Abstract
Cancer stem-like cells (CSCs) are a rare subpopulation of cancer cells capable of propagating the disease and causing cancer recurrence. In this study, we found that the cellular localization of PKB/Akt kinase affects the maintenance of CSCs. When Akt tagged with nuclear localization signal (Akt-NLS) was overexpressed in SKBR3 and MDA-MB468 cells, these cells showed a 10–15% increase in the number of cells with CSCs enhanced ALDH activity and demonstrated a CD44+High/CD24−Low phenotype. This effect was completely reversed in the presence of Akt-specific inhibitor, triciribine. Furthermore, cells overexpressing Akt or Akt-NLS were less likely to be in G0/G1 phase of the cell cycle by inactivating p21Waf1/Cip1 and exhibited increased clonogenicity and proliferation as assayed by colony-forming assay (mammosphere formation). Thus, our data emphasize the importance the intracellular localization of Akt has on stemness in human breast cancer cells. It also indicates a new robust way for improving the enrichment and culture of CSCs for experimental purposes. Hence, it allows for the development of simpler protocols to study stemness, clonogenic potency, and screening of new chemotherapeutic agents that preferentially target cancer stem cells. Summary: The presented data, (i) shows new, stemness-promoting role of nuclear Akt/PKB kinase, (ii) it underlines the effects of nuclear Akt on cell cycle regulation, and finally (iii) it suggests new ways to study cancer stem-like cells.
Abbreviations
| CSCs | = | cancer stem-like cells |
| CDK | = | cyclin-dependent kinase |
| GSK3 | = | glycogen synthase kinase-3 |
| PH | = | pleckstrin-homology |
| GPCR | = | G-protein-coupled receptor |
| RTK | = | receptor tyrosine kinase |
| PI3K | = | phoshatidylinositol-3-kinase |
| JAK | = | Janus kinase |
| STAT | = | signal transducer and activator of transcription |
| PTEN | = | phosphatase and tensin homolog |
| PH | = | pleckstrin-homology |
| PKB | = | protein kinase B |
| PDK | = | phosphoinositide dependent kinase |
| mTOR | = | mammalian target of rapamycin |
| Bcl2 | = | B cell lymphoma 2 |
| NLS | = | nuclear localization signal |
| WT | = | wild type |
| ALDH | = | aldehyde dehydrogenase |
| IGF1 | = | insulin like growth factor 1 |
| T-ALL | = | T-cell acute lymphoblastic leukemia |
| RPMI | = | Roswell Park Memorial Institute |
| poly-HEMA | = | poly-2-hydroxyethyl methacrylate |
| BPE | = | bovine pituitary epithelial |
| hEGF | = | human epidermal growth factor |
| RT | = | room temperature |
| RIPA | = | radioimmunoprecipitation |
| PVDF | = | polyvinylidene fluoride |
| FBS | = | fetal bovine serum |
| DEAB | = | diethylaminobenzaldehyde |
| 7-AAD | = | 7-aminoactinomycin D |
| GAPDH | = | glucose-6-phosphate dehydrogenase |
Introduction
Cancer stem-like cells (CSCs) are similar to normal stem cells and exhibit properties of both symmetric and asymmetric cell division, self-renewal and, to a certain degree, differentiation.Citation1,2 The origin of CSCs is still debated. They may either originate from normal (tissue) stem cells or form in a process similar to reprogramming (dedifferentiation). The earliest evidences of CSCs were reported in a breast cancerCitation3 and in acute myeloid leukemia.Citation4 The existence of CSCs was later also reported in neural,Citation5 colon,Citation6 pancreatic,Citation7 head and neck,Citation8 prostateCitation9 and other cancers.Citation10,11
CSCs remain largely resistant to the majority of current anti-cancer therapeutics (i.e. chemotherapeutic drugs) even though such therapies kill differentiated cancer cells that form the bulk of the tumor.Citation12,13 Though it is not clearly understood, several stemness-related cell signaling pathways including Notch, Wnt, Hedgehog and others that are vital to cell cycle, differentiation and metabolism may be deregulated when compared to normal stem cells.Citation14 Better knowledge about the (de)regulation of pathways like phosphatidylinositol 3-kinase (PI3K)/Akt and Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) could provide crucial insights into understanding the pathophysiology of CSCs in tumor and provide new clues helpful in the development of therapeutic strategies.Citation15 PI3K-Akt pathway is downstream of many signaling cascades including tyrosine receptor kinases (RTKs) and G-protein coupled receptors (GPCR) and up-regulated in several types of cancer.Citation16 The major reasons for the hyper-activated PI3K-Akt pathway are inactivating mutations in phosphatase and tensin homolog (PTEN), the inhibitory phosphatase responsible for the dephosphorylation of PI(3,4,5)P3 and inhibition of downstream signals.Citation17
PI3-Kinase consists of a catalytic subunit (P110) and an adaptor/regulatory subunit (P85). Both form a heterodimeric complex, which is activated either through receptor conformational changes or by RTK and GPCR.Citation18,19 The cytosolic PI3-Kinase is responsible for the phoshorylation of the inositol ring of PtdIns(4,5)P2 at the 3rd position to create PI(3,4,5)P3 which is a potent second messenger.Citation20 This function of PI3-Kinase is crucial for the recruitment of specific proteins containing pleckstrin-homology (PH) domain or FYVE domain to the cellular membrane.Citation21 Akt/protein kinase B (PKB) is a primary downstream target of PI3Kinase, carries the PH domain, and is recruited to the plasma membrane from the cytoplasm.Citation22 Membrane recruitment and binding to PtdIns(3,4,5)P3 causes conformational changes in Akt, resulting in exposure of its phoshorylation sites T308 and S473 which are then phosphorylated by phosphoinositide dependent kinase (PDK) and mammalian target of rapamycin (mTOR)/rictor, respectively.Citation23,24 Thus, the activated (phosphorylated) Akt stays mostly in the cytoplasm. However, under certain conditions it may also be transferred into the nucleus.Citation25 Here, it then triggers the regulation of downstream signaling molecules that play a role in cell survival, growth, migration, proliferation and cell death.Citation25
The cytoprotective function of Akt is attributed to its ability to both promote activation of anti-apoptotic molecules and to inhibiting the pro-apoptotic molecules of the B cell lymphoma 2 (Bcl2)-family member Bad and fork head transcription factor, FKHRL.Citation26 Akt is also involved in the phosphorylation of different cell proliferation factors such as cyclin-dependent kinase (CDK) inhibitors p21Waf1/Cip1 and p27kip1 and glycogen synthase kinase-3 (GSK3).Citation25,27 Akt mediated phosphorylation of p21Waf1/Cip1 and p27kip1 leads to their shift from nuclear localization to cytoplasmic localization and thus promoting the transition from G0 to S phase.Citation28-30 Akt mediated inhibition of GSK3 prevents the phoshorylation of β-catenin, which impedes its degradation, thus, enhancing cell proliferation.Citation28 In this study we have investigated the effects of Akt intracellular compartmentalization on the biology of cancer stem-like cell population. We have found that the intracellular localization of PKB/Akt kinase affects the maintenance of CSCs through up-regulation of Yamanaka factors Oct3/4, cMyc and Sox2 along with Nanog. When Akt-NLS was overexpressed in our model cell lines, breast cancer cells responded with 10–15% increase in number of cells with CSC characteristic in mammospheres.
