Spermidine, a natural polyamine the intracellular level of which declines during aging, participates in multiple cellular processes such as the maintenance of genomic DNA stability, gene transcription, translation, cell proliferation, and cell survival. For instance, it is an inhibitor of histone acetyltransferases, thereby leading to epigenetic hypoacetylation of histone H3 and triggering the initiation of gene transcription.Citation1 Alteration of the acetylation status of chromatin leads to changes in gene expression and has been regarded as an important anti-aging effect by spermidine. In neurons, spermidine has been reported to function in the modulation of neuronal excitability by blocking the ion channels. Spermidine can slow down the aging process and promote stress resistance in many species, including yeast, flies, worms, mice and human, through autophagy-dependent as well as autophagy-independent pathways,Citation1 and through its neuroprotective effect against neuron damages caused by oxidative stress, inflammation and ischemia.Citation2,3
It has been somewhat controversial as to whether polyamines, including spermidine, are beneficial for the prevention and delay of the progression of neurodegenerative diseases. Choi et al. showed that spermidine has an anti-inflammatory effect against lipopolysaccharide-stimulated oxidative stress in microglia cells, which mimics the pathological stress condition in neurodegenerative disorders.Citation3 On the other hand, Lewandowski et al.Citation4 has linked the polyamine pathway to α-synuclein toxicity in the pathogenesis of Parkinson's disease (PD). In particular, a disease-related decline in the expression of the spermine/ spermidine N1-acetyltrasferase 1 (SAT1), which is the rate-limiting enzyme of the polyamine metabolic pathway, has been found in human patients. Furthermore, enhancement of SAT1 activity in a transgenic PD mouse model, which lowers the level of spermine and likely that of spermidine as well, could reduce the PD histopathology.Citation4 Yet, Büttner et al. showed just last month that spermidine treatment not only rescued the motor dysfunction and shortening of lifespan induced by α-synuclein neurotoxicity in Drosophila, but it also prevented the severe anterior CEP dopaminergic neuronal loss caused by ectopic expression of α-synuclein in C. elegans. Interestingly, the alleviation of neurotoxicity in these PD-related neurodegeneration animal models by spermidine is autophagy-dependent, indicating that autophagy activation by spermidine underlies its neuroprotective effects of PD.Citation5
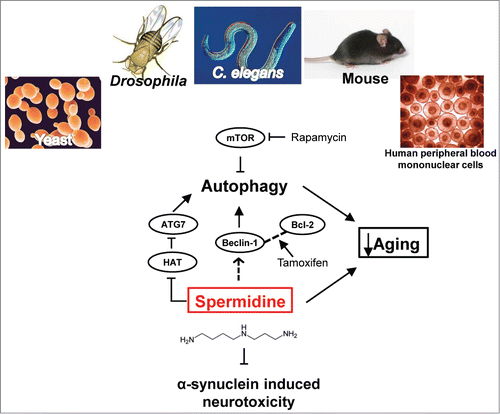
Spermidine induces autophagy not only through epigenetic regulation of the autophagy-related genes such as ATG7.Citation6 Knock-down of the autophagy gene Beclin-1/ATG6 abolished the life span extension of Drosophila by spermidine.Citation1 These studies suggest that spermidine triggers autophagy and lengthens the lifespan through mTOR-independent pathway. Besides PD, other neurodegenerative diseases hall-marked with intracellular inclusions, including Alzheimer's disease, Huntington's disease, amyotrophic lateral sclerosis (ALS) and frontotemporal lobe degeneration (FTLD), have also been reported to be associated with impairment or failure of protein degradation processes in the diseased cells. In interesting connection, the TAR-DNA binding protein-43 (TDP-43) is required for the maintenance of autophagy and TDP-43 proteinopathies in the forebrain of a FTLD-TDP mouse model could be alleviated by treatment with autophagy activators including spermidine.Citation7 In particular, the cytosolic ubiquitin-positive inclusions in the diseased neurons of the damaged mouse brain were diminished upon spermidine treatment, resulting in less severe neuron loss.Citation7 In view of the rescuing effects of spermidine in the variety of animal models of neurodegenerative diseases (Fig. 1), spermidine could serve as a potential, and likely promising, target for pharmacological development of drugs to treat different proteinopathy-induced neurodegenerative diseases through autophagy activation.
Figure 1. Schematic illustration of the targets of actions by spermidine in different pathways. The function of spermidine in anti-aging and resistance to stress is conserved in evolution. It acts through autophagy-independent pathway(s) as well as mTOR-independent autophagy pathway. By activating the autophagy, spermidine inhibits α-synuclein toxicity in two PD animal models, as demonstrated by Büttner et al.Citation5
Reference
- Eisenberg T, et al. Nature Cell Biol 2009; 11(11):1305-14; PMID:19801973; http://dx.doi.org/10.1038/ncb1975
- Minois N, et al. Cell Death Dis 2012; 3:e401; PMID:23059820; http://dx.doi.org/10.1038/cddis.2012.139
- Choi YH , Park HY. J Biomed Sci 2012; 19:31; PMID:22433014; http://dx.doi.org/10.1186/1423-0127-19-31
- Lewandowski NM, et al. Proc Natl Acad Sci U S A 2010; 107(39):16970-75; PMID:20837543; http://dx.doi.org/10.1073/pnas.1011751107
- Büttner S, et al. Cell Cycle, 2014; 13:3903-8; PMID:25483063; http://dx.doi.org/10.4161/15384101.2014.973309
- Madeo F,et al. Autophagy 2010; 6(1):160-2; PMID:20110777; http://dx.doi.org/10.4161/auto.6.1.10600
- Wang IF, et al. Proc Natl Acad Sci U S A 2012; 109(37):15024-9; PMID:22932872; http://dx.doi.org/10.1073/pnas.1206362109
