expression of Rictor-S1162A did not affect the phosphorylation of AKT-S473 either. In addition, overexpression of different Rictor constructs in Cos7 cells did not lead to any detectable change of phosphorylation levels of AKT and S6, either (). These results suggest that phosphorylation of Rictor-S1162 is not essential for AKT-S473 phosphorylation and activation of the canonical mTOR pathway.
Figure 4. Plk1 phosphorylation of Rictor at S1162 does not regulate the mTOR pathway. (A) HEK 293T cells were transfected with various Myc-Rictor constructs (WT, S1162A or S1162E) and subjected to IB with antibodies against pS473-AKT and pT389-S6K1. (B) HEK 293T cells expressing various forms of Myc-Rictor were harvested for IB with indicated antibodies. (C) HEK 293T cells were transfected with Myc-Rictor (WT or S1162A), treated with LY294002 or rapamysin, and harvested for IB. A, S1162A; E, S1162E. (D) Cos7 cells were transfected with Myc-Rictor constructs and harvested for IB.
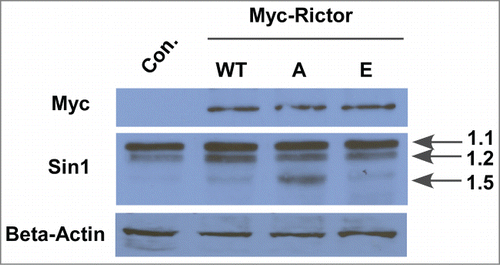
Rictor-S1162 phosphorylation is involved in Sin1.5-defined mTORC2 pathway. Mammalian stress-activated map kinase-interacting protein 1 (mSin1) has been identified as a crucial subunit of the mTORC2 protein complex.Citation26 In support, Rictor knockdown can dramatically decrease hSin1 protein level.Citation27 We can achieve the same result in PC3 cells (data not shown). Herein, we intend to investigate the effect on hSin1 expression by changing phosphorylation status of Rictor-S1162. After introducing different forms of Rictor (WT, S1162A, S1162E) into HEK 293T cells, we examined Sin1 protein levels by Western Blot. As indicated in , expression of Rictor-S1162A led to an elevated protein level of one isoform of Sin1 (1.5), but not the 2 other isoforms (1.1 and 1.2), suggesting that Plk1 phosphorylation of Rictor-S1162 might be involved in regulating a specific Sin1.5-containing mTORC2.
Discussion
The critical role of Plk1 during mitosis has been well established.Citation2 However, increasing evidence suggests that Plk1 might have additional functions outside of cell cycle. Because Plk1 is overexpressed in several kinds of cancers, Plk1 has been proposed as a potential therapeutic target during the last few years.Citation28 The mTOR kinase, involved in cell growth and proliferation via regulating protein translation, is activated by various stimuli including nutrients or growth factors and functions as a nutritional and energetic sensor.Citation29,30 Depending on their unique component Raptor or Rictor, 2 functionally different complexes mTORC1 and mTORC2 were identified.Citation20 Recent studies indicate that there might be a functional link between Plk1 and mTOR pathway, as inhibition of Plk1 activity by BI2536 or knowdown of Plk1 led to reduction of the phosphorylation of mTOR substrates.Citation14 In this study, we identified Rictor as a new Plk1 substrate and showed that Plk1 phosphorylates Rictor-S1162 in vitro and in vivo. To our knowledge, this is the first time a direct link between Plk1 and mTOR components has been established. Previous reports demonstrated that the Rictor-mTOR complex directly phosphorylates AKT-S473.Citation22 However, phosphorylation status of Rictor-Ser1162 did not affect the phosphorylation of AKT-S473, indicating that Plk1 phosphorylation of Rictor does not regulate the canonical PI3K/AKT/mTOR pathway. In support, additional mTORC2 downstream effectors, such as pT450-AKT, p-PKCα and GSK3β, were not affected by this phosphorylation event either. Rictor and mSin1 are necessary both for the assembly of mTORC2 and for its capacity to phosphorylate AKT.Citation31 Further, deficiency in Rictor expression results in reduced