Abstract
The present study analyzed the heterogeneous cell-cycle dependence and fate of single cancer cells in a population treated with UVB using a fluorescence ubiquitination-based cell-cycle (FUCCI) imaging system. HeLa cells expressing FUCCI were irradiated by 100 or 200 J/m2 UVB. Modulation of the cell-cycle and apoptosis were observed by time-lapse confocal microscopy imaging every 30 min for 72 h. Correlation between cell survival and factors including cell-cycle phase at the time of the irradiation of UVB, mitosis and the G1/S transition were analyzed using the Kaplan–Meier method along with the log rank test. Time-lapse FUCCI imaging of HeLa cells demonstrated that UVB irradiation induced cell-cycle arrest in S/G2/M phase in the majority of the cells. The cells irradiated by 100 or 200 J/m2 UVB during G0/G1 phase had a higher survival rate than the cells irradiated during S/G2/M phase. A minority of cells could escape S/G2/M arrest and undergo mitosis which significantly correlated with decreased survival of the cells. In contrast, G1/S transition significantly correlated with increased survival of the cells after UVB irradiation. UVB at 200 J/m2 resulted in a greater number of apoptotic cells.
Introduction
Tumor heterogeneity is a major problem for therapy and can result in chemosensitive and chemoresistant subpopulations within a single tumor whose interaction can result in unexpected responses. Shifts in the ratios within a tumor subpopulations can be detrimental to therapy.Citation1
Using an imageable cell colony-forming assay (iCFA), 2.6% of cells treated with a thymidylate synthetase inhibitor were shown to maintain a colony growth rate similar to that of untreated control colonies. Such minority resistant population could account for tumor recurrence.Citation2
Slocum et al.Citation3 determined single cell responses to a thymidylate synthetase inhibitor.Citation4,5 Although immediate loss of cell division occurred in 74% of treated cells, 6% of the cells could divide more than 2 times with the more proliferative sister cell sibling producing up to 73 times more cells than the other sister in the treated population. This is a striking example of heterogeneity in sister cells after mitosis.
Sakaue-Sawano et al.Citation6 have utilized oscillating proteins linked to spectrally-distinct fluorescent proteins that specifically label cell-cycle phases in living cells in a system termed FUCCI (fluorescent ubiquitination-based cell-cycle indicator). In a previous study, we utilized the FUCCI imaging system to investigate heterogeneity of response to chemotherapy at the single cancer-cell level. Time-lapse FUCCI imaging demonstrated that both doxorubicin (DOX) and cisplatinum (CDDP) could induce cell-cycle arrest in S/G2/M in almost all the cells, but a subpopulation of the cells could escape the block and undergo mitosis. The subpopulation which went through mitosis subsequently underwent apoptosis while the cells arrested in S/G2/M survived.Citation7
We previously compared the DNA damage repair (DDR) response of pancreatic cancer cells after UVB or UVC irradiation. The UV-induced DNA damage repair was imaged by focus formation of green fluorescent protein (GFP) fused to the DDR-related chromatin-binding protein 53BP1 in MiaPaCa-2 human pancreatic cancer cells growing in 3D Gelfoam® histoculture and in tumors grown in nude mice. 53BP1-GFP forms foci during DNA damage repair. Our results demonstrated that UVB has greater tissue penetration than UVC because of its longer wavelength and has clinical potential for eradicating superficial cancer.Citation8
In the present study, the modulation of the cell-cycle phase and apoptosis due to UVB irradiation were observed on HeLa cells by using the FUCCI imaging system to determine the correlation between cell-cycle progression and UVB sensitivity.
Results and Discussion
Time-lapse imaging of cell-cycle after low-dose UVB irradiation
To analyze the correlation between cell-cycle phase and sensitivity to UVB, HeLa-FUCCI cells were individually numbered. Mitosis, transition from G1 to S phase, and apoptosis were observed in the individual cells after 100 J/m2 UVB irradiation (, , ; Videos S2, 3). Time-lapse FUCCI imaging of HeLa cells demonstrated that UVB irradiation induced cell-cycle arrest in S/G2/M phase within 24 h (), and that a fraction of the cells irradiated by UVB escaped from cell-cycle arrest ().
