Abstract
The taxanes are used alone or in combination with anthracyclines or platinum drugs to treat breast and ovarian cancer, respectively. Taxanes target microtubules in cancer cells and modifiers of taxane sensitivity have been identified in vitro, including drug efflux and mitotic checkpoint proteins. Human epidermal growth factor receptor 2 (HER2/ERBB2) gene amplification is associated with benefit from taxane therapy in breast cancer yet high HER2 expression also correlates with poor survival in both breast and ovarian cancer. The pre-mRNA splicing factor 4 kinase PRP4K (PRPF4B), which we identified as a component of the U5 snRNP also plays a role in regulating the spindle assembly checkpoint (SAC) in response to microtubule-targeting drugs. In this study, we found a positive correlation between PRP4K expression and HER2 status in breast and ovarian cancer patient tumors, which we determined was a direct result of PRP4K regulation by HER2 signaling. Knock-down of PRP4K expression reduced the sensitivity of breast and ovarian cancer cell lines to taxanes, and low PRP4K levels correlated with in vitro-derived and patient acquired taxane resistance in breast and ovarian cancer. Patients with high-grade serous ovarian cancer and high HER2 levels had poor overall survival; however, better survival in the low HER2 patient subgroup treated with platinum/taxane-based therapy correlated positively with PRP4K expression (HR = 0.37 [95% CI 0.15-0.88]; p = 0.03). Thus, PRP4K functions as a HER2-regulated modifier of taxane sensitivity that may have prognostic value as a marker of better overall survival in taxane-treated ovarian cancer patients.
Abbreviations
| HER2 | = | human epidermal growth factor receptor 2 |
| PRP4K | = | pre-mRNA splicing factor-4 kinase |
| SAC | = | spindle assembly checkpoint |
| TMA | = | tissue microarray |
Introduction
Taxanes (docetaxel, paclitaxel and cabazitaxel) are a family of anticancer agents which function by binding to the β-tubulin subunit of assembled microtubules, leading to stabilization and an overall disruption of microtubule dynamics. The inability of a cell to properly segregate its sister chromatids during mitosis leads to prolonged spindle assembly checkpoint (SAC) activation and apoptosis.Citation1 While taxane-based regimens are commonly used as first-line therapies for many malignancies, including breast and ovarian cancer,Citation2,3 the emergence of clinical drug resistance remains the major limitation for their use. As a result, much research effort has been focused on determining both biological markers for predicting taxane response as well as the mechanisms underlying intrinsic and acquired resistance to taxane therapy.
The most common mechanism of taxane resistance encountered in vitro is the up-regulation of the ATP-binding cassette transporter, P-glycoprotein (P-gp).Citation4,5 While there is substantial evidence supporting the role of P-gp in the development of taxane resistance in vitro, the value of P-gp as a marker of taxane response in vivo remains unknown due to conflicting clinical reports.Citation6,7 Other mechanisms of resistance observed in vitro include alterations to the microtubules themselves including mutations, isotype switching and post-translation modifications of tubulin, which effect drug binding or reduces microtubule stability.Citation8 In addition, the upregulation of microtubule associate proteins (MAPs) such as MAPT/tau,Citation9 alteration in SAC function, and/or inhibition of taxane induced apoptosis can impart taxane resistance.Citation10-13 Much like P-gp, these mechanisms of resistance, while well characterized in vitro, have had limited success clinically as markers of taxane response.
In breast cancer, the amplification/overexpression of the HER2/ERBB2 gene has been widely explored for its potential application in predicting taxane response. HER2 amplification is observed in 15–20% of human breast cancers Citation14 and has been generally associated with poor survival.Citation15 In vitro, HER2 amplification has been shown to increase cellular resistance to taxanes in breast cancer cells,Citation16 while a number of clinical studies have shown HER2 amplification to correlate with increased response to taxanes, either as a single agent,Citation17,18 or as part of a combination therapy.Citation19-22 As a result, the use of HER2 as a biomarker for taxane sensitivity remains controversial, while the mechanism(s) through which HER2 signaling alter taxane sensitivity remain to be elucidated. A greater understanding of the genes acting downstream of HER2 with a more direct role in the modulation of taxane sensitivity is essential to improve our ability to predict taxane response.
In the present study, we have identified the pre-mRNA splicing factor-4 kinase (PRP4K) as a novel HER2-regulated gene in breast and ovarian cancer. In interphase cells, we previously identified PRP4K, also known as PRPF4B, as an essential pre-mRNA splicing kinase associated with the U5-snRNP,Citation23 which was later found to play a key role in spliceosomal assembly.Citation24 In mitotic cells, PRP4K has been identified as novel regulator of the SAC that plays a role in the recruitment of MPS1 kinase, MAD1 and MAD2 to the kinetochore. Loss of PRP4K expression leads to the failure of microtubule poisons to induce mitotic arrest in HeLa cells.Citation25 Furthermore, although not validated, PRP4K was identified as a potential modulator of taxane resistance in a large scale RNAi screen conducted in HCT-116 colon cancer cells.Citation26 In this study we demonstrate that decreased PRP4K protein level results in reduced sensitivity to paclitaxel in both breast and ovarian cancer cell lines, and that in vitro-derived and patient acquired taxane resistance correlates with reduced PRP4K expression. In addition, PRP4K protein expression in the biopsies of ovarian cancer patients with low HER2-expressing tumors correlates with better overall survival to treatment with platinum/taxane-based therapy. Taken together these results implicate PRP4K, through its role in the SAC, as a HER2-regulated modulator of taxane sensitivity.
