Abstract
Since their initial discovery, the intriguing proteins of the +TIP network have been the focus of intense investigation. Although many of the individual +TIP functions have been revealed, the capacity for +TIP proteins to regulate each other has not been widely addressed. Importantly, recent studies involving EBs, the master regulators of the +TIP complex, and several TOG-domain proteins have uncovered a novel mechanism of mutual +TIP regulation: allosteric interactions through changes in microtubule structure. These findings have added another level of complexity to the existing evidence on +TIP regulation and highlight the cooperative nature of the +TIP protein network.
+TIPs Form a Dynamic Protein Network at Microtubule Plus-Ends
Microtubules (MTs) are intrinsically polar filaments with 2 functionally distinct ends: the plus-end and the minus-end.Citation1,2 The polar nature of MTs is vital to their numerous functions in the cell, ranging from directional cell migration to cell division. In cells, the minus-ends of MTs are often anchored near the cell center by MT organizing centers (MTOCs),Citation3 while the plus-ends are oriented toward the cell edge and undergo phases of growth and shrinkage termed dynamic instability.Citation4,5 Plus-ends of MTs are hubs of activity that serve as a platform for the accumulation of a unique class of MT-associated proteins (MAPs). These proteins, known as MT plus-end tracking proteins (+TIPs), are essential regulators of MT dynamics and MT-based processes.Citation6 An understanding of the organization and regulation of this complex protein network is far from complete.
Within the +TIP network, EBs, a highly conserved family of +TIPs, are noted for their ability to autonomously recognize a transient feature characteristic of growing MT ends.Citation7 MTs grow by addition of GTP-bound tubulin subunits; once incorporated into the MT polymer, β-tubulin-bound GTP undergoes GTP-hydrolysis. In this way, growing MT ends maintain a ‘cap’ of GTP-tubulin, thought to provide stability against MT depolymerization. Recent work suggests that the specific localization of EBs to the ends of growing microtubules is due to their capacity to sense the nucleotide-dependent structure of growing MT ends.Citation8-10
Given their autonomous plus-end localization, EBs are particularly suited to serve as recruiters of other +TIP proteins to the growing MT ends (reviewed inCitation6). Indeed, to achieve their specific comet-like plus-end localization, the majority of +TIPs rely on direct binding to EBs through well characterized SxIP-motif or CAP-Gly domain interactions.Citation11,12 Thus, EBs have emerged as the “master regulators” of the +TIP network. Yet, the interrelationships between the diverse families of +TIP proteins are extensive, and their regulation of each other at MT tips is not well characterized. This review highlights recent efforts to investigate the cooperativity between several +TIPs and EBs, which uncovered an exciting new mode of +TIP-EB communication: allosteric interactions through modulation of the microtubule structure itself.
Allosteric Interaction Between EB and XMAP215 at MT Plus Ends
MT dynamics, and the proteins which regulate them, are exceedingly complex. MTs polymerized in vitro are different from those formed in cells,Citation13,14 indicating that we have yet to understand the scope of interactions that take place in cells. For example, MTs in vitro grow significantly slower than those in cells.Citation7,15,16 In vitro, addition of individual +TIP proteins, even the potent MT polymerase XMAP215, cannot account for the high MT growth rates observed in cells.Citation17 However, recent work has shown that cellular MT growth rates can be recapitulated in vitro through combined effects of 2 +TIP proteins: XMAP215 and EB1.Citation15
Interestingly, the high MT growth rates achieved by collective effects of XMAP215 and EB1 were synergistic, with growth promotion far exceeding the effects of either protein alone.Citation15 Even more striking was the finding that the synergistic effects of XMAP215 and EB1 on MT dynamics were not based on direct interactions between the 2 proteins; rather, they occurred allosterically through the MT end. EBs likely function to induce a structural change at the growing plus-ends of MTs, which results in straightening of protofilaments and/or a change in individual tubulin dimer curvature.Citation15 As tubulin-binding TOG domains of XMAP215 preferentially bind the curved conformation of tubulin dimers.Citation18 straightening of tubulin subunits at the microtubule end is expected to accelerate the polymerase activity of XMAP215. This result is additionally supported by the finding that taxol, which has been reported to induce protofilament straighteningCitation19 also displays synergistic effects on MT growth in combination with XMAP215.
