In eukaryotic cells the nucleus, harboring the genetic information, is separated from the cytoplasm by the nuclear envelope (NE), passage through which can only occur through the envelope-embedded nuclear pore complexes (NPCs), which allow passive diffusion of molecules < 60 kDa, and mediate active translocation of cargoes up to 59 nm in diameter. Most nuclear acting proteins need to be transported actively into the nucleus by cellular transporters of the importin (IMP) superfamily. IMPs recognize specific nuclear localization signals (NLSs) on cargo proteins, and mediate their translocation through the NPC prior to release within the nucleoplasm. In the best-characterized pathway, IMPβ1 binds to basic “classical” NLSs via the adapter molecule IMPα. In mammals, there are c. Six IMPα and 20 IMPβ types, which have distinct NLS binding specificities.
Since the delineation of the basic NLSs of Simian Virus 40 Large T-antigen (T-ag) and the yeast transcription factor GAL4, distinct NLSs binding directly to IMPβ1 or one of its homologues, have been identified.Citation1 Thus far a number of approaches, including high throughput methods and prediction algorithms, have contributed to delineation of the rules governing IMPα−NLS recognition for basic type NLSs.Citation2,3 Given the dynamic nature of most nuclear processes, however, it is clear that the nuclear import of particular signaling molecules needs to be tightly regulated according to cell state. Five years post discovery of the T-ag NLS, a cluster of proximal phosphorylation sites was shown to modulate both IMPα/β1 binding and nuclear import rate;Citation4 protein kinase CK2-mediated phosphorylation c. Eleven residues upstream of the T-ag NLS enhanced transport, whereas cdk1 phosphorylation adjacent to the NLS inhibited it. This constellation of CK2 and cdk phosphorylation sites in the vicinity of basic NLSs, dubbed “the CcN motif,” was identified in a number of cellular and viral proteins, and proposed to represent a module exploited by cells and viruses alike to ensure precisely coordinated nuclear regulation of certain cargoes in response to specific stimuli.Citation5,6 The nucleocytoplasmic distribution of a number of regulatory proteins in yeast has indeed been shown to be controlled by cdk1-mediated phosphorylation adjacent to the NLS.Citation2
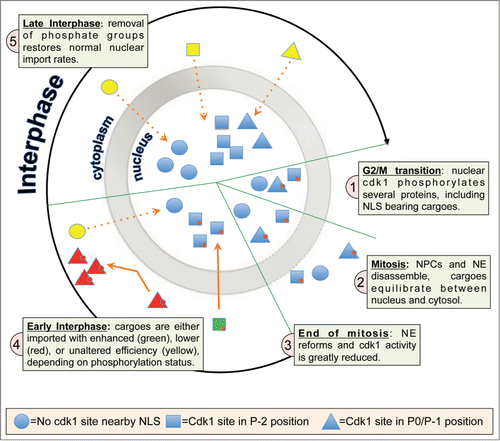
Rona et al.'s landmark studyCitation7 convincingly demonstrates that this also applies to mammalian cells, focusing on the impact of cdk1 in regulating nuclear import at the end of mitosis. After open mitosis, the segregation of genetic material to daughter cells is preceded by disassembly of the NE/NPCs, with cytosolic redistribution of most nuclear proteins, meaning that they subsequently need to be re-imported into the nucleus. Rona et al. performed a bioinformatics screen of the human proteome to identify 72 proteins bearing a cdk1 phosphorylation site adjacent to an NLS, and showed that S/T/Y residues located immediately upstream of the NLS basic core (P-1 or P0 positions), are more likely to be phosphorylated than random residues. These proteins include key regulators of the cell cycle (CCNL2), DNA repair (ATR), transcription (EP300) and epigenetic regulation (BAZ2A). Significantly, negative charge in place of the cdk1 site S/T markedly influences NLS function. A further crucial finding of the study is that the position of the cdk1 S/T relative to the NLS core determines whether phosphorylation inhibits (P-1 or P0 positions) or enhances (P-2 position) nuclear localization. Live cell imaging of a DsRed-tagged dUTPase (DUT) fusion protein shows that DUT is nuclear during S phase, but relocalizes to the cytosol in M phase due to cdk1 phosphorylation of S11 (P-1 position). At the end of mitosis when cdk1 is inactivated, DUT starts to accumulate in the nucleus, in contrast to a DUT S11 phosphomimetic mutant which is cytoplasmic regardless of the cell cycle phase, and a phosphonull mutant that accumulates much faster in the nucleus than wild-type.
In summary, cdk1 phosphorylation near NLSs can either stimulate or inhibit the rate of nuclear accumulation of specific cargoes once cellular DNA has been replicated, enabling the cell to coordinate the re-import of cargoes into the nucleus at the end of mitosis with high precision (). It will be important in the future to determine the molecular basis for differential recognition of NLSs by IMPα or other IMPs according to the precise distance of the phosphorylated cdk1 sites from the NLS, and whether cdk1 phosphorylation modulates the activity of other classes of NLSs to regulate the mammalian nucleome post cell division.
Figure 1. (A)cdk1-phosphorylation signature determines nuclear import rate after mitosis. The selective permeability of the nucleus, lost during open mitosis, is re-established at the end of mitosis through nuclear envelope and nuclear pore complex assembly. Cargoes can only be re-imported into the nucleus dependent on the presence of an efficient NLS, whose activity can be modulated by cdk1 phosphorylation (red circles). P-2 phosphorylated NLS–bearing cargoes (green) are imported rapidly, while P-1/P0 phosphorylated cargoes (red) remain cytosolic until dephosphorylated by specific phosphatases. Nonphosphorylated cargoes (yellow) are imported at a slower rate than P-2 NLS phosphorylated cargoes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kalderon D, et al. Cell 1984; 39(3 Pt 2):499–509; PMID:6096007; http://dx.doi.org/10.1016/0092-8674(84)90457-4
- Kosugi S, et al. Proc Natl Acad Sci U S A 2009; 106(25):10171-6; PMID:19520826; http://dx.doi.org/10.1073/pnas.0900604106
- Yang SN, et al. J Biol Chem 2010; 285(26):19935-46; PMID:20406804; http://dx.doi.org/10.1074/jbc.M109.079574
- Rihs HP, Peters R. EMBO J 1989; 8(5):1479-84; PMID:2670556
- Alvisi G, et al. Virology 2011; 417(2):259-67; PMID:21741668; http://dx.doi.org/10.1016/j.virol.2011.06.015
- Jans DA, et al. J Cell Biol 1991; 115(5):1203-12; PMID:1659575; http://dx.doi.org/10.1083/jcb.115.5.1203
- Rona G, et al. Cell Cycle 2014; 13(22):3551-64; PMID:25483092; http://dx.doi.org/10.4161/15384101.2014.960740