Results
High PRP4K protein level correlates with HER2 amplification in human breast and ovarian tumors
Despite PRP4K being an essential pre-mRNA splicing kinase, a number of studies have variously claimed that PRP4K may be oncogenic and a possible molecular target in cancer Citation27,28 or may be a predictive biomarker of treatment response to chemotherapy; in particular to taxane, although published studies are contradictory as to whether PRP4K increases or decreases sensitivity to taxanes.Citation26,27 Thus, the role of PRP4K as a biomarker or anti-cancer drug target remains controversial. To better evaluate the possible role of PRP4K in cancer we performed immunohistochemistry analysis of PRP4K expression in breast and ovarian tumors using tissue microarrays (TMAs) composed of pre-chemotherapy surgical samples. We scored the intensity of immunohistological staining for PRP4K using a relative scale from 0 to 3, and looked for correlation with a number of commonly used prognostic and therapeutic markers of breast and ovarian cancer (). A positive correlation was observed between PRP4K and the proliferation marker Ki-67, the recently identified prognostic marker BTF4/BTN3A2,Citation29 as well as the immunological cell markers CD3 and CD68 within the high-grade serous ovarian TMA, while a positive correlation between PRP4K and HER2 (ERBB2) was observed within both the ovarian and breast TMA ().
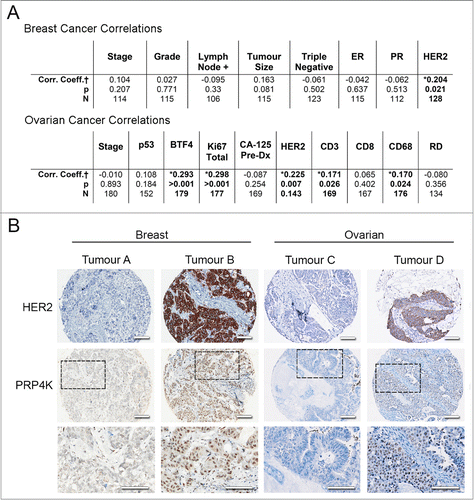
Figure 1. PRP4K correlates positively with HER2 expression in breast and ovarian tumors. (A) Breast and ovarian cancer tissue microarrays were used to correlate PRP4K protein levels with commonly used diagnostic and prognostic markers. Results are summarized in table format. † Spearman's Correlation coefficient (non-parametric); * = Significant; N= number of patients, RD = Residual Disease (B) Representative immunostaining of low (tumor A and C) and high (tumor B and D) PRP4K/HER2 in the breast and high-grade serous ovarian TMA. Dashed black boxes outline a region of the tumor in each TMA stained for PRP4K shown at higher magnification below each panel. Scale bars = 100 microns.
HER2 signaling positively regulates PRP4K
Since PRP4K levels measured by immunohistochemistry correlated with HER2 expression in breast and ovarian tumors, we next sought to determine if the positive correlation between PRP4K and HER2 observed was a result of direct regulation of PRP4K protein levels by HER2. To this end, HER2 expression was knocked down in 2 respective ovarian and breast cancer cell lines, IGROV-1 and SK-BR-3. As shown in , when cells were transduced with a HER2 targeting shRNA lentiviral vector, there is a significant reduction in both HER2 and PRP4K protein level. To determine if this regulation of PRP4K expression was mediated by active signaling through the HER2 receptor, IGROV-1 and SK-BR-3 cells were treated with the kinase inhibitor lapatinib for 48 h. In both cell lines, inhibition of HER2 signaling led to a decrease in PRP4K protein level (). Conversely, when the HER2 negative breast cancer cell line MCF-7 was transfected with constitutively active HER2,Citation30 PRP4K expression increased (), while the transfection of kinase dead HER2 Citation30 had no effect. Taken together, this data indicate that PRP4K protein expression is regulated by signaling through the HER2 receptor.
Figure 2. HER2 signaling regulates PRP4K expression. (A) IGROV-1 and SK-BR-3 cell lines were transduced with control or a HER2 targeting shRNA lentiviral vector and cultured for 48 h. Total cell lysates were prepared and subjected to Western blot analysis for HER2 and PRP4K expression. (B) IGROV-1 and SK-BR-3 cell lines were treated with 7.5 μM 0.1 μM lapatinib (respectively) for 48 h to inhibit HER2 signaling. Total cell lysates were subjected to Western blot analysis for PRP4K expression. (C) MCF-7 cells were transfected with constitutively active (CA) or kinase dead (KD) HER2 and analyzed by immunofluorescence confocal microscopy using an anti-HER2 (Red) and anti-PRP4K (Green) antibody. Nuclei were stained with DAPI. Scale bars = 10 microns.
Knockdown of PRP4K decreases the sensitivity of cancer cells to paclitaxel
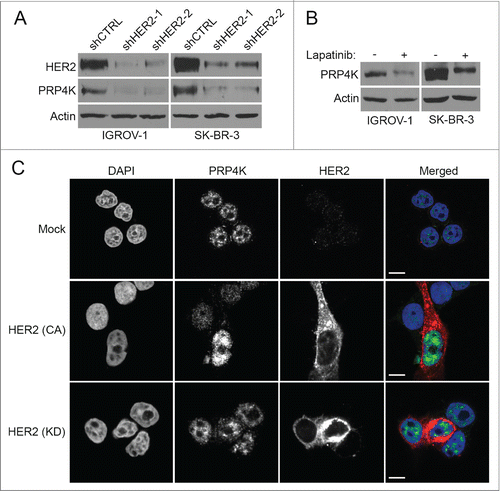
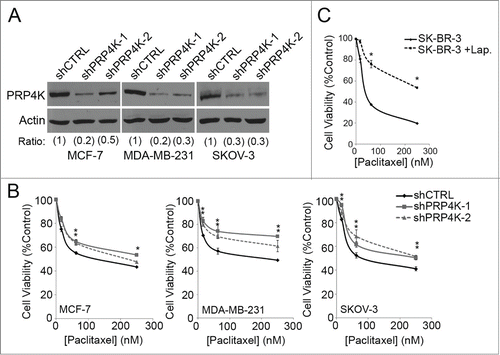
HER2 amplification has received a lot of attention for its potential application in predicting taxane response, although its use as predictive biomarker for taxane treatment remains controversial and the mechanism(s) that might govern the impact of HER2 over expression on the efficacy of taxane response, which might be used to guide therapy, remain to be elucidated. Since PRP4K is regulated by HER2 signaling, we next sought to explore the relationship between PRP4K and taxane response to determine if it could be playing a mechanistic role in the clinical link between HER2 amplification and a positive response to taxane treatment. Since PRP4K is an essential kinase, complete loss of kinase expression is lethal.Citation23 Therefore, to explore the role of PRP4K in the cellular response to paclitaxel we employed an inducible knock-down system that could be used to titrate PRP4K levels without killing the cell or impairing cell division; events that would confound interpretation of taxane sensitivity assays, and may be a factor in previously conflicting studies of taxane drug response in relation to this kinase.Citation26,27 To this end, we generated several breast (MCF-7, MDA-MB-231) and ovarian (SK-OV-3) cell lines stably expressing either a control hairpin (shCTRL), or one of 2 hairpins targeting PRP4K (shPRP4K-1 and shPRP4K-2) under the control of a doxycycline-inducible promoter. We found that the addition of 2 μg/ml doxycycline to the culture media for 96 h led to significant knockdown of PRP4K in all 3 cell lines without impacting cell viability (). Acute dosing (90 min) was chosen for the taxane response experiments, as described by Nguyen et al.,Citation31 as oppose to the commonly used chronic dosing (48 h), to more closely mimic the type of exposure a cell would experience in a clinical setting where patients are treated intravenously. Intravenous administration of paclitaxel results in a brief spike in serum taxane levels, followed by rapid drug clearance,Citation32 meaning cells would only be exposed to drug for a short period of time.Citation33 Knockdown of PRP4K expression over 96 h in each cell line resulted in significantly decreased sensitivity to paclitaxel, as measured by an alamarBlue cell viability assay following acute, low dose drug treatment (). Similarly, when PRP4K expression was decreased in SK-BR-3 cells by pre-treating for 48 h with 0.1 μM lapatinib (), cells showed decreased sensitivity to paclitaxel, as compared to the untreated control ().
