Neutrophils are short-lived effector cells of the innate immune system that form the first line of defense against microbial pathogens.Citation1 Recent studies show that, by migrating to bone marrow, these cells can also induce (VACV)-specific T cell responses.Citation2 Neutrophils polarize to distinct phenotypes in response to environmental cytokine/chemokine signals. When granulocyte macrophage-colony stimulating factor (GM-CSF) is present, neutrophils can acquire an antigen-presenting cell (APC) phenotype (by upregulating MHC class II and CD80/CD86 costimulatory molecules) to trigger CD8 T cell activationCitation3; when transforming growth factor (TGF)-β is found in a tumor context, tumor-associated neutrophils polarize from the anti-tumor N1 to the pro-tumor N2 subtype, whose ability to activate CD8 T cells is impaired.Citation4
In mice infected with the New York vaccina virus strain (NYVAC), we can alter neutrophil recruitment. For example, manipulation of this vector can activate NFκB, which generates a specific cytokine/chemokine milieu at the infection site; this influences the migration of distinct neutrophil subtypes (Nα and Nβ).Citation5 Nβ neutrophils are more lobulated and hypersegmented than the Nα subtype, which confirms neutrophil ability to acquire a distinct phenotype in specific cytokine/chemokine conditions. The APC markers CD80 and CD86 are upregulated in Nβ cells, which show a greater capacity to generate antigen-specific CD8 T cell responses than Nα cells.Citation5
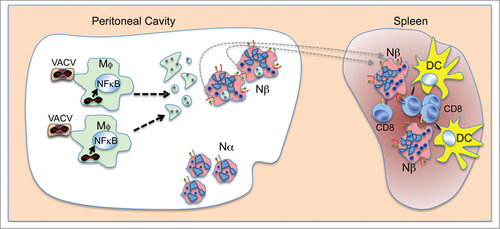
Although Nβ neutrophils present antigen to CD8 T effector cells to induce their activation, they cannot activate naïve CD8 T cells ex vivo.Citation5 Other APC-activating signals such as CD40, which aids dendritic cell (DC) stimulation of CD8 T cells, could help neutrophil activation of naïve CD8 T cells. In vivo studies confirmed that neutrophils do not prime VACV-specific CD8 T cell responses in the absence of myeloid DC,Citation2 and that neutrophils transport processed antigens and cross-prime naïve CD8 T cells in vivo to generate vigorous antigen-specific CD8 T cell responses.Citation6 These earlier findings suggest that antigen transport after peritoneal VACV infection is due not to direct neutrophil infection, but rather to neutrophil uptake of apoptotic infected cells in which antigen has been processed. These neutrophils then migrate to secondary lymphoid organs such as the spleen, where they might cross-present antigens; when typical APC such as DC are present, the neutrophils could prime naïve CD8 T cells (). Whether Nβ neutrophils cross-present antigens from virus-infected cells more efficiently than Nα neutrophils remains to be determined.
Figure 1. Model of neutrophil-dependent CD8 (T)cell activation. Apoptotic vaccinia virus (VACV)-infected macrophages (Mφ) are engulfed by neutrophils (Nβ) that cross-prime CD8 T cells with the help of dendritic cells (DC).
After infection with a human immunodeficiency virus (HIV) antigen-bearing NYVAC, murine neutrophils help to enhance CD8 T cell responses to non-secreted HIV antigens,Citation5 demonstrating a potential role in vaccine-induced immune responses and confirming their capacity to cross-prime antigens from virus-infected cells. Although neutrophils increase antigen-specific CD8 T cell responses, this is not the case for CD4 T cell responses.Citation5 It is possible that neutrophils prime CD4 T cells with difficulty, and probably downregulate CD4 T cell activation, which would explain the reduction in antigen-specific CD4 T cell responses. Another study of vaccine immunization confirmed that neutrophils generate poor antigen-specific CD4 responses, as their contacts with DC in the draining lymph nodes are very brief, which alters the quality of DC-T cell interactions.Citation7 An in vivo study of the mechanism of neutrophil-dependent control of T cell subset responses to VACV-delivered antigens would be of major interest for the generation of VACV-based vaccines that preferentially induce CD4 and CD8 T cell responses. How Nα and Nβ subtypes interact with typical APC and T cell subsets must be defined.
Inducing pathogen-specific T cell responses is one of the most important goals for achieving effective vaccines. The distinct ability of neutrophil subtypes to activate CD8 T cells suggests that virus-based vaccine efficacy might be improved by defining which cytokines/chemokines induce this functional switch, which would allow modulation of the Nβ/Nα neutrophil ratio after infection.
References
- Mantovani A, et al. Nat Rev Immunol 2011; 11:519-31; PMID:21785456; http://dx.doi.org/10.1038/nri3024
- Duffy D, et al. Immunity 2012; 37:917-29; PMID:23142782; http://dx.doi.org/10.1016/j.immuni.2012.07.015
- Matsushima H, et al. Blood 2013; 121:1677-89; PMID:23305731; http://dx.doi.org/10.1182/blood-2012-07-445189
- Fridlender ZG, et al. Cancer Cell 2009; 16:183-94; PMID:19732719; http://dx.doi.org/10.1016/j.ccr.2009.06.017
- Di Pilato M, et al. Proc Natl Acad Sci U S A 2015; 112:E1333-42; PMID:25739961; http://dx.doi.org/10.1073/pnas.1424341112
- Beauvillain C, et al. Blood 2007; 110:2965-73; PMID:17562875; http://dx.doi.org/10.1182/blood-2006-12-063826
- Yang CW, et al. J Immunol 2010; 185:2927-34; PMID:20679530; http://dx.doi.org/10.4049/jimmunol.1001289