Results
Nuclear localization signal (NLS-) tagged Akt activates downstream targets similar to wild type (WT) Akt
In this manuscript, we refer to Akt-1 as simply “Akt.” Akt constructs were transiently transfected into breast cancer cells, SKBR3 and MDA-MB468, leading to Akt overexpression of 20–25% and ˜50% respectively (Fig. S1A). We further checked for the intracellular localization of Akt upon transfection and observed that NLS-tagged Akt was profoundly localized to the nucleus where it displayed a punctate staining pattern, with some Akt still remaining in the cytoplasm (, and Fig. S1B). However, upon transfection of WT Akt construct lacking the NLS sequence the protein was predominantly localized to the cytoplasm (, and Fig. S1B).
Figure 1. Effect of Akt-WT and Akt-NLS on PI3K-mTOR signaling. (A) Fluorescent images showing nuclear localization of Akt-NLS in cells transfected to express the Akt-NLS construct. Cytoplasmic localization of Akt-WT in cells transfected with empty construct (control). The image data were collected in SKBR3 cells using confocal microscopy. (B) Western blot analysis showing the increased levels of phosphorylated Akt (pAkt) and mTOR in SKBR3 cells. Lower panels show quantitative (densitometric) assessment of the levels of pAkt and mTOR as compared to control * P < 0.05.
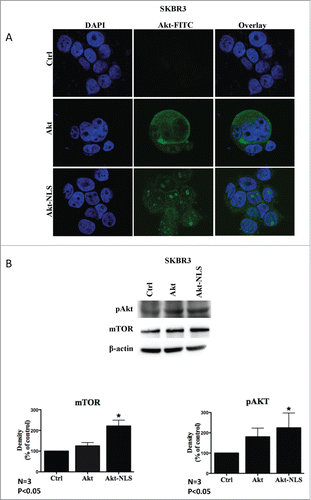
Once the localization of Akt protein expressed from the transfected constructs was established, we focused on the functional aspects of Akt intracellular compartmentalization. Akt localized to the nucleus was more frequently phosphorylated at T308 as compared to cytoplasmic WT Akt, implying an increase in Akt activity upon nuclear localization (). This observation was further confirmed with increased levels of downstream target of Akt, mTOR ().
Localization of Akt to the nucleus enhances the cancer stem-like cell population
Akt overexpression and its nuclear localization play a vital role in the maintenance of pluripotency among murine embryonic stem cells and cardiac progenitor cells.Citation31,32 We determined whether nuclear targeting of Akt would further enhance stemness of breast cancer cells. Transiently transfected breast cancer cells (SKBR3, MDA-MB468) with Akt-NLS resulted in an increased ALDH positive cell population (ALDH+/High) as compared to control plasmid transfected cells (, Figs. S2A and S3A). We further screened for breast cancer cell markers, CD44 and CD24 among Aldefluor-positive cells. Akt-NLS overexpressing and ALDH+/High cells displayed a CD44+/High/CD24−/Low phenotype (ALDH+/High/CD44+/High/CD24−/Low), which resembled the classical breast (cancer) stem cell phenotype.
Figure 2. Flow cytometric assessment of cancer stem-like cell populations by the combination of stem cell markers ALDH, CD24 and CD44, upon overexpression of Akt-WT and Akt-NLS. (A) Akt-NLS transfected SKBR3 cells showed enhanced presence of the ALDH+/High/CD44+/High/CD24−/Low CSCs phenotype when compared to control. Akt-WT cells failed to show a marked increase in the expression of CSCs markers in SKBR3 cells. (B) Graph representing quantification of markers assessed in “A.” Enhanced expression of Akt-NLS showed a significantly increase in the CSCs population compared to Akt-WT and control. (C) Presence of Akt-NLS in SKBR3-mammospheres coincided with a higher number in ALDH+/High/CD44+/High/CD24−/Low CSCs population compared to control. Akt-WT expressing cells failed to show a marked increase in ALDH+/High/CD44+/High/CD24−/Low CSCs in SKBR3 mammospheres. (D) Graph representing quantitative evaluation of expression of markers assessed in “C.” SKBR3 cells transfected with Akt-NLS showed a significantly increased CSCs population as compared to Akt-WT cells and mock controls, *P < 0.05.
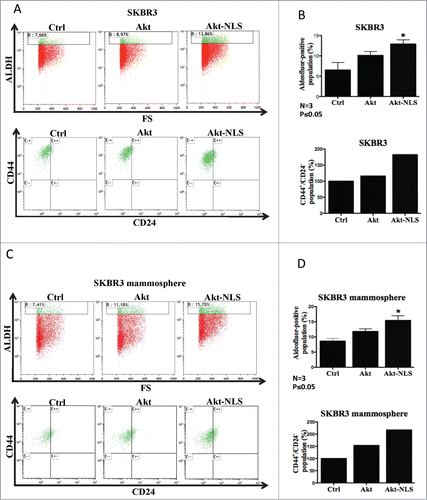
To further investigate the effects of Akt/NLS-Akt on cancer cell stemness, we cultured transiently transfected cells with Akt and Akt-NLS constructs under non-adherent conditions to allow for the formation of mammospheres, which formed within 3–5 days of culture. As expected, even control cells showed an increase in the CSCs population (ALDH+/High/CD44+/High/CD24−/Low) under those culture conditions when compared to adherent culture conditions. Intriguingly, Akt-NLS transfected cells showed a further significant increase in this CSCs population when compared to control mammosphere cultures (, Figs. S2B and S3B). Although Akt-WT overexpressing cells showed an increase in the cancer stem-like cell population compared to mock transfected cells, this enrichment in ALDH+/High/CD44+/High/CD24−/Low CSCs was lower when compared to Akt-NLS transfected cells (, and Fig. S3B).