Figure 3. Decreased PRP4K expression is associated with an increased cellular resistance to paclitaxel. (A) Cell lines stably expressing a tetracycline inducible shRNA targeting PRP4K were established using a lentiviral-based system. Hairpin expression was induced for 96 h with 2 μg/mL doxycycline and PRP4K levels analyzed by Western blot analysis. Band intensity was quantified by densitometry and represented as ratios with shCTRL normalized to 1. (B) PRP4K knock down was induced with doxycycline for 96 h followed by paclitaxel treatment at the indicated concentrations for 90 min. Cell viability was measured via an alamarBlue assay after a 72 h recovery in drug-free media. Data is presented as mean of triplicates ± SEM, *P < 0.05. (C) SK-BR-3 cells were treated with 0.1 μM lapatinib for 48 h to decrease PRP4K expression. The cells were then exposed to paclitaxel at the indicated concentration for 90 min. Cell viability was measured via an alamarBlue assay after a 72 h recovery in drug-free media. Data is presented as mean of triplicates ± SEM, *P < 0.05.
To try to determine the mechanism by which PRP4K regulates taxane sensitivity, PRP4K knockdown cells stably expressing a GFP-tagged histone H2B were treated with paclitaxel for 90 min and then monitored via live-cell microscopy. Under normal conditions, cells treated with taxane arrest in mitosis via activation of the SAC.Citation1 After prolonged activation (approximately 6 h), the cell typically undergoes apoptosis, or mitotic cell death (MCD) (Fig. S1, Video S1). Following the knockdown of PRP4K, taxane treated cells arrested in mitosis, but instead of undergoing apoptosis, underwent mitotic slippage, and re-entered the cell cycle (Fig. S1, Video S2). This is consistent with the previously published role of PRP4K in mitotic checkpoint control, where it was shown to recruit MPS1 kinase, MAD1 and MAD2 to the kinetochore in order to establish a functional SAC.Citation25 Thus, the decreased sensitivity of PRP4K knock-down cells to paclitaxel arises from impaired SAC activity and mitotic slippage.
Reduced PRP4K expression is associated with both in vitro derived and patient acquired resistance to taxanes
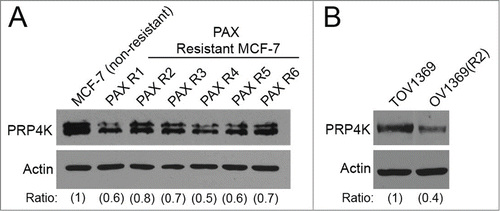
Breast and ovarian cancers can acquire resistance to taxanes, resulting in treatment failure in relapsed patients who have previously received taxane-containing chemotherapy regimens. To explore the role of PRP4K in acquired cellular resistance to paclitaxel, MCF-7 cells were maintained in several independent pools of cells cultured in increasing concentrations of paclitaxel for 15 weeks, which resulted in separate populations of cells with increased resistance to chronic exposure to 10 nM paclitaxel (). Six independent clones were isolated from these resistant pools and analyzed via Western blot for PRP4K protein level. As shown in , all of the paclitaxel resistant clones (PAXR1-PAXR6) express reduced levels of PRP4K, as compared to the starting, non-resistant population. In addition, we analyzed PRP4K levels in matched cell lines isolated from a patient diagnosed with high-grade serous ovarian cancer that had received paclitaxel as part of their treatment regimen, and subsequently relapsed and no longer responded to taxane-based therapy.Citation34 The cell line which was isolated prior to paclitaxel treatment (TOV1369) expressed a higher level of PRP4K (), as compared to the cell line that was isolated post-relapse (OV1369(R2)). Taken together, these results indicate that reduced PRP4K levels are associated with the acquisition of resistance to paclitaxel both in vitro and in vivo.
Figure 4. PRP4K expression is decreased in cells that have an acquired resistance to taxanes. (A) MCF-7 human breast cancer cells were exposed to a sub-lethal concentration of paclitaxel for 3 weeks. The concentration of paclitaxel in the growth media was tripled every 3 weeks for 15 weeks to create a resistant population. Individual clones were isolated from the resistant population, and analyzed via Western blot for PRP4K protein level. (B) TOV1369 and OV1369(R2) cell lines were isolated from an ovarian cancer patient pre-taxane treatment, and post-relapse, respectively. Whole cell lysates were prepared from each cell line and PRP4K protein levels were determined via Western blot. Band intensity was quantified by densitometry and represented as ratios.
