Class I cytokine receptors regulate a wide range of clinically relevant cellular processes. This family of receptors contains around 30 members including receptors for erythropoietin (EPO), prolactin (PRL), growth hormone (GH), thrombopoietin (TPO), granulocyte-macrophage colony-stimulating factor (GM-CSF), leukemia inhibitory factor (LIF), interleukin-3 (IL-3), IL-5, and IL-6.Citation1 The growth hormone receptor has been the archetype for the class I cytokine receptor family, as it was the first to be both cloned and have its extracellular domain crystal structure solved. The original paradigm for the mechanism of activation was based on biophysical and structural studies over 20 y ago showing that growth hormone binding to the extracellular domain of the receptor occurred in a sequential fashion, first binding to a high affinity site on a single receptor (site 1) and then binding to a lower affinity site (site 2) on a second receptor.Citation2 The subsequent publication of the crystal structure showed a single hormone bound to 2 receptor domains at the site 1 and site 2 positions, and also revealed the lower fibronectin III domain of the receptor locked together (site 3 or dimerization domain).Citation3 The conclusion from these studies was that hormone binding induced dimerization of the receptor and that this would bring the intracellular signaling domains together to allow association and activation of the associated janus kinases (JAKs). However, despite great progress in elucidating the structural details of ligand binding to the extracellular domain of receptors for several members of this family, the means by which these receptors activate their associated JAKs to initiate signaling had remained elusive. The original ligand-mediated activation model was undermined by several lines of evidence, particularly by studies showing that the GH receptor and other related receptors such as the PRL, EPO, and TPO receptors exist predominantly as dimers prior to ligand binding.Citation4 The question remained as to how ligand binding was able to transmit its signal through the transmembrane domain and to the intracellular domain of the pre-dimerized receptors to activate the associated kinases. Our group embarked on studies to unravel this mechanism, and recently were able to present the first complete mechanistic model of JAK2 activation by an archetypal class I cytokine receptor, the growth hormone receptor.Citation5
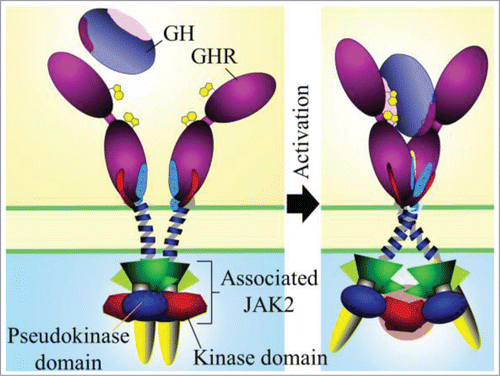
We confirmed that the GH receptor exists as a preformed dimer in vivo by using confocal microscopy anisotropy analysis at the surface of live mammalian cells. We then utilized a model system where we could lock receptors in different activation states. We did this by removing the extracellular domain of the receptor and replacing it with the dimerizing leucine zipper from the JUN transcription factor which results in a constitutively active receptor. We interrogated the role of the 12 residue linker that joins the transmembrane domain to the extracellular domain and found that as the zipper was brought closer to the membrane, the activation signal strength increased. We then monitored the relative positioning of the JAK2 binding region of the intracellular domains of the receptor by placing FRET reporters 37 amino acids below the Box1 motif in order to retain JAK2 binding to the receptor. By comparing the FRET signal from the leucine zipper construct with the weakest activation signal (containing the full 12 amino acid linker) to the construct with the strongest signal (no linker) we found that an increase in receptor signaling correlated with an increased distance between the JAK2 binding regions of the receptor, i.e., the intracellular domains were separating upon activation. We then confirmed that GH addition to its receptor induced a separation of the JAK2 binding regions by using a GH receptor construct with FRET reporters placed at the same intracellular position as before. Since the activation of a pre-dimerized receptor must be transmitting a signal through the transmembrane domain, we identified the interaction face of the transmembrane domains of the receptor in the absence of GH by cysteine crosslinking analysis and molecular modeling. Further in silico modeling showed that the transmembrane domains are in a parallel interaction in the inactive state and that transition to the active state involves a rotation resulting in a left-handed crossover dimer. This brings the residues at the extracellular juxtamembrane in close proximity, while C-termini of the transmembrane domains separate, explaining the repositioning of the intracellular domains that we observed with FRET upon activation. Previous models of JAK2 activation did not identify a separation of the JAK2 binding Box1 motifs, therefore we proposed a new model of JAK2 activation where in the inactive state of the predimerized receptor the pseudokinase domain from a JAK2 molecule that is bound to one receptor, interacts with and inhibits the kinase domain of the JAK2 bound to the other receptor. Upon activation, the intracellular domains of the receptor where the JAK2 FERM domain is bound separate, which brings the kinase domains together, facilitating trans-activation (). We were able to use FRET, molecular modeling as well as interaction and JAK2 activation assays to support this model.Citation5 The key remaining question is: does such a mechanism generalize to other class I cytokine receptors?
References
- Liongue C, Ward AC. BMC Evol Biol 2007; 7:120; PMID:17640376; http://dx.doi.org/10.1186/1471-2148-7-120
- Cunningham BC,et al. Science 1991; 254:821–5; PMID:1948064; http://dx.doi.org/10.1126/science.1948064
- de Vos AM,et al. Science 1992; 255:306–12; PMID:1549776; http://dx.doi.org/10.1126/science.1549776
- Waters MJ, et al. Biochem J 2015; 466:1-11.
- Brooks AJ, et al. Science 2014; 344:1249783; PMID:24833397; http://dx.doi.org/10.1126/science.1249783