Akt function is necessary for the proliferation of cancer stem-like cells
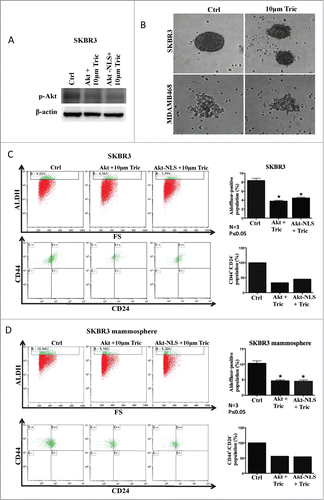
Having demonstrated the effects on cancer stem-like cell proliferation by increased expression of Akt and nuclear Akt compartmentalization, we investigated how Akt function and intracellular localization affect cancer stem-like cell maintenance/viability and proliferation. To manipulate Akt activity, we employed the Akt inhibitor triciribine that blocks the phosphorylation of Akt. Inhibition of Akt function by triciribine was confirmed by western blot probing phosphorylated Akt in Akt-WT and Akt-NLS overexpressing cells (). Furthermore, we checked the ability of MDA-MB468 and SKBR3 to form mammospheres in the presence of triciribine. As shown in the , triciribine (10 μM) attenuated mammosphere formation by SKBR3 and MDA-MB468 cells. Moreover, treatment with triciribine (10 μm) for 24 hours strongly reduced the cancer stem-like cell population (ALDH+/High) in MDA-MB468 and SKBR3 cells grown under adherent conditions ( and Fig. S4A). The decrease of the ALDH+/High/CD44+/High/CD24−/Low CSCs population was further confirmed in SKBR3 cells upon triciribine treatment (). Similar experiments were carried out using SKBR3 mammosphere assays. Measurement of the CSC-like phenotype (ALDH+/High/CD44+/High/CD24−/Low) in SKBR3-mammospheres confirmed that Triciribine (10 μm) drastically reduced the CSCs phenotype in SKBR3, which had been transfected with Akt-WT or Akt-NLS ( and Fig. S4A).
Figure 3. Role of Akt in CSC proliferation. (A) Western blot results displaying decreased phosphorylation of Akt (pAkt) after treatment with Akt inhibitor triciribine in the presence of Akt-WT and Akt-NLS when compared to control SKBR3 cells. (B) Phase-contrast images showing the inhibition of mammosphere formation upon treatment with triciribine. Inhibition of Akt phosphorylation/ activation resulted in attenuated mammosphere formation capacity in SKBR3 and MDA-MB468 cells. (C) Triciribine treatment significantly reduced the ALDH+/High/CD44+/High/CD24−/Low CSCs population in the presence of Akt-WT and Akt-NLS as compared to control SKBR3 cells. Left part of the panel shows graphs representing the quantitative assessment of CSCs marker expression after pAKT inhibition. (D) Triciribine treatment significantly reduced the ALDH+/High/CD44+/High/CD24−/Low CSCs population in mammospheres derived from Akt-WT and Akt-NLS SKBR3 as compared to mock control. Similarly, the left part of the panel shows graphs representing the quantitative evaluation of CSC marker expression after AKT inhibition *P < 0.05.
Akt-NLS over expression increases expression of stem cell reprogramming factors
Following the above findings that Akt is necessary for increasing CSCs population and its nuclear localization further enhances CSCs, we investigated to find if Akt's nuclear localization further enhances expression and/or stabilization of known pluripotent factors like Oct3/4, cMyc, Nanog, Sox2 and KLF4. We observed that both wild type and NLS tagged Akt constructs showed an increase in the expression of Oct3/4, cMyc and Nanog protein levels (). We did however observe marked difference between Akt and Akt-NLS expressing cells in the mRNA levels of Oct3/4, cMyc, Sox2 and Nanog. While Akt-WT increased the transcription of Oct4, cMyc, Sox2 and Nanog, Akt-NLS showed significant up-regulation of only cMyc mRNA levels and a partial increase in Oct4 and Nanog levels (). However both Akt constructs didn't show any such alteration in KLF4 both at transcriptional and translational levels ().
Figure 4. Expression of stem cell reprogramming markers. (A) Western blot detection of Oct4, cMyc, Nanog and Sox2 were increased in Akt-WT and Akt-NLS compared to control. In case of KLF4, a protein level was approximately the same as in Akt-WT and Akt-NLS cells compared to control. (B) Gene expression of Oct4, cMyc, Nanog and Sox2 were increased in Akt WT compared to Akt-NLS and control. In contrast, KLF4 expression is increased in Akt-NLS as compared to Akt WT and control but not significant in SKBR3 mammospheres. (C) Nuclear Akt enhanced the colony forming ability (mammosphere formation) of SKBR3 and MDA-MB468 as compared to mock cells. The mammosphere formation assay was conducted in soft agar. (D) Quantitative representation of data “C” a significantly increased number of colonies (mammosphere formation) of SKBR3 and MDA-MB468 as compared to, *P < 0.05 and ** P < 0.005.
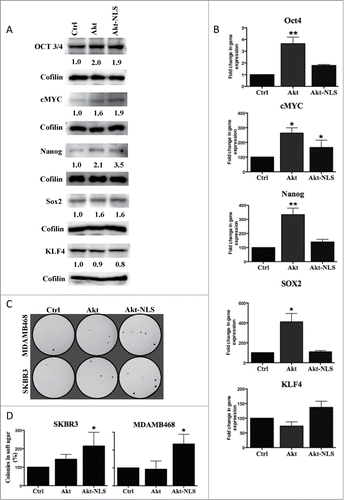
To further assess the functional translation of Akt-WT and Akt-NLS enhanced expression of pluripotent factors, we conducted 3D- soft agar colony formation assay. Mammospheres of SKBR3 and MDA-MB468 breast cancer cells transiently transfected with control (mock), Akt-WT and Akt-NLS were cultured in soft agar for 3 weeks. As shown in Akt-NLS overexpressing cells produced significantly more colonies than controls. This difference in the number of colonies was not significant among Akt-WT transfected cells and mock control cells ().