Figure 1. UVB irradiation and time-lapse FUCCI imaging of cell-cycle progression. HeLa-FUCCI cells were cultured in 35 mm dishes for 24 h. The cells were irradiated with 100 or 200 J/m2 UVB using a Benchtop 3UV transilluminator (UVP, LLC, Upland, CA) with an emission peak at 302 nm. After irradiation, cell-cycle progression, as well as mitotsis and apoptosis were observed every 30 min for 72 h by FV1000 confocal microscopy (Olympus). The UVB dose was measured with a UVX Radiometer (UVP). After UVB irradiation, cell-cycle progression and apoptosis were observed every 30 min for 72 h using the FV1000. Scanning and image acquisition were controlled by FluoView software (Olympus).
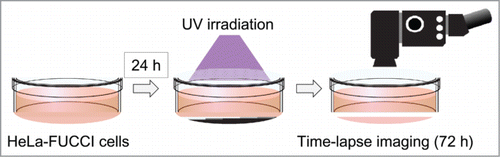
Figure 2. Single-cell time-lapse imaging in HeLa-FUCCI cells after irradiation with 100 J/m2 UVB. (A) Individualization of cancer cells. Each cell was individualized by numbering. The cell-cycle phase of each cell was observed every 30 min for 72 hours by confocal imaging. (B) Time-lapse imaging of the cell-cycle and apoptosis after irradiation with 100 J/m2 UVB. (C) Apoptosis after irradiation with 100 J/m2 UVB. The cells circled with white dotted lines entered apoptosis after mitosis. (D) Distribution of cell-cycle phase after irradiation with 100 J/m2 UVB.
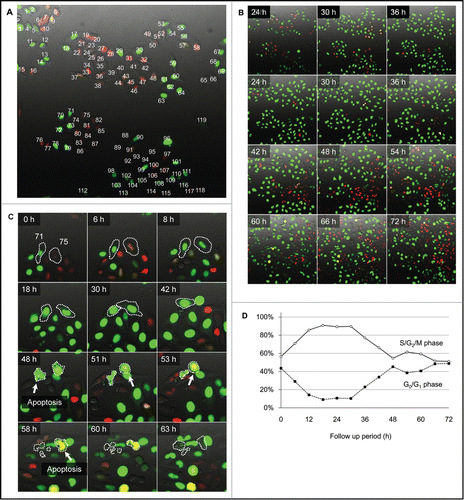
Figure 3. Survival analysis of individual cells after irradiation with 100 J/m2 UVB. (A) Kaplan-Meier survival curve for G0/G1 phase cells and S/G2/M phase cells at the onset of UVB irradiation. (B) Kaplan-Meier survival curve for the cells entering mitosis within 24 h after UVB irradiation compared to non-mitotic cells. (C) Kaplan-Meier survival curve for the cells which transitioned from G1 phase to S phase within 24 h after UVB irradiation, compared with cells without G1/S transition.
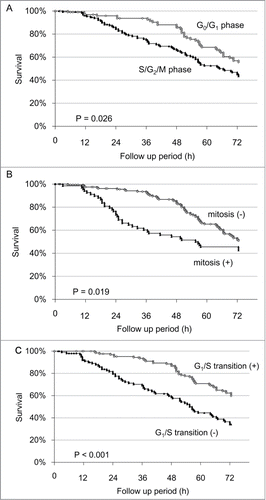
Table 1. Time-lapse cell-cycle imaging of individual HeLa-FUCCI cells after UVB irradiation
To determine the correlation between the cell-cycle phase when UVB irradiation was initiated and sensitivity to UVB, the cells were divided into 2 groups according to the cell-cycle phase at the onset of time-lapse imaging: Green and yellow fluorescence defined S/G2/M phase, and red fluorescence defined G0/G1 phase in the FUCCI imaging system. Survival analysis of the HeLa-FUCCI cells revealed that the initiation of irradiation of 100 J/m2 UVB during S/G2/M phase significantly decreased the survival of the HeLa-FUCCI cells compared to initiation of irradiation in G0/G1 phase (P = 0.026) (Fig. 3A). To determine the correlation between mitosis and survival of the cells, the cells were divided into 2 groups according to the presence or absence of mitosis within 24 h after irradiation of 100 J/m2 UVB: Survival analysis demonstrated that mitosis significantly decreased the survival of the cells after irradiation with 100 J/m2 UVB (P = 0.019) (Fig. 3B).