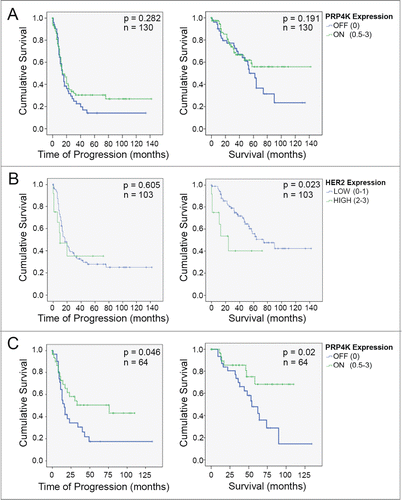
PRP4K expression correlates with better overall survival in ovarian cancer patients treated with taxanes with low HER2-expressing tumors
Given that decreased PRP4K expression is associated with reduced sensitivity to paclitaxel in vitro, that PRP4K is regulated by HER2 (a controversial predictive biomarker of taxane response), and that acquired taxane resistance is associated with reduced PRP4K levels, we next wanted to determine if PRP4K could serve as a more direct biomarker for taxane response among high-grade serous epithelial ovarian cancer patients receiving taxanes as first-line therapy. The taxane treated cohort of patients from the ovarian tumor TMA from were divided into 2 categories according to PRP4K status; PRP4K OFF (TMA score of 0) or PRP4K ON (TMA score of 0.5 to 3). The PRP4K ON subclass of patients appear to have a better disease-free and overall survival (), although this was not statistically significant. In breast and ovarian cancer, HER2/ERBB2 gene amplification is generally associated with poor survival. While HER2 protein expression by immunohistochemistry has less prognostic power than direct detection of ERBB2 gene amplification,Citation35 previous studies have demonstrated that typically only the highest scoring tumors for HER2 expression by immunohistology are found to harbour ERBB2 gene amplification.Citation36 Therefore, we rationalized that perhaps the patients with the highest expression of HER2 in our TMA analysis (i.e. score of 2 or 3 out of 3) were likely to progress early irrespective of PRP4K status because of greater ERBB2 gene amplification. Indeed, when we compared HER2 high (2-3) versus HER2 low (0-1) patients within our cohort of high-grade serous epithelial ovarian cancer patients, the HER2 high patients display a significantly worse overall survival, as compared to the HER2 low cohort (). Therefore, we repeated our analysis removing patients with the high HER2 staining from the cohort. Under these criteria, a significant increase in both time to progression (HR = 0.53 [95% CI 0.27–1.01]; p = 0.05) and overall survival (HR = 0.37 [95% CI 0.15–0.88]; p = 0.03) was observed among PRP4K ON patients, as compared to PRP4K OFF (). Thus, in the context of low HER2 expression, PRP4K is a positive prognostic biomarker for high-grade serous ovarian cancer patients treated with platinum/taxane-based therapies. The same analysis could not be done for the breast cancer TMA as those patients did not receive taxanes as part of their treatment regimen.
Figure 5. Evaluation of PRP4K as a biomarker of platinum/taxane response in high-grade serous ovarian cancer. (A) Kaplan Meier curves of overall disease free survival (left) and overall survival (right) in a cohort of 130 ovarian cancer patients treated with platinum/taxane chemotherapy. (B) Kaplan Meier curves of overall disease free survival (left) and overall survival (right) in 103 platinum/taxane treated ovarian cancer patients with either low (0–1), or high (2-3), expression of HER2. Significance (p) is indicated by Log Rank. (C) Kaplan Meier curves of overall disease free survival (left) and overall survival (right) in 64 patients (i.e. a subset of the 130 in A) with no to moderate expression of HER2. Significance (p) is indicated by Log Rank.
Discussion
Despite the challenges associated with the development of resistance, taxanes remain one of the most widely used therapies for a range of human malignancies, including breast, lung and ovarian cancer. Biomarkers which can accurately predict taxane response are needed to identify those patients that will benefit from the inclusion of taxanes into their treatment regimen, and those who will not. Numerous biomarkers have been identified that correlate with in vitro taxane resistance, however their use clinically to stratify patients according to predicted taxane response has been limited.
In this study we have identified PRP4K as a novel HER2-regulated gene, and investigated its role in the cellular response to taxanes. Immunostaining for PRP4K in human breast and ovarian cancer TMAs revealed a significant correlation between PRP4K and HER2 (), which we confirmed was a result of PRP4K expression being regulated by HER2 signaling (). While the role of HER2 in the cellular response to taxanes in vitro remains controversial, the clinical data seems to support a link between HER2 expression and positive response to taxanes in breast cancer.Citation17-22 However, due to the discrepancies between in vitro and in vivo data, the mechanism(s) linking HER2 signaling to taxane sensitivity remain unknown. To determine if PRP4K was contributing to the observed HER2 mediated taxane sensitivity, we generated breast and ovarian cancer cell lines stably expressing a doxycycline inducible hairpin targeting PRP4K. Across all 3 cell lines generated, knockdown of PRP4K led to a decrease in cellular sensitivity to paclitaxel. Furthermore, when PRP4K expression was decreased through inhibition of HER2 signaling, cellular sensitivity to paclitaxel also decreased. Together, these data support the hypothesis that HER2 signaling may contribute to taxane response in part by modulating PRP4K gene expression.
In breast cancer, HER2-based therapies target this receptor tyrosine kinase either by antibody (i.e., trastuzumab) or small molecule (i.e. lapatinib) inhibition, and the combination of HER2 pathway inhibition with taxane-based chemotherapy has shown improved outcomes in patients with HER2-positive tumors.Citation37,38 In our experiments, it is important to note that chemical inhibition of HER2 was used as a tool to manipulate PRP4K expression, and reduced taxane effectiveness when given sequentially. This is in contrast to how these drugs are administered in recent clinical trials showing efficacy of combined trastuzumab or lapatinib treatment with taxanes; where treatment is initiated with trastuzumab or lapatinib being given concurrently with taxane treatments.Citation39,40 It should also be noted that patient outcome is statistically better and patients exhibit fewer side-effects when treated periodically with trastuzumab (given concurrently with taxane) rather than with chronic dosing of lapatinib, which is taken daily with periodic taxane treatment.Citation39 Given our results, it is tempting to speculate that when lapatinib is given daily the chronic exposure to drug may reduce PRP4K expression and as a consequence reduce taxane sensitivity over time; which, could provide a potential explanation for the increased efficacy of trastuzumab periodic treatment over chronic lapatinib treatment regimes.