The ability of EBs to modify the microtubule structure has been previously reported; in vitro studies of MTs grown in the presence of EB1 or its S. pombe homolog Mal3 have found MT lattices with preferentially 13-protofilament structures, as well as a higher proportion of A-lattice structures.Citation9,20,21 Given its localization at the interface between protofilaments in close proximity of the GTP-binding site,Citation9 and reports of it serving as a ‘maturation’ factor at the microtubule end,Citation22 EB1 is likely to accelerate the structural transitions accompanying GTP hydrolysis.Citation23 In such ways EBs encode MT structural changes recognized by XMAP215, indicating that regulatory mechanisms at the MT tips are more varied than previously thought.
Allosteric Regulation of EB Localization at the MT Lattice
The capacity for EBs to autonomously track MT plus-ends in vitro implies that other proteins are not needed in the determination of EB localization at MTs. Initial examination revealed that the presence of other +TIP proteins did not alter EB plus-end localization (Citation7,15,24 and others). However, the list of +TIP proteins is ever growing,Citation25 and the majority of +TIPs have yet to be tested for a potential role in the regulation of EBs at MTs and, thus, the establishment of the +TIP network.
A novel study on the role of CLASPs in the regulation of EB proteins has revealed that this family of +TIPs is a major determinant of exclusive EB plus-end localization. Both in cells and in vitro, EB localization was significantly altered in the absence of CLASPs, with EBs localizing along the entire MT lattice in addition to their normal enrichment at MT plus-endsCitation26 (). Similarly to the findings on studies between XMAP215 and EB1,Citation15 interactions between CLASP and EB were not required for CLASP-dependent regulation of EB at MTs.Citation26 The data presented suggests that CLASPs influence the MT lattice itself, in turn reducing EB binding along the length of MTs polymerized in the presence of CLASPs.
Figure 1. Altered EB localization at microtubules in CLASP-depleted cells. (A and B) Immunofluorescence images of A7r5 cells stained for α-tubulin31 and EB3 (green). (A) EB3 localizes to MT plus-ends in NT control cells. (B) EB3 extensively coats the MT lattice, in addition to its normal plus-end localization, in CLASP-depleted cells. CLASP-depletion is a combination of siRNA against CLASP1 and CLASP2. (A and B) Merge zoomed region is indicated by boxed corners in Merge. White box indicates scale bar.
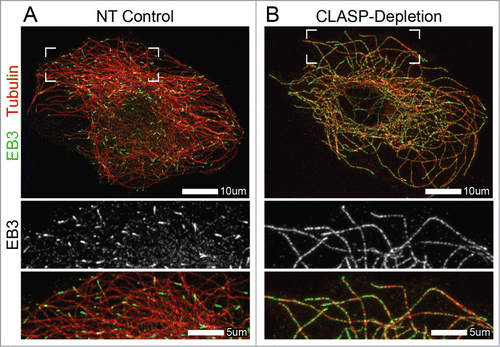
Interestingly, like XMAP215, CLASPs are also members of the XMAP215/Dis1 family of TOG-domain containing proteins.Citation27 Although CLASP TOG domains have a distinct structure,Citation28 it is the interactions of these TOGs with MTs that change the microtubule lattice, potentially by promoting a particular GTP-hydrolysis state of tubulin, leading to specific restriction of high-affinity EB-binding sites to the MT growing end.Citation26 Whether and how this new function of CLASPs relates to their reported role in promoting MT rescuesCitation29 remains an intriguing question. Although experiments involving XMAP215 and EB1 showed that XMAP215 had no effect on the plus-end localization of EB1,Citation15,22,30 the capacity of XMAP215 to regulate EB lattice binding has not been addressed to date. Further studies are necessary to determine if allosteric interactions with EBs are unique to the TOG-containing family of proteins.
