One of the most universal environmental conditions that impacts longevity is dietary restriction (DR). DR is a reduction in caloric uptake without malnutrition; DR can increase health and life span in many organisms, from yeast to invertebrates and mammals. Studies in C. elegans have identified novel genetic regulators of DR, including transcription factors (TFs) and environmental sensors, such as pha-4, skn-1, ampk, and hsf-1. In addition, our group uncovered for the first time a role for the ubiquitin pathway in DR-mediated longevity by identifying the C. elegans wwp-1 HECT E3 ligase and its associated E2 ubiquitin conjugating enzyme, ubc-18, as positive regulators of lifespan in response to DR.Citation1 E2 and E3 enzymes facilitate the ubiquitylation of substrate proteins to alter their function in various ways. Therefore, the identification of targets of UBC-18/WWP-1 would yield an understanding of how this E2/E3 complex regulates the response to nutrient intake and longevity in worms.
To identify substrates of WWP-1 that regulate DR-induced longevity, we performed a targeted RNAi screen by selecting worm orthologues of known ubiquitylated substrates of the mammalian WWP-1 orthologues- WWP1, WWP2, and ITCH.Citation2 None of the 12 genes we analyzed was able to extend lifespan in wild-type (WT) worms when knocked-down. However, we found that loss of one gene, the Krüppel-like TF klf-1, was able to suppress the extended longevity of a DR genetic model (eat-2 mutant animals) while not affecting the lifespan of WT worms. Like wwp-1, we found that klf-1 was essential for the DR-longevity response, and that overexpression of klf-1 in the intestines, a site where longevity cues are expressed, led to lifespan extension dependent on wwp-1. Using biochemical approaches, we were able to demonstrate a direct physical interaction between WWP-1 and KLF-1 that results in the multi-monoubiquitylation of KLF-1 both in vitro and in vivo.Citation2 Together, our data support a model in which modulation of KLF-1 by WWP-1 regulates DR-mediated longevity. While the mechanism through which KLF-1 ubiquitylation drives lifespan extension is not known, it is reasonable to speculate that multi-monoubiquitylation modifies KLF-1 transcriptional output, possibly altering how nutrient stores are utilized in the worm. Given that wwp-1 is evolutionarily conserved and that mammalian WWP-1 orthologues target multiple KLFs,Citation3 we can speculate that a similar signaling pathway regulates DR-induced longevity in higher organisms.
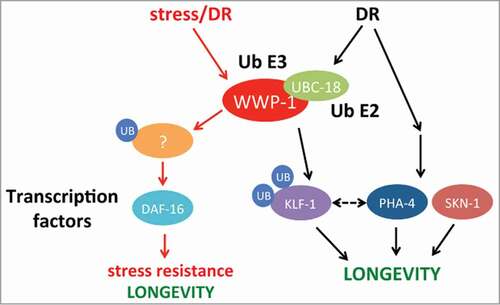
Many mutations or treatments that prolong lifespan in C. elegans also increase resistance to environmental stressors such as heat, UV, and oxidative conditions. Previously, we found that loss of wwp-1 decreased stress resistance in the adult worm.Citation1 To our surprise, reduced klf-1 expression did not affect the stress response in worms. Also in unpublished results, we found that loss of pha-4, which we had shown to be downstream of both wwp-1 and klf-1,Citation1,2 caused no change in stress resistance in worms, much like loss of klf-1. This would suggest that wwp-1 is upstream of 2 pathways: one that regulates DR-longevity requiring klf-1 and pha-4, and one that modulates stress response independent of these 2 factors (). Our results would indicate that WWP-1 targets a different substrate(s) for ubiquitylation to regulate stress functions. How WWP-1 regulates stress resistance independent of KLF-1 and if this stress function is required for the DR response are still open questions.
Figure 1. Proposed model on how the WWP-1/UBC-18 complex modulates both stress response and dietary restriction pathways. Upon DR, a signaling cascade is initiated that results in the ubiquitylation of KLF-1 by the WWP-1/UBC-18 E3/E2 complex. KLF-1, together with PHA-4 and SKN-1, launch a transcriptional response that results in increased longevity. In a distinct pathway, environmental stress and/or DR induces WWP-1/UBC-18 complex activity resulting in the ubiquitylation of an unknown substrate that feeds into a DAF-16 dependent signaling pathway, leading to an increased resistance to multiple types of stress as well as increased lifespan with some DR regimens (see text). The dotted line represents a possible interaction of klf-1 and pha-4.
To date, the exact mechanism by which DR extends lifespan remains unclear. Studies are further complicated by the numerous methods used to manipulate the worm diet[4]. Multiple approaches have been used to mimic DR in worms including eat-2 mutant worms and bacteria dilution in liquid culture or on agar plates. Furthermore, the distinct methods used to manipulate the worm diet to extend lifespan have different effects on stress responses in worms.Citation4 For instance, worms grown on agar plates with lower bacteria concentrations have increased resistance to the oxidative-damaging agent paraquat.Citation5 Here, the FOXO TF daf-16 is essential for the increased longevity induced by this method; however, there is no requirement for skn-1 and pha-4.Citation4,5 In contrast, worms grown in liquid culture with lower bacteria concentrations are not more resistant to paraquat and H2O2,Citation6 and eat-2 mutant worms do not have increased thermotolerance.Citation7 Indeed, skn-1 and pha-4, but not daf-16, are required for the longevity response induced by these 2 methods of DR[4]. It is interesting to note that wwp-1 is essential for lifespan extension mediated by all 3 methods described above,Citation1 suggesting that these different DR regimens evoke at least 2 distinct genetic pathways involving WWP-1.
It is now obvious that lifespan extension by DR is not achieved by a single genetic/biochemical pathway, but likely through multiple pathways that act together to form a “DR network.” Possibly, different sensors for key nutrients couple to the DR pathways in distinct ways such that different DR regimens can exploit discrete/overlapping pathways to extend lifespan. Currently, the specific downstream components for each DR method are still elusive. Monitoring global changes using transcriptome, ChIP-seq and/or ubiquitome analysis of worm populations lacking critical regulators, like WWP-1 and KLF-1, subjected to different DR protocols will not only identify downstream targets required for longevity, but will also distinguish how these regulators connect as signaling modules to form this DR network.
References
- Carrano AC, et al. Nature 2009; 460(7253):396-9; PMID:19553937
- Carrano AC, et al. Nat Commun 2014; 5:3772; PMID:24805825; https://doi.org/10.1038/ncomms4772
- Scheffner M, Kumar S. Biochim Biophys Acta 2014; 1843(1):61-74; PMID:23545411; https://doi.org/10.1016/j.bbamcr.2013.03.024
- Greer EL, Brunet A. Aging Cell 2009; 8(2):113-27; PMID:19239417; https://doi.org/10.1111/j.1474-9726.2009.00459.x
- Greer EL, et al. Curr Biol 2007; 17(19): p. 1646-56; PMID:17900900; https://doi.org/10.1016/j.cub.2007.08.047
- Houthoofd K, et al. Exp Gerontol 2002; 37(12):1359-69; PMID:12559405; https://doi.org/10.1016/S0531-5565(02)00172-9
- Houthoofd K, et al. Exp Gerontol 2002; 37(12):1371-8; PMID:12559406; https://doi.org/10.1016/S0531-5565(02)00173-0