How PRP4K contributes to taxane sensitivity may be multifactorial considering its critical role in pre-mRNA splicing,Citation23,24 and as a consequence we are actively pursuing how changes in PRP4K expression affect alternative pre-mRNA splicing. However, to date there has been little evidence for splicing factors playing a role in taxane resistance; whereas paclitaxel induced apoptosis has been clearly demonstrated to be dependent on the prolonged activation of the SAC,Citation12,41 and disruption of checkpoint function has been shown to increase resistance to taxanes in vitro.Citation10,26 With PRP4K playing an important role in SAC function in response to microtubule poisons like nocodazole,Citation25 it is most likely that loss of PRP4K leads to taxane resistance through mitotic checkpoint failure.
Given our in vitro data indicating that low PRP4K expression, induced either by inhibition of HER2 signaling or by knock-down of PRP4K, reduces the sensitivity of breast and ovarian cancers to taxanes, we also evaluated PRP4K as a potential prognostic biomarker for outcome in ovarian cancer patients treated with taxane-containing therapies. Although we had PRP4K immunohistochemistry data on both breast and ovarian tumors, none of the patients in the breast cohort were treated first-line with taxane-based chemotherapy. Thus, we focused on 130 tumors in the ovarian cohort from patients that received taxanes as part of their treatment regime and found that again PRP4K (i.e., PRP4K ON) correlated with positive prognosis markers such as BTF4/BTN3A2 Citation29 (r = 0.24, p = 0.005, Spearman correlation) and that although not significant, a trend of better survival and increased time to progression was seen in patients with tumors expressing PRP4K (). In interpreting these results, we noted that although breast cancer patients with tumors harboring a HER2 amplification have better overall survival when their treatment regimen includes taxanes,Citation21 overall, HER2 amplification is associated with poor prognosis in breastCitation15 and ovarian cancer.Citation35,42 Given the strong correlation between HER2 expression and PRP4K within this cohort, we would expect to preferentially select for HER2 amplified tumors within the PRP4K ON group, and thus, select for patients with overall a poorer prognosis (), irrespective of treatment, as compared to the majority of patients that do not have amplified HER2. Using this rationale, we decided to remove the highest HER2-expressing tumors from the cohort and to evaluate the prognostic power of PRP4K in patients with tumors exhibiting low HER2 levels. In this cohort, PRP4K ON tumors exhibited significant increases in both time to progression (HR = 0.53 [95% CI 0.27–1.01]; p = 0.05) and overall survival (HR = 0.37 [95% CI 0.15–0.88]; p = 0.03), as compared to patients with tumors that exhibited no detectable PRP4K expression by immunohistochemistry (PRP4K OFF).
In summary, this study identifies PRP4K as a HER2-regulated modifier of taxane sensitivity, and as a potential prognostic marker for better survival in ovarian cancer patients treated with taxanes that harbour low HER2 expressing tumors. Our data also indicates that reduced PRP4K expression correlates with acquired taxane resistance post-treatment (). Thus, in addition to being a positive prognostic marker for survival in ovarian cancer patients with low or HER2-negative tumors at diagnosis, PRP4K expression could be of value in determining which patients might receive additional benefit (or not) from continued taxane treatment following relapse from previous taxane-based therapy.
Materials and Methods
Patients and tissue specimens
Ethics approval was obtained by the local institutional ethics board (Comité d’éthique de la recherche du Center hospitalier de l’Université de Montréal). Tumor samples were collected and banked following appropriate written consent from patients undergoing surgery at the Center Hospitalier de l’Université de Montréal from 1993 to 2010. An independent pathologist scored tumor grade and stage and a gynecologic oncologist scored tumor residual disease according to criteria from the International Federation of Gynecologists and Obstetricians. Less than 10% of specimens were excluded on quality, and this was not correlated to age of sample. Clinical data on progression-free interval were defined based on imaging and CA125 blood levels. Overall survival was defined as the time from surgery to death from ovarian cancer. Patients known to be alive at the time of analysis were censored to the time of their last follow-up. Patient disease free survival was calculated from the time of surgery until the first progression. Eligibility criteria for inclusion in the study were as follows: no pre-operative chemotherapeutic treatment for ovarian cancer; platinum-based post-operative chemotherapy treatment, high grade tumors, serous histopathology subtype and completed informed consent. All patients received a platinum-based chemotherapy as an initial therapy after surgery with the exception of patients who died shortly (<3 months) after surgery. Patients who died from other causes were censored at time of last follow-up.
Tissue Microarray and Immunohistochemistry
This tissue array has previously been described.Citation43 Briefly, areas of tumor were selected based on review of a hematoxylineosin-stained slide. Formalin fixed paraffin embedded tumor blocks were then biopsied using a 0.6 mm needle and resultant cores were arrayed into a grid in a recipient paraffin block using a Pathology Device Tissue Microarrayer (Pathology Devices Inc.). The tissue array contained samples from 260 ovarian cancer patients, and each patient is represented by 2 cores. After review of clinical data 61 patients were excluded from the final analysis as they did not meet the inclusion criteria. The tissue array was then sectioned, stained with hematoxylin-eosin and reviewed by an expert pathologist to confirm tumor content. Tissue arrays were sectioned at 4 μm and the slides were stained using the BenchMark XT automated stainer (Ventana Medical System Inc.). Antigen retrieval was carried out with Cell Conditioning 1 (VMSI; #950-123) for 60 min. Prediluted sheep anti-PRP4K (1:100) antibody (H143),Citation23 or Her2 (DAKO #A0485) was automatically dispensed, and the slides were incubated at 37°C for 120 min or 30 min, respectively, and developed by the UltraView DAB detection kit (VMSI#760-091). Slides were counterstained with hematoxylin (VMSI#760-2021). All sections were observed by light microscopy at 400× magnification.