Concluding Remarks
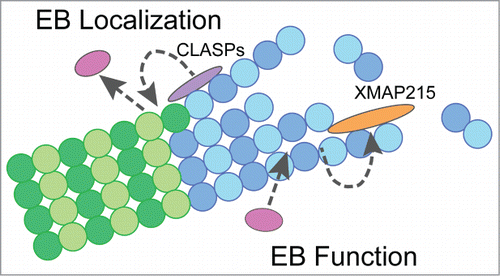
The ubiquitous nature of +TIP proteins and their high level of involvement in countless fundamental cellular processes have lead to intense investigation into +TIP protein function and regulation. However, many important questions remain unanswered, including how +TIP proteins work together and regulate each other's function. Recent investigations have revealed novel, allosteric mechanisms by which individual +TIPs encode the MT lattice with structural features that can be recognized by other +TIPs. Specifically, multiple TOG-domain containing proteins and EBs interact allosterically by promoting specific MT lattice structures, resulting in proper EB localization and enhanced dynamics of MT ends (). These studies contribute to a better understanding of the regulation governing the unique behavior of EBs and have major implications for understanding the establishment of the +TIP network.
Figure 2. Model for allosteric interactions between EBs and TOG-containing proteins. (A) Model depicting the role of TOG-containing proteins, CLASPs and XMAP215, in allosteric interactions with EB proteins. Gray dotted-arrows indicate allosteric mechanisms at MTs involved in the regulation of EB localization (CLASPs) and microtubule dynamics (EB & XMAP215). Interactions between +TIP proteins are not shown in this model. Purple = CLASPs, orange = XMAP215, pink = EBs, dark blue = GTP-tubulin, light blue = GDP-tubulin after hydrolysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by National Institutes of Health grant GM078373 (to IK), American Heart Association grant-in-aid 13GRNT16980096 (to IK), and American Heart Association pre-doctoral fellowship 12PRE12040153 (to ADG).
References
- Fan J, Griffiths AD, Lockhart A, Cross RA, Amos LA. Microtubule minus ends can be labelled with a phage display antibody specific to α-tubulin. J Mol Biol 1996; 259:325–30; PMID:8676371
- Mitchison TJ. Localization of an exchangeable GTP binding site at the plus end of microtubules. Science 1993; 261:1044–7; PMID:8102497
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 1997; 13:83–117; PMID:9442869
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature 1984; 312:237–42; PMID:6504138
- Mitchison T, Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature 1984; 312:232–7; PMID:6504137
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 2008; 9:309–22; PMID:18322465; http://dx.doi.org/10.1038/nrm2369
- Bieling P, Laan L, Schek H, Munteanu EL, Sandblad L, Dogterom M, Brunner D, Surrey T. Reconstitution of a microtubule plus-end tracking system in vitro. Nature 2007; 450:1100–5; PMID:18059460
- Maurer SP, Bieling P, Cope J, Hoenger A, Surrey T. GTPgammaS microtubules mimic the growing microtubule end structure recognized by end-binding proteins (EBs). Proc Natl Acad Sci U S A 2011; 108:3988–93; PMID:21368119; http://dx.doi.org/10.1073/pnas.1014758108
- Maurer SP, Fourniol FJ, Bohner G, Moores CA, Surrey T. EBs recognize a nucleotide-dependent structural cap at growing microtubule ends. Cell 2012; 149:371–82; PMID:22500803; http://dx.doi.org/10.1016/j.cell.2012.02.049
- Zanic M, Stear JH, Hyman AA, Howard J. EB1 recognizes the nucleotide state of tubulin in the microtubule lattice. PloS one 2009; 4:e7585; PMID:19851462; http://dx.doi.org/10.1371/journal.pone.0007585
- Honnappa S, Okhrimenko O, Jaussi R, Jawhari H, Jelesarov I, Winkler FK, Steinmetz MO. Key interaction modes of dynamic +TIP networks. Molecul Cell 2006; 23:663–71; PMID:16949363
- Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell 2009; 138:366–76; PMID:19632184; http://dx.doi.org/10.1016/j.cell.2009.04.065
- McEwen B, Edelstein SJ. Evidence for a mixed lattice in microtubules reassembled in vitro. J Mol Biol 1980; 139:123–45; PMID:7411629
- Wade RH, Chretien D. Cryoelectron microscopy of microtubules. J Structural Biol 1993; 110:1–27; PMID:8494670