Nuclear localized Akt increases cell proliferation and cell survival
Akt nuclear localization was shown to decrease G0/G1 phase by phosphorylation of cell cycle inhibitory proteins p21Waf1/Cip1 and p27kip1, resulting in their cytoplasmic transport and attenuation of nuclear cell cycle inhibitory functions.Citation29,30 Using protein gel blot analysis, we observed that Akt-NLS overexpressing breast cancer cells contained more of p21Waf1/Cip1 and p27kip1 total protein levels but only p21Waf1/Cip1 showed an increase in phosphorylation by Akt-NLS. However, both p21Waf1/Cip1 and p27kip1 showed increased phosphorylation by Akt-WT (). Subsequently, we performed qRT-PCR and found a similar significant increase in p21Waf1/Cip1 and p27kip1 mRNA by Akt-WT as protein expression but in the presence of Akt-NLS only p21Waf1/Cip1 showed significantly increased expression ().
Figure 5. Expression of cell cycle regulatory proteins. (A) Western blot detection of p21 and p27 cell cycle inhibitory proteins. Levels of phosphorylated p21 were increased in Akt-WT and Akt-NLS cells compared to control. In contrast, detectable levels of phosphorylated p27 were increased in Akt-WT compared to Akt-NLS and control in MDA-MB468 mammospheres. (B) Gene expression of p21 and p27 was increased in Akt-WT as compared to Akt-NLS and control; only p21 expression was increased in Akt-NLS as compared to control in SKBR3 mammospheres. (C) Western blot analysis of cyclin D1, cyclin E1, cyclin A1 and cyclin B1 cell cycle regulatory proteins. Levels of cyclin D1, cyclin E1 and cyclin A1 were increased in Akt-WT and Akt-NLS as compared to control. In case of cyclin B1, protein expression was similar in Akt-WT and Akt-NLS as compared to control in SKBR3 mammospheres, *P < 0.05 and **P < 0.005.
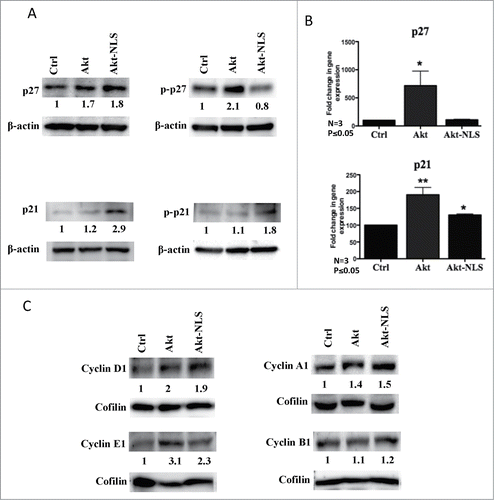
Given the observed difference in expression and activity of cyclin dependent kinase inhibitors p21Waf1/Cip1 and p27kip1 among Akt-WT and Akt-NLS transfected cells, a further regulation in the expression and functional activity of cell cycyle regulatory proteins were studied. We noticed a marked upregulation in the expression of cell cycle enhancing proteins cyclin D1 and cyclin E1 representing a G0/G1 to S phase transition upon Akt and Akt-NLS over-expression (). Similarly, mRNA levels of cyclin D1 showed significant increase upon Akt-NLS expression and a similar trend was observed with Akt-WT expression even though no statistical significance was observed (Fig. S5A). Contrastingly, cyclin E1 mRNA levels were increased both by Akt-WT and Akt-NLS but no statistical significant difference was observed with the latter (Fig. S5A). We further analyzed cyclin A1 and cyclin B1 expression, which monitor the transition from S phase through G2 to mitotic phase. Cyclin A1 protein expression showed an increment in Akt-WT and Akt-NLS over-expressing cells (), but mRNA levels were significantly up-regulated among Akt-WT and significantly down-regulated by Akt-NLS overexpression (Fig. S5A). Cyclin B1 protein levels however didn't show any significant change by either of Akt contructs (), but its mRNA showed significantly higher levels in the Akt-NLS overexpressed cells than control and Akt-WT (Fig. S5A). The above described observations concerning the expression of cyclins need to be treated with caution, as it has been previously observed that manipulation of the cell cycle, may artificially affect the quantity of the expressed cyclins.Citation33
Because of the above similarities and differences in the expression and function of CKI's (p21Waf1/Cip1 and p27kip1) and cyclins among Akt and Akt-NLS transfected cells, we further assessed the impact of such changes on cell cycle profiles of Akt and Akt-NLS over expressing cells. As shown in the , Akt-NLS overexpression showed a significant decrease in G0/G1 phase cells and distinct increase in G2/M phase cells as compared to controls. A similar trend was observed with Akt-WT overexpressing breast cancer cells but without statistical significance compared to the corresponding control cells ().
Figure 6. Akt and nuclear Akt enhance cell proliferation and reduce cell death. (A) Cell cycle analysis showed a significant decrease in G0/G1 phase and increase in G2/M phase in the presence of Akt-NLS in SKBR3 derived from mammospheres. *P < 0.05. (B) Cell death was assessed using Po-Pro (apoptotic) and 7-AAD (necrotic) markers. Presence of Akt-WT and Akt-NLS resulted in reduced cell death compared to control SKBR3 cells, *P < 0.05. Control hsitogram are marked black.
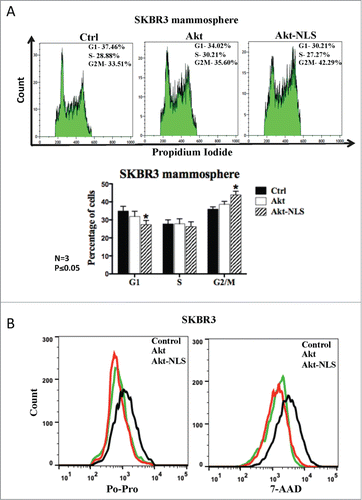
Finally, we assessed whether the cell survival functions of Akt were enhanced in our model and could contribute to the increase in the CSCs population. We assessed cell death in our experimental system, using apoptotic dye Po-Pro and necrotic cell death marker 7-AAD. As shown in the , cells overexpressing Akt-WT or Akt-NLS showed lower staining for Po-Pro and 7-AAD, implying an increase in cell survival in the presence of Akt-WT and Akt-NLS.
