It is often overlooked that genes that play well-characterized, essential roles during one stage of the cell cycle may also perform completely unrelated, but still critical functions during other cell cycle stages. Alternate functions of well-studied proteins are often difficult to define because they are outside the area of expertise of laboratories that focus on pathways and processes in which the protein serves its canonical role. However, a full understanding of the roles a protein plays throughout the cell cycle is critical for accurate interpretation of loss of function and overexpression studies that occur over multiple cell cycles, particularly experiments in whole animals during development and aging.
Mitotic arrest deficient 1 (Mad1) was first identified as one of the genes essential for the mitotic checkpoint (also known as the spindle assembly checkpoint), which prevents chromosome missegregation and aneuploidy by delaying anaphase onset until all chromosomes have made stable attachments to spindle microtubules. We have focused our attention on this protein because overexpression of Mad1 is common in tumors and is a marker of poor prognosis.Citation1 The function of Mad1 during mitosis has been well studied and is largely dependent on its association with another mitotic checkpoint component, Mad2. Mad1 accumulates at kinetochores on unattached chromosomes that have not yet made stable attachments to spindle microtubules and would therefore be randomly segregated if the cells entered anaphase. At unattached kinetochores, Mad1 recruits and converts Mad2 from an inactive, open form into an active, closed form that inhibits the Anaphase Promoting Complex/Cyclosome (APC/C) bound to its specificity factor Cdc20.Citation2
Although the function of Mad1 in mitosis has been well studied, Mad1 is expressed throughout the cell cycle and its protein levels do not exhibit cell cycle regulation.Citation1 Previous evidence indicated that Mad1 interacts with Mad2 throughout the cell cycle.Citation2 In interphase, both Mad1 and Mad2 are associated with the nuclear pore complex. Nuclear pore binding stabilizes both proteins and helps to scaffold production of APC/C-Cdc20 inhibitors during interphase, which delays activation of APC/C-Cdc20 in mitosis.Citation3 It remains unclear whether nuclear pore-associated pools of Mad1 and Mad2 perform functional roles during interphase in vertebrates.
Recently we identified an unsuspected Golgi-localized pool of Mad1 ().Citation4 Golgi localization of Mad1 was confirmed by immunofluorescence experiments and cell fractionation. The perinuclear Mad1 signal dispersed after treatment with the microtubule poison vinblastine or with an inhibitor of protein trafficking, Brefeldin A, both of which cause disassembly of the Golgi. Transient and stable depletion of Mad1 removed the Golgi localized pool. Interestingly, unlike kinetochore and nuclear pore bound pools of Mad1, Golgi associated Mad1 is independent of Mad2 ().
Figure 1. Mad1 localizes to the Golgi where it regulates secretion of α5 integrin and cell migration. (Left) Unlike Mad1 localization to the nucleus and nuclear envelope, the localization of Mad1 on the Golgi is independent of Mad2. Golgi-associated Mad1 facilitates the secretion of α5-integrin, which along with its binding partner β1-integrin, forms the cell surface receptor for the extracellular matrix (ECM) protein fibronectin. (Right) Down-regulation of Mad1 causes accumulation of α5-integrin in the Golgi and decreases cell surface localization of α5-integrin and migration on fibronectin.
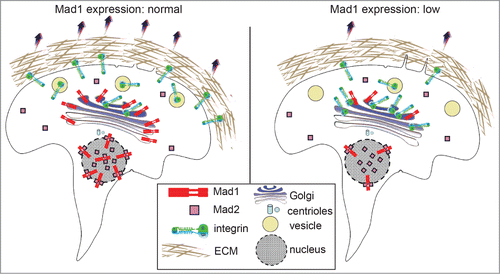
To determine whether Mad1 functions in secretion at the Golgi, we generated several cell lines in which Mad1 expression was stably knocked down (Mad1-KD cells). Previous studies have identified the proteins required for global secretion, which did not include Mad1.Citation5 Consistent with this, we found that the depletion of Mad1 did not affect secretion of VSVG or EGFR. However, testing of a variety of additional secretory proteins revealed that Mad1 knockdown results in impaired secretion of α5 integrin. In complex with β1 integrin, α5 integrin serves as a key molecule on the plasma membrane to anchor cells to the extracellular matrix (ECM) component fibronectin. In Mad1-KD cells, the α5 integrin subunit was enriched in the Golgi and showed less accumulation at the cell surface than in wild type cells ().
The defects in α5 integrin secretion suggested that Mad1-KD cells exhibit impaired cellular adhesion and migration on fibronectin. Consistent with this, fewer Mad1-KD cells adhered to and spread on fibronectin coated plates compared to wild type cells. Mad1-KD cells also exhibited impaired migration on fibronectin in cell culture wounding and transwell migration assays.Citation4 These effects were not due to decreased proliferation, and were also apparent in single cell migration tracking assays. Overexpression of Mad1 enhanced migration on fibronectin, further supporting a role for Mad1 in secretion of α5 integrin. Notably, cells depleted of Mad2 did not show defects in secretion of α5 integrin or spreading on fibronectin.Citation4
In the future, it will be important to gain a mechanistic understanding of Mad1 localization to the Golgi and its regulation of integrin secretion. It will also be of interest to determine whether Mad1 upregulation, which frequently occurs in human breast cancer, potentiates cellular migration and metastasis.
Since Mad1 plays a well-established role in the mitotic checkpoint, mouse models expressing altered levels of Mad1 (and multiple other mitotic checkpoint genes) have been used to assess the effects of chromosome missegregation and aneuploidy on tumorigenesis. Our data demonstrating a role for Mad1 in secretion and cellular motility highlight the fact that proteins that are present throughout the cell cycle have the potential to participate in additional functions that may influence tumor phenotype. With respect to aneuploidy, certain animals with mitotic checkpoint defects are tumor prone, others are not, and some actually exhibit fewer tumors than controls, depending on the specific genetic mutations involved.Citation6,7 This highlights the more general caveat that unknown functions during other stages of the cell cycle may have a significant impact on phenotypic outcomes of targeted genetic manipulations.
References
- Ryan SD, et al. Proc Natl Acad Sci U S A 2012; 109:E2205–14; PMID:22778409; http://dx.doi.org/10.1073/pnas.1201911109
- Chen RH, et al. Mol Biol Cell 1999; 10:2607–18; PMID:10436016; http://dx.doi.org/10.1091/mbc.10.8.2607
- Rodriguez-Bravo V, et al. Cell 2014; 156:1017–31; PMID:24581499; http://dx.doi.org/10.1016/j.cell.2014.01.010
- Wan J, et al. Curr Biol 2014; 24:2687–92; PMID:25447996; http://dx.doi.org/10.1016/j.cub.2014.09.052
- Simpson JC, et al. Nat Cell Biol 2012; 14:764–74; PMID:22660414; http://dx.doi.org/10.1038/ncb2510
- Zasadil LM, et al. Semin Cell Dev Biol 2013; 24:370–9; PMID:23416057; http://dx.doi.org/10.1016/j.semcdb.2013.02.001
- Ricke RM, et al. Trends Genet 2008; 24:457–66; PMID:18675487; http://dx.doi.org/10.1016/j.tig.2008.07.002
