During precursor B-cell differentiation, heavy (H) and light chain genes of an immunoglobin (Ig) molecule are somatically assembled from germline DNA. This V(D)J recombination process occurs in the bone marrow prior to antigenic challenge (). In germinal centers, variable (V) regions become the target of somatic hypermutation (SHM) in activated B-cells generating high-affinity Ig (). In mature B-cells, class-switch recombination (CSR) deletes the constant (C) μ region and replaces it with a downstream CH gene. This enables B-cells to express various Ig isotypes without affecting antigen specificity (). Once activated, B-cells differentiate into Ig-secreting plasma cells. During B-cell development, V(D)J recombination, SHM, CSR and Ig synthesis are coupled with transcriptional accessibility of the IgH loci. IgH transcription is controlled by complex functional interactions of multiple enhancers, promoters and insulators spread among the 2.5 megabases of the locus. Among them, the 3′ regulatory region (3′RR) stands out as a major player during late stages of B-cell maturation (i.e., SHM, CSR and Ig synthesis).Citation1,2
Figure 1. DNA recombination and mutations occur during B-cell maturation. RAG-induced (during VDJ recombination) and AID-induced (during CSR and SHM) DNA breaks are potential sites of oncogene translocations. The IgH 3′RR may act as an oncogene deregulator by its long range transcriptional activity. Targeting 3′RR transcriptional activity might be a therapeutic strategy for the treatment of mature B-cell lymphomas.
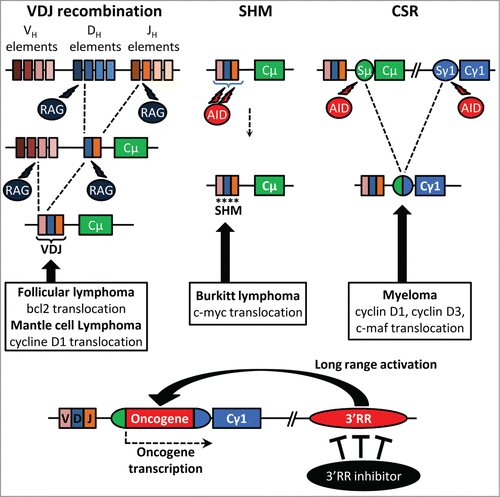
Ongoing recombination and mutation all along B-cell development make the IgH locus a hotspot for translocations. Numerous lymphomas are marked by proto-oncogene translocation into the IgH locus. Cyclin D1 and Bcl-2 translocations, found respectively in mantle cell lymphoma and follicular lymphoma, take place during the V(D)J recombination. Cyclin D1/D3 or c-maf translocations observed in myeloma are obviously linked to CSR. c-myc translocation, the typical hallmark of Burkitt lymphoma, is linked to SHM or CSR. The mouse 3′RR, located downstream of the IgH Cα gene, shares a strong structural homology with the regulatory regions located downstream of each human IgH Cα gene (Cα1 and Cα2). Mouse models exploring the role of the 3′RR in B-cell physiology and in B-cell malignancies should provide useful indications about the pathophysiology of human B-cell proliferations. Convincing demonstration of the key contribution of the 3′RR in mature B-cell lymphomagenesis has been done by transgenic animal models. c-myc-3′RR transgenics developed Burkitt lymphoma-like proliferation,Citation3 and the knock-in of a 3′RR cassette upstream of the endogenous c-myc gene induced B-cell lymphomas.Citation4 Interestingly the phenotype of lymphoproliferations induced by the c-myc-3′RR transgene is affected by the presence of associated mutations in key cell cycle dependent genes such as p53 or Cdk4.Citation5
Knock-out models have clarified the functions of the 3′RR as essential for high-rate IgH transcription at the plasma cell stage.Citation2 3′RR may thus be a potent activator of IgH-translocated oncogene transcription, even when the breakpoints lie several hundred kb away from the 3′RR. Long-range interactions between the 2 regions of chromatin, through formation of a loop structure, constitute an important mechanism of normal and abnormal gene transcription regulation by the 3′RR. Studies have reported interactions between the 3′RR and the IgH variable region in normal and lymphomagenetic contexts. Therefore, targeted inhibition of the 3′RR could theoretically provides a therapeutic strategy for the treatment of a wide range of mature B-cell lymphomas. However, the first step before considering the 3′RR as a potential suitable target for anti-lymphoma pharmacological therapy is to demonstrate the innocuousness of such an approach, and notably the absence of potent adverse effect on normal immune and inflammatory B-cell mediated responses. Induction of negative alterations on the physiological anti-lymphoma immune or inflammatory networks would be obviously a counterproductive approach. We have recently tested this prerequisite by investigating the in vivo pristane-induced inflammatory response in 3′RR-deficient BALB/c and wt BALB/c mice.Citation6 The lack of the 3′RR in BALB/c mice has no significant effect on the incidence, the kinetic of development and the cellular composition (IgM+IgD+ B-cells, CD4+ T cells, CD8+ T cells, monocyte/macrophage cells) of peritoneal ascites. Moreover ascite pro- and anti-inflammatory cytokine levels (IL-6, IL-21, IL-12/23, TNF-α IL-10, interferon-γ) are unaffected by the 3′RR-deficiency. Thus, a fully functional 3′RR is dispensable for the efficient recruitment of immune cells and the development of a normal inflammatory response in the in vivo pristane-induced inflammatory model.
In conclusion, the 3′RR is considered as a major lymphoma oncogene deregulator,Citation3-5 and its deletion has no effect on immune and inflammatory responses in the pristane mouse model. It is, thus, tempting to speculate that the 3′RR might be considered as a potential suitable target for anti-lymphoma pharmacological therapy without significant impact on the normal immune and inflammatory networks. Previous results have reported that the 3′RR is a sensitive immunological target and that 3′RR activation and transcriptional activity can be altered by a diverse range of chemicals, including ones with anti-carcinogenic properties such as isothiocyanates,Citation7 strengthening the hypothesis that altering 3′RR activity by chemicals could modulate the occurrence and severity of lymphomas. A limitation of the pristane mouse model is that inflammation is restricted to the peritoneal cavity. It is of evidence that other mouse models of inflammatory reactions must be tested before definitive validation of this hypothesis. Finally given the strong sequence homology between human and mouse 3′RR enhancers, mouse models could reveal useful tools for an in vivo study of lymphoma treatments based on IgH 3′RR down-regulation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Vincent-Fabert C, et al. Blood 2010; 116:1895–8; PMID:20538806; http://dx.doi.org/10.1182/blood-2010-01-264689
- Rouaud P, et al. J Exp Med 2013; 210:1501-7; PMID:23825188; http://dx.doi.org/10.1084/jem.20130072
- Truffinet V, et al. J Immunol 2007; 179:6033-42; PMID:17947677; http://dx.doi.org/10.4049/jimmunol.179.9.6033
- Wang J, et al. J Biol Chem 2006; 280:12766-73; http://dx.doi.org/10.1074/jbc.M412446200
- Rouaud P, et al. Oncotarget 2012; 3:586-93; PMID:22592113
- Saad F, et al. Oncoscience 2014; 1:591-8; PMID:25594069
- Henseler RA, et al. Toxicology 2009; 261:9-18; PMID:19447539; http://dx.doi.org/10.1016/j.tox.2009.03.015