To determine the correlation between G1/S transition and survival, the cells were divided into 2 groups: the cells which were in G1/S transition within 24 h after the irradiation of 100 J/m2 UVB and cells which were not. G1/S transition significantly correlated with increased survival of the cells after irradiation with 100 J/m2 UVB (P < 0.001) (Fig. 3C).
Time-lapse imaging of cell-cycle progression after high-dose UVB irradiation
Time-lapse imaging of HeLa-FUCCI cells after UVB irradiation demonstrated that more than 90% of the cells underwent cell-cycle arrest in S/G2/M phase within 24 h after 200 J/m2 UVB irradiation (, , Videos S4, 5A–C). The cell-cycle arrest in S/G2/M phase continued until 36 h in more than 80% of the cells (Fig. 4D).
Figure 4. Single cell time-lapse imaging of HeLa-FUCCI cells after irradiation with 200 J/m2 UVB. (A) Individualization of cancer cells. Each cell was individualized by numbering. The cell-cycle phase of each cell was observed every 30 min for 72 hours by confocal microscopy imaging. (B) Time-lapse imaging of the cell-cycle phase and apoptosis after irradiation with 200 J/m2 UVB. (C) Apoptosis after irradiation with 200 J/m2 UVB. Cell 96 entered apoptosis after mitosis. Cell 97 cell became apoptotic without mitosis. (D) Distribution of cell-cycle phase after irradiation with 200 J/m2 UVB.
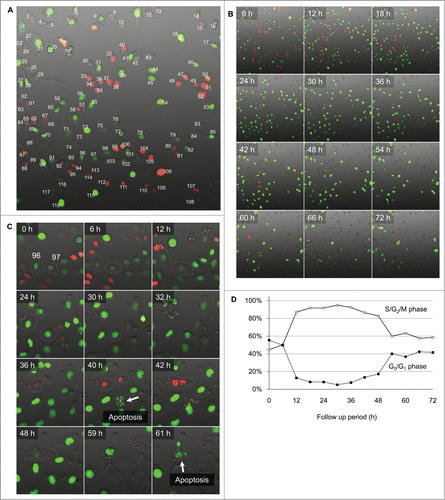
Figure 5. Survival analysis of individual cells after irradiation with 200 J/m2 UVB. (A) Kaplan-Meier survival curve for G0/G1 and S/G2/M cells at the onset of UVB irradiation. (B) Kaplan-Meier survival curve for cells which entered mitosis within 24 h after irradiation with UVB compared to non-mitotic cells. (C) Kaplan-Meier survival curve for the cells which transitioned from G1 to S phase within 24 h after irradiation with UVB, compared to cells without G1/S transition.
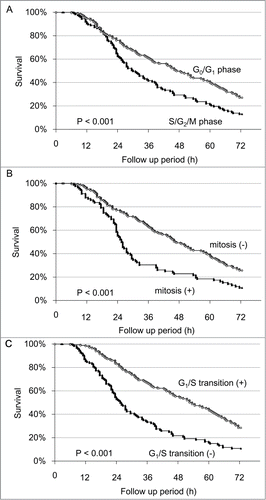
Survival analysis of the HeLa-FUCCI cells after 200 J/m2 UVB irradiation demonstrated that cells irradiated during S/G2/M phase are more sensitive than G0/G1 phase cells (P < 0.001, ). Mitosis within 24 h after 200 J/m2 UVB irradiation significantly correlated with decreased survival of the cells (P < 0.001, ). Transition from G1 to S phase within 24 h after the irradiation significantly increased the survival of the cells (P < 0.001, ). UVB irradiation at 200 J/m2 increased apoptosis of HeLa cells compared to 100 J/m2 ().
In our previous study, single cell time-lapse FUCCI imaging enabled observation of the heterogeneous effect of chemotherapy on cell-cycle progression of single cancer cells.Citation7
In the present study, single-cell time-lapse FUCCI imaging of HeLa cells showed heterogeneous responses to UVB including cell-cycle arrest, escape from the arrest, mitosis and apoptosis in individual cells. The cell-cycle arrest after 200 J/m2 UVB irradiation lasted longer compared with the cells irradiated by 100 J/m2 UVB.
The present study also showed that mitosis has significant correlation with decreased survival of the cells after UVB irradiation, and that G1/S transition has significant association with increased survival of the cells after the UVB irradiation. Furthermore, survival analyses of the HeLa-FUCCI cells revealed that cells in S/G2/M phase cells had higher sensitivity to UVB than G0/G1 phase cells.