Staining Quantification
Tumor sections were scanned and digitally visualized. Epithelial zones were scored according to the staining intensity (value 0 for absent, 1 for weak, 2 for moderate and 3 for high intensity). Each array was independently analyzed in a blind study by 2 independent observers. Correlation was >80%. The average of all cores with cancer from the same patient was used for the final analysis.
Cell culture
SK-BR-3, IGROV-1, MCF-7, MDA-MB-231 and SKOV-3 cell lines used were cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal calf serum, 1% penicillin/streptomycin at 37°C with 5% CO2. All cell lines were obtained originally from American Type Culture Collection (ATCC) and validated by STR profiling (DDC Medical) within 6 months of experimentation. MCF-7, MDA-MB-231 and SKOV-3 cell lines stably expressing shRNAs were cultured as above with the addition of 1 μg/mL puromycin. TOV1369 and OV1369(R2) cell lines were cultured in OSE medium (Wisent, 316-030-CL) supplemented with 10% fetal calf serum, 0.5 μg/mL amphotericin B (Life Technologies, 15290-018), and 50 μg/mL gentamicin (Life Technologies, 15750-078) at 37°C with 5%O2 and 5%CO2, as previously described.Citation34
Western Blot Analysis
Cells were lysed in ice-cold lysis buffer (20 mM Tris-HCl pH8, 300 mM KCl, 10% Glycerol, 0.25% Nonidet P-40, 0.5 mM EDTA, 0.5 mM EGTA, 1x protease inhibitors). Protein concentrations were determined using Bio-Rad Protein Reagent (Bio-Rad, 500-0006). Lysates, with exception to those ran in , were dephosphorylated with calf intestinal phosphatase (Sigma, A2356) as previously described.Citation44 This allowed for more accurate quantification as PRP4K is present in lysates in several phospho-forms (Fig. S2). Lysates were then mixed with 2× sample buffer and boiled prior to separation by SDS-PAGE and Western blot analysis with sheep anti-PRP4K antibody (H143),Citation23 rabbit anti-HER2 antibody (Cell Signaling, #2165), or mouse anti-actin antibody (Sigma, A3853). Quantification of band intensities was performed by densitometry analysis using ImageJ (NIH).
shRNA Lentiviral Transduction
To knockdown HER2 in the IGROV-1 and SK-BR-3 cell lines, HER2 targeting GIPZ Lentiviral shRNAs (shHER2-1 = clone: V3LHS_315855, shHER2-2 = clone: V3LHS_315852) were purchased from Thermos Scientific. To create the MCF7, MDA-MB-231 and SKOV3 PRP4K knockdown cell lines, PRP4K targeting TRIPZ Inducible Lentiviral Human shRNAs (Thermos Scientific) (shPRP4K-1 = clone:V2THS_47787, shPRP4K-2 = clone:V3THS_383960) were purchased. Lentivirus was obtained by co-transefection of the TRIPZshRNA, pMD2.G, pCMV-8.92, and pCMV-8.93 vectors (described previouslyCitation45) into human HEK-293T cells via calcium-phosphate transfection (Promega, E1200), according to manufacturer's directions. After 48 h, media from the transfected cells was filter sterilized using a 0.45 μ filter, and the viral media added to the target cell line for 48 h. To induce expression of the inducible TRIPZ PRP4K shRNA, 2 μg/mL doxycycline was added to culture media for 96 h with the drug being replaced every 24 h.
Immunofluorescence
Cells were plated onto sterile coverslips in a 6-well dish and allowed to adhere overnight. The cells were then transfected with the indicated plasmids using Lipofectamine2000 (Life Technologies, 11668027), according to the manufacturer's directions. HER2CA (V659E) (Addgene plasmid # 16259), and HER2KD (K753M) (Addgene plasmid #16258). Forty-eight hours post-transfection, cells were washed with PBS and fixed in 3% paraformaldehyde for 20 min. Immunolabeling was carried out as previously described.Citation46 Fluorescent images were captured with a Zeiss Cell Observer Microscope under a 63× immersion oil objective lens. Images were processed using only linear adjustments (e.g.brightness/contrast) with Slidebook (Intelligent Imaging Innovations, Boulder, CO) and Adobe Photoshop CS5.
HER2 Inhibition
SK-BR-3 and IGROV-1 cells were maintained in the presence of 0.1 μM, and 7.5 μM Lapatinib Ditosylate (Selleck, S1028), respectively, for 48 h.
In Vitro Cell Viability Assay
Paclitaxel (Sigma, T7402) was reconstituted in dimethyl sulfoxide (DMSO) and diluted in growth media so that the DMSO concentration was 0.05% or less. To evaluate paclitaxel response in vitro, 7 000 cells were plated in individual wells of a 96 well plate, and allowed to adhere for 24 h prior to incubation with the indicated concentration of paclitaxel for 90 min. Following acute paclitaxel treatment, the drug was removed and cells were allowed to recover in fresh medium for 72 h, at which point cell viability was measured using the alamarBlue cell viability assay (Life Technologies, DAL1100) according to the manufactures protocol. Fluorescence was measured using an Infinite M200 Pro plate reader (Tecan Group Ltd) 4 h after the addition of alamarBlue® reagent. To determine the effect of PRP4K knockdown on paclitaxel response, cell lines stably expressing an shRNA were incubated with 2 μg/ml doxycycline for 92 h prior to plating in the 96 well plate, and maintained in doxycycline for the remainder of the experiment.
Statistical Analysis
Survival curves were plotted using the Kaplan-Meier curve analysis and the log-rank test was used to test for significance. Univariate Cox proportional hazard models were used to estimate the hazard ratio for PRP4K expression. For the in vitro viability assays a Student's T-test was used to compare viability between cell lines. A Spearman correlation test was applied to compare biomarkers staining. All statistical analyses were done using the Statistical Package for the Social Sciences software version 11.0 (SPSS, Inc.), and statistical significance was set at p = 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1007775_Supplementary_Materials.zip
Download Zip (3.2 MB)Acknowledgments
We would like to thank the Gynecology-Oncology and Pathology services of the CHUM-Hôpital Notre-Dame for tumor procurement.