mSin1 levels and vice versa, whereas the level of mTOR remains unchanged.Citation26,27,32 Therefore, we investigated whether Plk1 phosphorylation of Rictor regulates mSin1 expression. Interestingly, loss of Plk1-associated phosphorylation in Rictor led to an increase of protein level of mSin1.5, but not 2 other isoforms of mSin (1.1 or 1.2). It was reported that the mTOR complex containing mSin1.5 is insensitive to serum starvation and insulin stimulation and might even not be associated with growth factor-mediated activation of AKT. In agreement, we showed that Rictor phosphorylation by Plk1 did not affect the status of pS473-AKT under normal condition. Because the mSin1.5-containing mTORC2 was suggested to play a role in stress signaling,Citation31 we propose that Plk1 phosphorylation of Rictor-S1162 might also be involved in stress signaling pathways, such as SAPK (stress-activated protein kinase) signaling cascade. On the other hand, more and more studies suggest the mTOR pathway plays important roles in cellular senescence and longevity.Citation33-35 However, how mTORC1 and mTORC2 activity regulate cellular senescence respectively remains not fully understood as they don't share the same principle. Since stress and aging are causally linked, our findings may shed light of the functional significance of mTORC2 in aging through stress response regulated by Plk1.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant R01CA157429 (X.L.), NSF grant MCB-1049693 (X.L.), ACS grant RSG-13–073 (X.L.), and NIH grant P30 CA023168 for Purdue Center for Cancer Research.
References
- Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol 2004; 166:167-72; PMID:15263015; http://dx.doi.org/10.1083/jcb.200403084
- Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov 2010; 9:643-60; PMID:20671765; http://dx.doi.org/10.1038/nrd3184
- van de Weerdt BC, Medema RH. Polo-like kinases: a team in control of the division. Cell Cycle 2006; 5:853-64; PMID:16627997; http://dx.doi.org/10.4161/cc.5.8.2692
- Yim H, Erikson RL. Polo-like kinase 1 depletion induces DNA damage in early S prior to caspase activation. Mol Cell Biol 2009; 29:2609-21; PMID:19289504; http://dx.doi.org/10.1128/MCB.01277-08
- Trenz K, Errico A, Costanzo V. Plx1 is required for chromosomal DNA replication under stressful conditions. Embo J 2008; 27:876-85; PMID:18309293; http://dx.doi.org/10.1038/emboj.2008.29
- Lei M, Erikson RL. Plk1 depletion in nontransformed diploid cells activates the DNA-damage checkpoint. Oncogene 2008; 27:3935-43; PMID:18297112; http://dx.doi.org/10.1038/onc.2008.36
- Song B, Liu XS, Davis K, Liu X. Plk1 phosphorylation of Orc2 promotes DNA replication under conditions of stress. Mol Cell Biol 2011; 31:4844-56; PMID:21947279; http://dx.doi.org/10.1128/MCB.06110-11
- Keppner S, Proschak E, Schneider G, Spankuch B. Fate of primary cells at the G(1)/S boundary after polo-like kinase 1 inhibition by SBE13. Cell Cycle 2011; 10:708-20; PMID:21301227; http://dx.doi.org/10.4161/cc.10.4.14898
- Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A 2003; 100:5789-94; PMID:12732729; http://dx.doi.org/10.1073/pnas.1031523100
- Tamura Y, Simizu S, Muroi M, Takagi S, Kawatani M, Watanabe N, Osada H. Polo-like kinase 1 phosphorylates and regulates Bcl-x(L) during pironetin-induced apoptosis. Oncogene 2009; 28:107-16; PMID:18820703; http://dx.doi.org/10.1038/onc.2008.368
- Liu X, Lei M, Erikson RL. Normal cells, but not cancer cells, survive severe Plk1 depletion. Mol Cell Biol 2006; 26:2093-108; PMID:16507989; http://dx.doi.org/10.1128/MCB.26.6.2093-2108.2006
- Keppner S, Proschak E, Kaufmann M, Strebhardt K, Schneider G, Spankuch B. Biological impact of freezing Plk1 in its inactive conformation in cancer cells. Cell Cycle 2010; 9:761-73; PMID:20139717; http://dx.doi.org/10.4161/cc.9.4.10644