- Zanic M, Widlund PO, Hyman AA, Howard J. Synergy between XMAP215 and EB1 increases microtubule growth rates to physiological levels. Nat Cell Biol 2013; 15:688–93; PMID:23666085; http://dx.doi.org/10.1038/ncb2744
- Walker RA, O'Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol 1988; 107:1437–48; PMID:3170635; http://dx.doi.org/10.1083/jcb.107.4.1437
- Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell 2008; 132:79–88; PMID:18191222; http://dx.doi.org/10.1016/j.cell.2007.11.043
- Ayaz P, Ye X, Huddleston P, Brautigam CA, Rice LM. A TOG:alphabeta-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science 2012; 337:857–60; PMID:22904013; http://dx.doi.org/10.1126/science.1221698
- Elie-Caille C, Severin F, Helenius J, Howard J, Muller DJ, Hyman AA. Straight GDP-tubulin protofilaments form in the presence of taxol. Curr Biol 2007; 17:1765–70; PMID:17919908; http://dx.doi.org/10.1016/j.cub.2007.08.063
- Vitre B, Coquelle FM, Heichette C, Garnier C, Chretien D, Arnal I. EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat Cell Biol 2008; 10:415–21; PMID:18364701; http://dx.doi.org/10.1038/ncb1703
- des Georges A, Katsuki M, Drummond DR, Osei M, Cross RA, Amos LA. Mal3, the Schizosaccharomyces pombe homolog of EB1, changes the microtubule lattice. Nat Structural Mol Biol 2008; 15:1102–8; PMID:18794845; http://dx.doi.org/10.1038/nsmb.1482
- Maurer SP, Cade NI, Bohner G, Gustafsson N, Boutant E, Surrey T. EB1 accelerates two conformational transitions important for microtubule maturation and dynamics. Curr Biol 2014; 24:372–84; PMID:24508171; http://dx.doi.org/10.1016/j.cub.2013.12.042
- Alushin GM, Lander GC, Kellogg EH, Zhang R, Baker D, Nogales E. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 2014; 157:1117–29; PMID:24855948; http://dx.doi.org/10.1016/j.cell.2014.03.053
- Montenegro Gouveia S, Leslie K, Kapitein LC, Buey RM, Grigoriev I, Wagenbach M, Smal I, Meijering E, Hoogenraad CC, Wordeman L, et al. In vitro reconstitution of the functional interplay between MCAK and EB3 at microtubule plus ends. Curr Biol 2010; 20:1717–22; PMID:20850319; http://dx.doi.org/10.1016/j.cub.2010.08.020
- Jiang K, Toedt G, Montenegro Gouveia S, Davey NE, Hua S, van der Vaart B, Grigoriev I, Larsen J, Pedersen LB, Bezstarosti K, et al. A Proteome-wide screen for mammalian SxIP motif-containing microtubule plus-end tracking proteins. Curr Biol 2012; 22:1800–7; PMID:22885064; http://dx.doi.org/10.1016/j.cub.2012.07.047
- Grimaldi AD, Maki T, Fitton BP, Roth D, Yampolsky D, Davidson MW, Svitkina T, Straube A, Hayashi I, Kaverina I. CLASPs are required for proper microtubule localization of end-binding proteins. Dev Cell 2014; 30:343–52; PMID:25117684; http://dx.doi.org/10.1016/j.devcel.2014.06.026
- Al-Bassam J, Chang F. Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP. Trend Cell Biol 2011; 21:604–14; PMID:21782439; http://dx.doi.org/10.1016/j.tcb.2011.06.007
- Leano JB, Rogers SL, Slep KC. A cryptic TOG domain with a distinct architecture underlies CLASP-dependent bipolar spindle formation. Structure 2013; 21:939–50; PMID:23727231; http://dx.doi.org/10.1016/j.str.2013.04.018
- Al-Bassam J, Kim H, Brouhard G, van Oijen A, Harrison SC, Chang F. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev Cell 2010; 19:245–58; PMID:20708587; http://dx.doi.org/10.1016/j.devcel.2010.07.016
- Nakamura S, Grigoriev I, Nogi T, Hamaji T, Cassimeris L, Mimori-Kiyosue Y. Dissecting the nanoscale distributions and functions of microtubule-end-binding proteins EB1 and ch-TOG in interphase HeLa cells. PloS one 2012; 7:e51442; PMID:23251535; http://dx.doi.org/10.1371/journal.pone.0051442
- Buey RM, Sen I, Kortt O, Mohan R, Gfeller D, Veprintsev D, Kretzschmar I, Scheuermann J, Neri D, Zoete V, et al. Sequence determinants of a microtubule tip localization signal (MtLS). J Biol Chem 2012; 287:28227–42; PMID:22696216; http://dx.doi.org/10.1074/jbc.M112.373928