Funding
This work was funded by a Canadian Breast Cancer Foundation (CBCF)-Atlantic operating grant awarded to GD. GD is a Senior Scientist of the Beatrice Hunter Cancer Research Institute (BHCRI), and DPC was supported by a CIBC Graduate Scholarship in Medical Research trainee award from the BHCRI with funds provided by CBCF-Atlantic, and The Canadian Cancer Society, Nova Scotia Division as part of The Terry Fox Foundation Strategic Health Research Training (STIHR) Program in Cancer Research at the Canadian Institutes of Health Research (CIHR). DPC also was supported by funds to GD from ClicGear International Ltd. via the Dalhousie Medical Research Foundation's “Adopt-a-Researcher” program. Tumor banking was supported by the Banque de tissus et de données of the Réseau de recherche sur le cancer of the Fonds de la recherche en santé du Québec (FRSQ), affiliated with the Canadian Tumor Repository Network (CTRNet).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta 2008; 1785:96-132; PMID:18068131
- Lage H, Denkert C. Resistance to chemotherapy in ovarian carcinoma. Recent Results Cancer Res 2007; 176:51-60; PMID:17607916
- Choy H. Taxanes in combined modality therapy for solid tumors. Crit Rev Oncol 2001; 37:237-47; PMID:11248579
- Gerlach JH, Endicott JA, Juranka PF, Henderson G, Sarangi F, Deuchars KL, Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature 1986; 324:485-9; PMID:2878368; http://dx.doi.org/10.1038/324485a0
- Fojo AT, Menefee M. Microtubule targeting agents: basic mechanisms of multidrug resistance (MDR). Semin Oncol 2005; 32:S3-8; PMID:16360716; http://dx.doi.org/10.1053/j.seminoncol.2005.09.010
- Noguchi S. Predictive factors for response to docetaxel in human breast cancers. Cancer Sci 2006; 97:813-20; PMID:16805818
- Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst 1997; 89:917-31; PMID:9214671; http://dx.doi.org/10.1093/jnci/89.13.917
- Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003; 22:7280-95; PMID:14576838; http://dx.doi.org/10.1038/sj.onc.1206934
- Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, Ayers M, Ross JS, Zhang P, Buchholz TA, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A 2005; 102:8315-20.
- Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res 2004; 64:2502-8; PMID:15059905; http://dx.doi.org/10.1158/0008-5472.CAN-03-2013
- Takahashi T, Yamasaki F, Sudo T, Itamochi H, Adachi S, Tamamori-Adachi M, Ueno NT. Cyclin A-associated kinase activity is needed for paclitaxel sensitivity. Mol Cancer Ther 2005; 4:1039-46; PMID:16020661; http://dx.doi.org/10.1158/1535-7163.MCT-04-0282
- Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell 2003; 3:51-62; PMID:12559175; http://dx.doi.org/10.1016/S1535-6108(02)00235-0
- Masuda A, Maeno K, Nakagawa T, Saito H, Takahashi T. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol 2003; 163:1109-16; PMID:12937152; http://dx.doi.org/10.1016/S0002-9440(10)63470-0
- Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res 2008; 14:8019-26; PMID:19088018; http://dx.doi.org/10.1158/1078-0432.CCR-08-0974
- Joensuu H, Isola J, Lundin M, Salminen T, Holli K, Kataja V, Pylkkanen L, Turpeenniemi-Hujanen T, von Smitten K, Lundin J. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: a nationwide population-based study. Clin Cancer Res 2003; 9:923-30; PMID:12631589
- Yu D, Liu B, Tan M, Li J, Wang SS, Hung MC. Overexpression of c-erbB-2/neu in breast cancer cells confers increased resistance to Taxol via mdr-1-independent mechanisms. Oncogene 1996; 13:1359-65; PMID:8808711
- Baselga J, Seidman AD, Rosen PP, Norton L. HER2 overexpression and paclitaxel sensitivity in breast cancer: therapeutic implications. Oncology 1997; 11:43-8; PMID:9110342
- Di Leo A, Chan S, Paesmans M, Friedrichs K, Pinter T, Cocquyt V, Murray E, Bodrogi I, Walpole E, Lesperance B, et al. HER-2/neu as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Breast Cancer Res Treat 2004; 86:197-206; PMID:15567936; http://dx.doi.org/10.1023/B:BREA.0000036783.88387.47
- Konecny GE, Thomssen C, Luck HJ, Untch M, Wang HJ, Kuhn W, Eidtmann H, du Bois A, Olbricht S, Steinfeld D, et al. Her-2/neu gene amplification and response to paclitaxel in patients with metastatic breast cancer. J Natl Cancer Inst 2004; 96:1141-51; PMID:15292386; http://dx.doi.org/10.1093/jnci/djh198
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, Tomiak E, Al-Tweigeri T, Chap L, Juhos E, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005; 352:2302-13; PMID:15930421; http://dx.doi.org/10.1056/NEJMoa043681
- Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, Broadwater G, Goldstein LJ, Martino S, Ingle JN, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med 2007; 357:1496-506; PMID:17928597; http://dx.doi.org/10.1056/NEJMoa071167
- Camerini A, Donati S, Viacava P, Siclari O, Puccetti C, Tartarelli G, Valsuani C, De Luca F, Martini L, Cavazzana A, et al. Evaluation of HER2 and p53 expression in predicting response to docetaxel-based first-line chemotherapy in advanced breast cancer. J Exp Clin Cancer Res 2011; 30:38; PMID:21481270; http://dx.doi.org/10.1186/1756-9966-30-38
- Dellaire G, Makarov EM, Cowger JM, Longman D, Sutherland HGE, Luhrmann R, Torchia J, Bickmore WA. Mammalian PRP4 Kinase Copurifies and Interacts with Components of Both the U5 snRNP and the N-CoR Deacetylase Complexes. Mol Cell Biol 2002; 22:5141-56; PMID:12077342; http://dx.doi.org/10.1128/MCB.22.14.5141-5156.2002