Discussion
The presence of cancer stem-like cells is now widely accepted and reported among most cancers.Citation13 Some types of (cancer) stem cells may even be differentially visualized by certain fluorescent dyes.Citation34,35 Even though fewer in numbers, cancer stem-like cells show resistance to currently available radiation and chemotherapeutic interventions and thus cause cancer reoccurrence. Previous studies revealed that the PI3K/Akt pathway plays a pivotal role in oncogenesis by inhibiting pro-apoptotic signaling molecules and maintaining pluripotency among murine and cynomolgus monkey embryonic stem cells.Citation31,32 The intracellular localization of Akt has attracted significant interest in the last decade because of Akt displaying diverse functions when present in cytoplasm as opposed to its compartmentalization in the nucleus or mitochondria. While cytoplasmic Akt is well known for its cell survival effects and metabolic regulation, the role of nuclear localization of Akt is less clear. Nuclear Akt may support cell proliferation or cell death depending upon the trigger and stage of the cell cycle.Citation25,36 Akt mitochondrial localization, i.e., as a result of activation of PI3K by Insulin like Growth Factor 1(IGF1), regulates the β-subunit of ATP-synthase and inhibits GSK-3β function.Citation37 Because of its critical and diverse functions, depending on Akt intracellular localization, we explored the functional aspects of Akt in the nucleus and its ability to promote maintenance of stemness in human breast cancer cells. Our work shows that the introduction of Akt-WT and Akt-NLS into breast cancer cells resulted in an increase of the CSC population which was, at least in part, the result of increased CSCs proliferation. Similarly, a higher percentage of breast cancer cells with characteristic stem-like phenotype and increased ability to form mammospheres was found in samples transfected with vectors to express Akt-WT and Akt-NLS. Similar to previous findings that Akt promotes pluripotency through the regulation and/or stabilization of Oct4, Sox2 and cMyc, in this study we observed that Akt-NLS also showed increased protein levels of reprogramming factors either similar or even more compared to wild type Akt.Citation38–40 However, Akt-NLS markedly differed from wild type Akt in the transcriptional ability of the reprogramming factors. While Akt-WT upregulated the mRNA levels of Oct4, Sox2, cMyc and Nanog drastically Akt-NLS showed no increased expression except for cMyc mRNA. This shows that Akt's role in stem cell maintenance is through stabilization of pluripotent factors as compared to increase in transcription.
To confirm the correlation between Akt expression and nuclear localization in CSCs, inhibition studies using triciribine, an inhibitor of Akt phosphorylation and activation, were conducted. Evangelisti and colleagues (2011) had recently shown that treatment with triciribine significantly decreased the CSC population in a T-cell acute lymphoblastic leukemia (T-ALL) cell line and in patient samples.Citation41 Similarly, in this study, triciribine served as an effective inhibitor of Akt activation independent of its cytoplasm or nuclear localization. The fact that inhibition of Akt led to a significant decrease in the CSC population and CSCs clonogenicity, as determined by mammosphere cultures, indicates the crucial function of Akt in the maintenance of stemness. Triciribine treatment reduced stemness potential even in cells over-expressing Akt-WT or Akt-NLS. Hence this study shows that Akt activity is necessary for the maintenance of pluripotency and nuclear localization of Akt further increases stemness potential.
The phoshorylation of Akt leads to the activation of the downstream regulator of PI3K/Akt pathway, mTOR, which also is the key regulator of autophagy.Citation42,43 The mTOR pathway can form 2 multiprotein complexes with distinct functionalities: mTORC1 (leads to Akt inhibition) and mTORC2 (leads to Akt activation). Phoshorylated Akt induces mTORC1 activation through intermediate molecules and Akt itself is phoshorylated by the mTORC2 complex. Thus, the net Akt phoshorylation/activation status seems to dependent, at least in part, on the balance of mTORC1 and mTORC2 activities. The detailed mechanism behind the mTORC1 and −2 mediated regulation of Akt activation is not yet fully understood. Our study shows significant enhancement of mTOR-activity and Akt-phosphorylation in Akt-WT and Akt-NLS overexpressing cells as compare to control CSCs. These observations strongly suggest that PI3K/Akt/mTOR pathway plays a role in CSC proliferation.
One of the prominent functions of nuclear Akt is to promote cell proliferation through the phosphorylation of cell cycle inhibitory proteins p21Waf1/Cip1 and p27kip1, resulting in their cytoplasmic export and degradation.Citation29,30 Nuclear presence of Akt also leads to an increase in the levels of cell cycle promoters cyclin D1 and CDK2.Citation25,44 Since nuclear Akt may affect the status of cell cycle-regulating proteins, we investigated whether the observed increase in cancer stem-like cell population with nuclear Akt coincided with those same activities. We hypothesized that nuclear Akt may reduce G0/G1 cell-cycle phase, thus, promoting cell cycle progression in CSCs. Indeed, the presence of Akt-NLS coincided with a short G0/G1 phase and a relative increase in the percentage of cells in G2 phase. In agreement with the above, we observed increased phosphorylation of p21Waf1/Cip1 upon Akt-NLS expression leading to p21Waf1/Cip1 cytoplasmic translocation and removal of its inhibitory effect on cell cycle progression. Even though Akt-NLS expression resulted in a decrease in phosphorylated p27kip1, this effect was not statistically significant when comparing to p27kip1 phosphorylation status in control cells. Based on these evidences, we further looked for the active role of nuclear Akt in the cell cycle. Akt-NLS, similar to wild type Akt showed an increase in cyclin D1, E1 and A1 proteins. Similar to mRNA profile of reprogramming factors Akt and Akt-NLS differed in the transcriptional regulation of cyclins and CKI's. Akt-NLS triggered an increase in mRNA levels of G0/G1 to S phase regulating cyclin D1, E1 and p21Waf1/Cip1 along with G2 to mitotic transition regulating cyclin B1. It also showed strong decrease in cyclin A1 mRNA levels. Akt-WT on the other hand induced an increase only in the case of cyclin A1 and E1 mRNA levels. Thus, Akt and Akt-NLS mediated stabilization of cyclin proteins similar to reprogramming factors along with inhibition of p21Waf1/Cip1 correspond to an increase in the number of CSCs population.