Our study is the first report which determined the correlation between sensitivity to UVB and cell-cycle progression by using survival analyses for individual cells whose cell-cycle phase was determined by FUCCI imaging.
The cell-cycle effect and dependence of UVB irradiation described in the present report can be synergistically used with previously-developed tumor-targeting strategies.Citation9-16
Materials and Methods
Establishment of HeLa cells stably transfected with FUCCI plasmids
The FUCCI system was used to visualize the cell-cycle phase in individual HeLa cells.Citation6 Plasmids expressing mKO2-hCdt1 (green-yellow fluorescent protein) or mAG-hGem (orange-red fluorescent protein) were obtained from the Medical & Biological Laboratory (Nagoya, Japan). Plasmids expressing mKO2-hCdt1 were transfected into HeLa cells using Lipofectamine™ LTX (Invitrogen, Carlsbad, CA). The cells were sorted by green-yellow (S, G2, and M phase) using a FACS-Aria cell sorter (Becton Dickinson). The first-step-sorted green-fluorescent cells were then re-transfected with mAG-hGem (orange-red) and then sorted by orange fluorescence.Citation7,17-21
Confocal imaging of cell-cycle behavior
After UVB irradiations, cell-cycle progression, mitosis and apoptosis were observed every 30 min for 72 h using the FluoView FV1000 confocal laser microscope (Olympus Corp., Tokyo, Japan) to determine the correlation between cell-cycle progression, mitosis and apoptosis induced by UVB. Scanning and image acquisition were controlled by FluoView software (Olympus).Citation22
Cellular irradiation with UVB
HeLa-FUCCI cells were cultured in high-glucose DMEM (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Sigma-Aldrich) at 37°C with 95% air and 5% CO2 for 24 h. The cells were irradiated with UVB from the bottom of a chamber using a Benchtop 3UV transilluminator (UVP, LLC, Upland, CA) with an emission peak at 302 nm.Citation8 The UVB dose was measured with a UVX Radiometer (UVP, LLC, Upland, CA).
Statistical analysis
The survival period of each cell was defined as the time from initial imaging to the time of the appearance of apoptosis. Survival was calculated using the Kaplan–Meier method along with the log rank test. Statistical significance was defined as P < 0.05. Statistical analyses were performed using EZR statistical software (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Dedication
This study dedicated to the memory of Dr. AR Moossa.
Disclosure of Potential Conflicts of Interest
SM, SY, HK, MY, MT, TM, KH, NY, and RMH are or were unsalaried associates of AntiCancer Inc. There are no other competing financial interests.
1033598_supplmental_movies__5_.zip
Download Zip (13 MB)Supplemental Material
Supplemental data for this article can be accessed on the publisher's website
References
- Heppner GH, Miller BE. Therapeutic implications of tumor heterogeneity. Semin Oncol 1989; 16:91-105.
- Schöber C, Gibbs JF, Yin MB, Slocum HK, Rustum YM. Cellular heterogeneity in DNA damage and growth inhibition induced by ICI D1694, thymidylate synthase inhibitor, using single cell assays. Biochem Pharmacol 1994; 48:997-1002; PMID:8093112; http://dx.doi.org/10.1016/0006-2952(94)90370-0
- Slocum HK, Parsons JC, Winslow EO, Broderick L, Minderman H, Tóth K, Greco WR, Rustum YM. Time-lapse video reveals immediate heterogeneity and heritable damage among human ileocecal carcinoma HCT-8 cells treated with raltitrexed (ZD1694). Cytometry 2000; 41:252-60; PMID:11084610; http://dx.doi.org/10.1002/1097-0320(20001201)41:4%3c252::AID-CYTO3%3e3.0.CO;2-X
- Yin MB, Voigt W, Panadero A, Vanhoefer U, Frank C, Pajovic S, Azizkhan J, Rustum YM. p53 and WAF1 are induced and Rb protein is hypophosphorylated during cell growth inhibition by the thymidylate synthase inhibitor ZD1694 (Tomudex). Mol Pharmacol 1997; 51:630-6; PMID:9106628
- Matsui SI, Arredondo MA, Wrzosek C, Rustum YM. DNA damage and p53 induction do not cause ZD1694-induced cell cycle arrest in human colon carcinoma cells. Cancer Res 1996; 56:4715-23; PMID:8840989
- Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 2008; 132:487-98; PMID:18267078; http://dx.doi.org/10.1016/j.cell.2007.12.033
- Miwa S, Yano S, Kimura H, Yamamoto M, Toneri M, Matsumoto Y, Uehara F, Hiroshima Y, Murakami T, Hayashi K, et al. Cell-cycle fate-monitoring distinguishes individual chemosensitive and chemoresistant cancer cells in drug-treated heterogeneous populations demonstrated by real-time FUCCI imaging. Cell-Cycle 2015; 14:621-9; PMID:25551170; http://dx.doi.org/10.4161/15384101.2014.991604, Epub ahead of print.