- Schneider M, Hsiao HH, Will CL, Giet R, Urlaub H, Luhrmann R. Human PRP4 kinase is required for stable tri-snRNP association during spliceosomal B complex formation. Nat Struct Mol Biol 2010; 17:216-21; PMID:20118938; http://dx.doi.org/10.1038/nsmb.1718
- Montembault E, Dutertre S, Prigent C, Giet R. PRP4 is a spindle assembly checkpoint protein required for MPS1, MAD1, and MAD2 localization to the kinetochores. J Cell Biol 2007; 179:601-9; PMID:17998396; http://dx.doi.org/10.1083/jcb.200703133
- Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell 2007; 11:498-512; PMID:17560332; http://dx.doi.org/10.1016/j.ccr.2007.04.011
- Duan Z, Weinstein EJ, Ji D, Ames RY, Choy E, Mankin H, Hornicek FJ. Lentiviral short hairpin RNA screen of genes associated with multidrug resistance identifies PRP-4 as a new regulator of chemoresistance in human ovarian cancer. Mol Cancer Ther 2008; 7:2377-85; PMID:18687998; http://dx.doi.org/10.1158/1535-7163.MCT-08-0316
- Gao Q, Mechin I, Kothari N, Guo Z, Deng G, Haas K, McManus J, Hoffmann D, Wang A, Wiederschain D, et al. Evaluation of cancer dependence and druggability of PRP4 kinase using cellular, biochemical, and structural approaches. J Biol Chem 2013; 288:30125-38; PMID:24003220; http://dx.doi.org/10.1074/jbc.M113.473348
- Le Page C, Ouellet V, Quinn MC, Tonin PN, Provencher DM, Mes-Masson AM. BTF4/BTNA3.2 and GCS as candidate mRNA prognostic markers in epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 2008; 17:913-20; PMID:8398031UND; http://dx.doi.org/10.1158/1055-9965.EPI-07-0692
- Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 2004; 6:459-69; PMID:15542430; http://dx.doi.org/10.1016/j.ccr.2004.09.027
- Nguyen DM, Chen GA, Reddy R, Tsai W, Schrump WD, Cole G, Jr., Schrump DS. Potentiation of paclitaxel cytotoxicity in lung and esophageal cancer cells by pharmacologic inhibition of the phosphoinositide 3-kinase/protein kinase B (Akt)-mediated signaling pathway. J Thorac Cardiovasc Surg 2004; 127:365-75; PMID:14762343; http://dx.doi.org/10.1016/j.jtcvs.2003.09.033
- Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Lacatelli A, Bonadonna G, Egorin MJ. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 1995; 13:180-90; PMID:7799018
- Gascoigne KE, Taylor SS. How do anti-mitotic drugs kill cancer cells? J Cell Sci 2009; 122:2579-85; PMID:19625502; http://dx.doi.org/10.1242/jcs.039719
- Letourneau IJ, Quinn MC, Wang LL, Portelance L, Caceres KY, Cyr L, Delvoye N, Meunier L, de Ladurantaye M, Shen Z, et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer 2012; 12:379; PMID:22931248; http://dx.doi.org/10.1186/1471-2407-12-379
- Lassus H, Leminen A, Vayrynen A, Cheng G, Gustafsson JA, Isola J, Butzow R. ERBB2 amplification is superior to protein expression status in predicting patient outcome in serous ovarian carcinoma. Gynecol Oncol 2004; 92:31-9; PMID:14751135; http://dx.doi.org/10.1016/j.ygyno.2003.10.010
- Raspollini MR, Amunni G, Villanucci A, Castiglione F, Rossi Degl'Innocenti D, Baroni G, Paglierani M, Taddei GL. HER-2/neu and bcl-2 in ovarian carcinoma: clinicopathologic, immunohistochemical, and molecular study in patients with shorter and longer survival. Appl Immunohistochem Mol Morphol 2006; 14:181-6; PMID:16785787
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783-92; PMID:11248153; http://dx.doi.org/10.1056/NEJM200103153441101
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr., Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353:1673-84; PMID:16236738; http://dx.doi.org/10.1056/NEJMoa052122
- Alba E, Albanell J, de la Haba J, Barnadas A, Calvo L, Sanchez-Rovira P, Ramos M, Rojo F, Burgues O, Carrasco E, et al. Trastuzumab or lapatinib with standard chemotherapy for HER2-positive breast cancer: results from the GEICAM/2006-14 trial. Br J Cancer 2014; 110:1139-47; PMID:24457911
- Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A, et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 2012; 30:1989-95; PMID:22493419; http://dx.doi.org/10.1200/JCO.2011.39.0823
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature 1998; 392:300-3; PMID:9521327; http://dx.doi.org/10.1038/32688
- Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, et al. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer research 1990; 50:4087-91; PMID:1972347
- Le Page C, Marineau A, Bonza PK, Rahimi K, Cyr L, Labouba I, Madore J, Delvoye N, Mes-Masson AM, Provencher DM, et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PloS One 2012; 7:e38541; PMID:22685580; http://dx.doi.org/10.1371/journal.pone.0038541
- Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation 2000; 102:1221-6; PMID:10982534; http://dx.doi.org/10.1161/01.CIR.102.11.1221
- Bedard K, Attar H, Bonnefont J, Jaquet V, Borel C, Plastre O, Stasia MJ, Antonarakis SE, Krause KH. Three common polymorphisms in the CYBA gene form a haplotype associated with decreased ROS generation. Hum Mutat 2009; 30:1123-33; PMID:19388116; http://dx.doi.org/10.1002/humu.21029
- Salsman J, Pinder J, Tse B, Corkery D, Dellaire G. The translation initiation factor 3 subunit eIF3K interacts with PML and associates with PML nuclear bodies. Exp Cell Res 2013; 319:2554-65; PMID:24036361; http://dx.doi.org/10.1016/j.yexcr.2013.09.001