Understanding the biology of CSCs will have a profound impact on the design of new therapeutic approaches. Preferential targeting of CSCs is a challenging task, especially within a rapidly changing and genetically unstable environmentCitation45 prevalent in advanced cancers. A majority of available anti-cancer drugs either inhibit cell proliferation or actively induce apoptosis in cancer cells, often leading to relative enrichment of CSCs.Citation46,47 It is crucial to target CSCs populations in order to permanently eradicate tumor.Citation46,47 Our study indicates that nuclear Akt is required for, and enhances the proliferation of CSCs. Targeting nuclear Akt opens new avenues for selective or preferential targeting of CSCs. Currently available Akt inhibitors do not distinguish between cytoplasmic and nuclear Akt, resulting in major side effects. These pharmacological inhibitors also are not selective for different Akt subtypes; for example, Akt inhibitors also block the insulin dependent glucose transport that leads to hyperglycemia.Citation48 Based on our findings, we propose to focus on the development of strategies to specifically or at least preferentially inhibit nuclear Akt.
In conclusion, this study shows the importance of subcellular localization of Akt in maintenance of stemness in breast cancer cells. We demonstrated that nuclear localized Akt enriches the population of breast cancer stem-like cells. p21Waf1/Cip1 and p27kip1 are involved in the cancer cell proliferation and cell cycle progression through Akt regulation.Citation25 Our study shows that subcellular localization of Akt hindered the CSCs proliferation by opposite regulation of p21Waf1/Cip1 and p27kip1. As we have used several cellular models, the a bove findings are likely relevant for other cell types. They may be utilized for the development of methodologies of growing CSCs in vitro and/or enrich CSCs population for experimental purposes (e.g. testing of drugs preferentially targeting CSCs). To better understand the functions of nuclear Akt,Citation27 the identification of its nuclear interaction partners as well as the way it is transported to the nucleus (wild type Akt lacks NLS) are among the most pressing questions within this research area.
Materials and Methods
Cell culture and mammosphere culture
Breast cancer cell lines, MDA-MB468 and SKBR3, (checked for authenticity every 2 years by caryotyping), were grown in Roswell Park Memorial Institute (RPMI), media (PAA, Pasching, Austria) with 10% fetal bovine serum (FBS) (PAA, Pasching, Austria) and 1% penicillin-streptomycin (Gibco, USA) and incubated at 37°C with 5% CO2 in a humidified atmosphere. For mammosphere culture, suspension of single cells were plated in poly-2-hydroxyethyl methacrylate (poly-HEMA) (Sigma-Aldrich, USA) coated flask and maintained in serum free basal epithelial cell medium (Promocell, Heidelberg, Germany) supplemented with Bovine Pituitary Epithelial (BPE) (0.004 ml/ml), Recombinant human Epidermal Growth Factor (hEGF) (10 ng/ml), Recombinant human insulin (5 μg/ml) and Hydrocortisone (0.5 μg/ml) (Promocell, Heidelberg, Germany) for 3 to 5 days to form mammospheres.
Chemical inhibitor and antibodies
Triciribine was obtained from Sigma and dissolved in PBS to make a stock concentration of 10 μM. The primary antibodies used: Akt1 and Cyclin A1 obtained from Sigma-Aldrich (USA), Cyclin B1, Cyclin E1, pAkt and mTOR from Cell Signaling (Beverly, USA), KLF4, cMYC, Cyclin D1, β-actin, p27 and phospho-p27 from Abcam (Cambridge, UK), hSOX2, OCT3/4 and Nanog from R&D systems (Minneapolis, USA), and p21 and phospho-p21 from Santa-Cruz (USA). The secondary antibodies used were Alexafluor488 obtained from Life Technologies, anti-rat HRP-conjugate from Jackson Immuno Research Laboratories, Inc. (USA), anti-goat -conjugate from Abcam (Cambridge, UK), anti-rabbit HRP-conjugate from Biorad (USA) and anti-mouse HRP-conjugate from GE Heath Care (Buckinghamshire, UK).
Plasmids and transient transfection
Akt1 cDNA was inserted into the BamHI and EcoR1 sites of pLVX-Tight-Puro (Clontech, USA). Further, synthetic nuclear localization sequence “RRKRQR”Citation49 was fused to 3′ end of Akt1 by PCR based cloning technique to generate Akt-NLS construct. The construction of cloning was confirmed by sequence analysis and restriction digestion. Empty plasmid pLVX-Tight-Puro was used as control. X-treme GENE HP DNA Transfection Reagent (Roche, Mannheim, Germany) was used for the transient transfection according to manufacturer's instructions.
Immunocytochemistry
Cells were grown on coverslip in 12-well plate. Post-transfection, cells were fixed in 4% paraformaldehyde solution (Santa-Cruz, USA) for 30 min at room temperature (RT) and washed 3 times with PBS. Cells were permeabilized with 0.1% Triton X for 10 min at RT and blocked using 1% BSA, intervened by 3 times with 1× PBS wash. Further, cells were incubated in primary antibody overnight at 4°C and followed by 3 times wash with 1× PBST. Then respective secondary antibody was added and incubated for 1 h at RT and followed by a triple wash with 1× PBST. DAPI was used for nuclear counter staining, intervened by 3 times washing with 1× PBST and cells were mounted on a slide with a mounting medium. Images were captured using a Laser Scanning Confocal Microscope (Zeiss).
Western blot
Transfected cells were washed with PBS and lysed with radioimmunoprecipitation (RIPA) buffer with protease inhibitors (cOm-plete, Roche Diagnostics, Mannheim, Germany). Cell debris was removed by centrifuging at 10,000 g for 10 min. Protein concentration was determined by performing Bradford assay and loaded into 10% polyacrylamide gel and ran at 100 V for 3–4 h. Further, proteins were transferred to Polyvinylidene fluride (PVDF) membrane (Millipore, Darmstadt, Germany) at 80 V for 2–3 h. Blocking was done by using 5% non-fat milk and incubated with primary antibody overnight at 4°C. The blots were washed for 3 times with 1× TBST and incubated in their respective secondary antibody for 1 h at RT. Blots were washed again with 1× TBST for 3 times and developed with Amersham ECL plus (GE Technologies, Buckinghamshire, UK).
Aldofluor assay and cancer stem-like cell characterization
Aldefluor assay was performed to determine the elevated aldehyde dehydrogenase (ALDH) enzymatic activity in CSCs. Cells were stained using Aldefluor kit (StemCell Technologies, Canada) according to manufacturer's instructions and single cells were resuspended in an aldefluor assay buffer containing ALDH substrate. For a negative control, cells were treated with the diethylaminobenzaldehyde (DEAB), a specific inhibitor for ALDH. The presence of CSCs was further confirmed by using CD24-PE and CD44-APC surface markers (BD, Bioscience, USA). Single cell suspension was prepared in PBS with 1% FBS. Cells were incubated with the fore mentioned fluorochrome conjugated markers for 30 min on ice as per the manufacturer's instructions. Further, the stained cells were washed with PBS and analyzed using flow cytometry (Gallios, Beckman Coulter Inc.). Kaluza software (Beckman Coulter Inc.) was used for data analysis and compensation was conducted for ALDH (maximum emission spectrum 512 nm) and CD24-PE (maximum emission spectrum 575 nm). The spectral overlap signal was subtracted from the total fluorescence detected in each channel.