- Uehara F, Miwa S, Tome Y, Hiroshima Y, Yano S, Yamamoto M, Efimova E, Matsumoto Y, Maehara H, Bouvet M, et al. Comparison of UVB and UVC effects on the DNA damage-response protein 53BP1 in human pancreatic cancer. J Cell Biochem 2014; 115: 1724-8; PMID:24819034; http://dx.doi.org/10.1002/jcb.24837
- Blagosklonny MV. How cancer could be cured by 2015. Cell Cycle 2005; 4:269-78; PMID:15655345
- Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003; 89:1147-51; PMID:14520435; http://dx.doi.org/10.1038/sj.bjc.6601256
- Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-7; PMID:14678733; http://dx.doi.org/10.1016/S1359-6446(03)02806-X
- Blagosklonny MV. “Targeting the absence” and therapeutic engineering for cancer therapy. Cell Cycle 2008; 7:1307-12; PMID:18487952; http://dx.doi.org/10.4161/cc.7.10.6250
- Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005; 4:1518-21; PMID:16258270; http://dx.doi.org/10.4161/cc.4.11.2208
- Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID:11417480; http://dx.doi.org/10.1038/sj.leu.2402127
- Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia 2006; 20:385-91; PMID:16357832; http://dx.doi.org/10.1038/sj.leu.2404075
- Blagosklonny MV. Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biol Ther 2007; 6:1684-90; PMID:18344680; http://dx.doi.org/10.4161/cbt.6.11.5167
- Yano S, Miwa S, Mii S, Hiroshima Y, Uehara F, Yamamoto M, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, et al. Invading cancer cells are predominantly in G0/G1 resulting in chemoresistance demonstrated by real-time FUCCI imaging. Cell Cycle 2014; 13:953-60; PMID:24552821; http://dx.doi.org/10.4161/cc.27818
- Yano S, Zhang Y, Miwa S, Tome Y, Hiroshima Y, Uehara F, Yamamoto M, Suetsugu A, Kishimoto H, Tazawa H, et al. Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle 2014; 13: 2110-19; PMID:24811200; http://dx.doi.org/10.4161/cc.29156
- Yano S, Miwa S, Mii S, Hiroshima Y, Uehara F, Kishimoto H, Tazawa H, Zhao M, Bouvet M, Fujiwara T, Hoffman RM. Cancer cells mimic in vivo spatial-temporal cell-cycle phase distribution and chemosensitivity in 3-dimensional Gelfoam® histoculture but not 2-dimensional culture as visualized with real-time FUCCI imaging. Cell Cycle 2015; 14: 808-19: PMID:25564963; http://dx.doi.org/10.4161/15384101.2014.989946
- Yano S, Zhang Y, Zhao M, Hiroshima Y, Miwa S, Uehara F, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, et al. Tumor-targeting Salmonella typhimurium A1-R decoys quiescent cancer cells to cycle as visualized by FUCCI imaging and become sensitive to chemotherapy. Cell Cycle 2014; 13:3958-63; PMID:25483077; http://dx.doi.org/10.4161/15384101.2014.964115, in press
- Miwa S, Yano S, Tome Y, Sugimoto N, Hiroshima Y, Uehara F, Mii S, Kimura H, Hayashi K, Efimova EV, et al. Dynamic color-coded fluorescence imaging of the cell-cycle phase mitosis, and apoptosis demonstrates how caffeine modulates cisplatinum efficacy. J Cell Biochem 2013; 114:2454-60; PMID:23696238; http://dx.doi.org/10.1002/jcb.24593
- Uchugonova A, Duong J, Zhang N, König K, Hoffman RM. The bulge area is the origin of nestin-expressing pluripotent stem cells of the hair follicle. J Cell Biochem 2011; 112: 2046-50; PMID:21465525; http://dx.doi.org/10.1002/jcb.23122