- Lu LY, Wood JL, Minter-Dykhouse K, Ye L, Saunders TL, Yu X, Chen J. Polo-like kinase 1 is essential for early embryonic development and tumor suppression. Mol Cell Biol 2008; 28:6870-6; PMID:18794363; http://dx.doi.org/10.1128/MCB.00392-08
- Renner AG, Creancier L, Dos Santos C, Fialin C, Recher C, Bailly C, Kruczynski A, Payrastre B, Manenti S. A functional link between polo-like kinase 1 and the mammalian target-of-rapamycin pathway? Cell Cycle 2010; 9:1690-6; PMID:20404504; http://dx.doi.org/10.4161/cc.9.9.11295
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 2002; 110:177-89; PMID:12150926; http://dx.doi.org/10.1016/S0092-8674(02)00833-4
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 2002; 110:163-75; PMID:12150925; http://dx.doi.org/10.1016/S0092-8674(02)00808-5
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004; 14:1296-302; PMID:15268862; http://dx.doi.org/10.1016/j.cub.2004.06.054
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12 :21-35; PMID:21157483; http://dx.doi.org/10.1038/nrm3025
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009; 10:307-18; PMID:19339977; http://dx.doi.org/10.1038/nrm2672
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 2002; 10:457-68; PMID:12408816; http://dx.doi.org/10.1016/S1097-2765(02)00636-6
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004; 6:1122-8; PMID:15467718; http://dx.doi.org/10.1038/ncb1183
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005; 307:1098-101; PMID:15718470; http://dx.doi.org/10.1126/science.1106148
- Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. Embo J 2008; 27:1932-43; PMID:18566586; http://dx.doi.org/10.1038/emboj.2008.120
- Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. Embo J 2008; 27:1919-31; PMID:18566587; http://dx.doi.org/10.1038/emboj.2008.119
- Iliuk A, Liu XS, Xue L, Liu X, Tao WA. Chemical visualization of phosphoproteomes on membrane. Mol Cell Proteomics 2012; 11:629-39; PMID:22593177; http://dx.doi.org/10.1074/mcp.O112.018010
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006; 127:125-37; PMID:16962653; http://dx.doi.org/10.1016/j.cell.2006.08.033
- Yang CS, Song CH, Lee JS, Jung SB, Oh JH, Park J, Kim HJ, Park JK, Paik TH, Jo EK. Intracellular network of phosphatidylinositol 3-kinase, mammalian target of the rapamycin/70 kDa ribosomal S6 kinase 1, and mitogen-activated protein kinases pathways for regulating mycobacteria-induced IL-23 expression in human macrophages. Cell Microbiol 2006; 8:1158-71; PMID:16819968; http://dx.doi.org/10.1111/j.1462-5822.2006.00699.x
- Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer 2006; 6:321-30; PMID:16557283; http://dx.doi.org/10.1038/nrc1841
- Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 2000; 103:253-62; PMID:11057898; http://dx.doi.org/10.1016/S0092-8674(00)00117-3
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev 2001; 15:807-26; PMID:11297505; http://dx.doi.org/10.1101/gad.887201
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol 2006; 16:1865-70; PMID:16919458; http://dx.doi.org/10.1016/j.cub.2006.08.001
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 2006; 11:859-71; PMID:17141160; http://dx.doi.org/10.1016/j.devcel.2006.10.007
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012; 4:159-65; PMID:22394614
- Serrano M. Dissecting the role of mTOR complexes in cellular senescence. Cell Cycle 2012; 11:2231-2; PMID:22714590; http://dx.doi.org/10.4161/cc.21065
- Khapre RV, Kondratova AA, Patel S, Dubrovsky Y, Wrobel M, Antoch MP, Kondratov RV. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany NY) 2014; 6:48-57; PMID:24481314