Cell cycle analysis
Mammospheres obtained from the transfected cells were trypsinized and fixed in ice cold 70% ethanol and kept overnight at −20°C. After washing with PBS, cells were resuspended in 50 μg/ml RNAse and incubated for 30 min at 37°C. Then the samples were stained with 100 μg/ml propidium iodide (Sigma-Aldrich, USA) for 10 min and run through the flow cytometer (Gallios, Beckman Coulter Inc.). Kaluza software (Beckman Coulter Inc.) was used for data analysis.
PO-PRO and 7-AAD cell death assay
Transfected cells and their respective mammospheres were trypsinized and washed with PBS. Further, the resuspended cells in PBS were treated with Po-Pro and 7-AAD (7-aminoactinomycin D) dyes (Life Technologies Ltd, USA) for 30 min as per the manufacturer's instruction. The florescent intensities of the samples were quantified in Gallios flow cytometer (Beckman Coulter Inc.) and the data was analyzed using Kaluza software (Beckman Coulter Inc.).
Soft agar assay
Soft agar assay was performed in a 6-well plate with each well containing 2 layers of agarose (Calbiochem, Darmstadt, Germany) in maintenance medium. The lower layer of well contains 0.5% agarose while the upper layer contains 0.35% agarose. Single cell suspensions were prepared from mammospheres, which are obtained from the transfected cells and added into the upper layer of agarose. The cells were incubated for 2–3 weeks and fed twice a week with fresh maintenance medium. Colonies formation was observed and stained with 0.005% crystal violet (Acros Organics), and counted using ImageJ software.
Quantitative RT-PCR
For the quantitative RT- PCR, total RNA was extracted using High Pure RNA Isolation Kit (Roche, Germany) according to the manufacture's instructions from mammospheres obtained by transfecting the breast cancer cells. Total RNA concentration was measured by NanoDrop™ spectrophotometer (Thermo Scientific, USA) and stored at −80°C. Then, 0.5 ug total RNA was reverse transcribed into cDNA using Maxima® First Strand cDNA Synthesis Kit for qRT-PCR (Thermo Scientific, USA) using CFX96™ real-time PCR detection system (Biorad, USA). Next, cDNA was added to the reaction mix containing iQ™ SYBR® Green Supermix (Biorad, USA) and primers (). Each gene reaction were performed in triplicates using a 2-step PCR amplification program with initial denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 10 s and 30 s at different primer-specific annealing/extension temperature using CFX96™ real-time PCR detection system (Biorad, USA). Glucose-6-phosphate dehydrogenase (GAPDH) was used as housekeeping gene to normalize the amount of cDNA between different samples. Cycle threshold (CT) values from the logarithmic amplification phase were used for calculating relative quantification of each gene by applying the −2ΔΔCt method.
Table 1. Primers utilized for the qRT-PCR experiment performed in this study
Statistics
All the statistics (One way Anova including Bonferroni post hoc test) were performed using Prism (version 5.0 d) and P-value <0.05 is considered as statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1041692_supplemental_files.zip
Download Zip (1.9 MB)Funding
MJL kindly acknowledges the core/startup support from Linkoping University, from Integrative Regenerative Medicine Center (IGEN), from VR-NanoVision (K2012-99X-22325-01-5), and from Cancerfonden (2013/391). ACP thankfully acknowledges the support from BK/265/RAU1/2014/t.10. TK acknowledges the support of the Canadian Breast Cancer Foundation (CBCF) and the Natural Sciences and Engineering Research Council of Canada (NSERC).
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, Batakis P, Wiechec E. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis 2014; 35:747-59; PMID:24531939; http://dx.doi.org/10.1093/carcin/bgu045
- Wasik AM, Grabarek J, Pantovic A, Cieslar-Pobuda A, Asgari HR, Bundgaard-Nielsen C, Rafat M, Dixon IM, Ghavami S, Los MJ. Reprogramming and carcinogenesis–parallels and distinctions. Intl Rev Cell Mol Biol 2014; 308:167-203; PMID:24411172; http://dx.doi.org/10.1016/B978-0-12-800097-7.00005-1
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003; 100:3983-8; PMID:12629218; http://dx.doi.org/10.1073/pnas.0530291100
- Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol 2004; 5:738-43; PMID:15170211; http://dx.doi.org/10.1038/ni1080
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature 2004; 432:396-401; PMID:15549107; http://dx.doi.org/10.1038/nature03128
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445:106-10; PMID:17122772; http://dx.doi.org/10.1038/nature05372
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res 2007; 67:1030-7; PMID:17283135; http://dx.doi.org/10.1158/0008-5472.CAN-06-2030
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A 2007; 104:973-8; PMID:17210912; http://dx.doi.org/10.1073/pnas.0610117104
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005; 65:10946-51; PMID:16322242; http://dx.doi.org/10.1158/0008-5472.CAN-05-2018
- Hombach-Klonisch S, Paranjothy T, Wiechec E, Pocar P, Mustafa T, Seifert A, Zahl C, Gerlach KL, Biermann K, Steger K, et al. Cancer stem cells as targets for cancer therapy: selected cancers as examples. Archivum Immunol Ther Exp 2008; 56:165-80; PMID:18512024; http://dx.doi.org/10.1007/s00005-008-0023-4
- Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers – therapeutic implications. Trends Mol Med 2008; 14:450-60; PMID:18775674; http://dx.doi.org/10.1016/j.molmed.2008.08.003
- Jangamreddy JR, Ghavami S, Grabarek J, Kratz G, Wiechec E, Fredriksson BA, Rao Pariti RK, Cieslar-Pobuda A, Panigrahi S, Los MJ. Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: differences between primary and cancer cells. Biochim Biophy Acta 2013; 1833:2057-69; PMID:23639289; http://dx.doi.org/10.1016/j.bbamcr.2013.04.011
- Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cell Mol Life Sci 2008; 65:3145-67; PMID:18581055; http://dx.doi.org/10.1007/s00018-008-8111-5
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008; 27:5497-510; PMID:18794884; http://dx.doi.org/10.1038/onc.2008.245
- Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta 2013; 1830:2481-95; PMID:23196196; http://dx.doi.org/10.1016/j.bbagen.2012.11.008
- Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, Hung MC. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem 2000; 275:8027-31; PMID:10713122; http://dx.doi.org/10.1074/jbc.275.11.8027
- Nassif NT, Lobo GP, Wu X, Henderson CJ, Morrison CD, Eng C, Jalaludin B, Segelov E. PTEN mutations are common in sporadic microsatellite stable colorectal cancer. Oncogene 2004; 23:617-28; PMID:14724591; http://dx.doi.org/10.1038/sj.onc.1207059
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Ann Rev Cell Dev Biol 2001; 17:615-75; PMID:11687500; http://dx.doi.org/10.1146/annurev.cellbio.17.1.615
- Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res 1999; 253:239-54; PMID:10579926; http://dx.doi.org/10.1006/excr.1999.4701
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Ann Rev Biochem 1998; 67:481-507; PMID:9759495; http://dx.doi.org/10.1146/annurev.biochem.67.1.481
- Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev 2000; 14:1027-47; PMID:10809663
- Ye K. PIKE/nuclear PI 3-kinase signaling in preventing programmed cell death. J Cell Biochem 2005; 96:463-72; PMID:16088938; http://dx.doi.org/10.1002/jcb.20549
- Martelli AM, Faenza I, Billi AM, Manzoli L, Evangelisti C, Fala F, Cocco L. Intranuclear 3′-phosphoinositide metabolism and Akt signaling: new mechanisms for tumorigenesis and protection against apoptosis? Cell Signal 2006; 18:1101-7; PMID:16516442; http://dx.doi.org/10.1016/j.cellsig.2006.01.011
- Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 2007; 26:1338-45; PMID:17322919; http://dx.doi.org/10.1038/sj.onc.1210202
- Maddika S, Panigrahi S, Wiechec E, Wesselborg S, Fischer U, Schulze-Osthoff K, Los M. Unscheduled Akt-triggered activation of cyclin-dependent kinase 2 as a key effector mechanism of apoptin's anticancer toxicity. Mol Cell Biol 2009; 29:1235-48; PMID:19103742; http://dx.doi.org/10.1128/MCB.00668-08
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol 2004; 15:177-82; PMID:15209377; http://dx.doi.org/10.1016/j.semcdb.2004.01.002
- Los M, Maddika S, Erb B, Schulze-Osthoff K. Switching Akt: from survival signaling to deadly response. BioEssays 2009; 31:492-5; PMID:19319914; http://dx.doi.org/10.1002/bies.200900005
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis 2004; 9:667-76; PMID:15505410; http://dx.doi.org/10.1023/B:APPT.0000045801.15585.dd
- Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med 2002; 8:1153-60; PMID:12244302; http://dx.doi.org/10.1038/nm761
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol 2001; 3:245-52; PMID:11231573; http://dx.doi.org/10.1038/35060032
- Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006; 25:2697-707; PMID:16407845; http://dx.doi.org/10.1038/sj.onc.1209307
- Fischer KM, Din S, Gude N, Konstandin MH, Wu W, Quijada P, Sussman MA. Cardiac progenitor cell commitment is inhibited by nuclear Akt expression. Circ Res 2011; 108:960-70; PMID:21350213; http://dx.doi.org/10.1161/CIRCRESAHA.110.237156
- Gong J, Traganos F, Darzynkiewicz Z. Growth imbalance and altered expression of cyclins B1, A, E, and D3 in MOLT-4 cells synchronized in the cell cycle by inhibitors of DNA replication. Cell Growth Differ 1995; 6:1485-93.
- Cieslar-Pobuda A, Back M, Magnusson K, Jain MV, Rafat M, Ghavami S, Nilsson KP, Los MJ. Cell type related differences in staining with pentameric thiophene derivatives. Cytometry Part A 2014; 85:628-35; PMID:24500794; http://dx.doi.org/10.1002/cyto.a.22437
- Magnusson K, Appelqvist H, Cieślar-Pobuda A, Wigenius J, Karlsson T, Łos MJ, Kågedal B, Jonasson J, Nilsson KPR. Differential vital staining of normal and transformed cells by an anionic conjugated polyelectrolyte. Cytometry Part A 2015; 87(3):262-72:( #14–120, provisionally accepted); PMID:25605326
- Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 2004; 94:884-91; PMID:14988230; http://dx.doi.org/10.1161/01.RES.0000124394.01180.BE
- Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem 2003; 87:1427-35; PMID:14713298; http://dx.doi.org/10.1046/j.1471-4159.2003.02113.x
- Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X, Zhou L, Liu C, Chen C, He J, et al. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol Cell 2012; 48:627-40; PMID:23041284; http://dx.doi.org/10.1016/j.molcel.2012.08.030
- Peltier J, Conway A, Keung AJ, Schaffer DV. Akt increases sox2 expression in adult hippocampal neural progenitor cells, but increased sox2 does not promote proliferation. Stem Cell Dev 2011; 20:1153-61; PMID:21028992; http://dx.doi.org/10.1089/scd.2010.0130
- Zhu J, Blenis J, Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A 2008; 105:6584-9; PMID:18451027; http://dx.doi.org/10.1073/pnas.0802785105
- Evangelisti C, Ricci F, Tazzari P, Chiarini F, Battistelli M, Falcieri E, Ognibene A, Pagliaro P, Cocco L, McCubrey JA, et al. Preclinical testing of the Akt inhibitor triciribine in T-cell acute lymphoblastic leukemia. J Cell Physiol 2011; 226:822-31; PMID:20857426; http://dx.doi.org/10.1002/jcp.22407
- Chaabane W, User SD, El-Gazzah M, Jaksik R, Sajjadi E, Rzeszowska-Wolny J, Los MJ. Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp 2013; 61:43-58; PMID:23229678; http://dx.doi.org/10.1007/s00005-012-0205-y
- Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 2014; 112:24-49; PMID:24211851; http://dx.doi.org/10.1016/j.pneurobio.2013.10.004
- Badve S, Collins NR, Bhat-Nakshatri P, Turbin D, Leung S, Thorat M, Dunn SE, Geistlinger TR, Carroll JS, Brown M, et al. Subcellular localization of activated AKT in estrogen receptor- and progesterone receptor-expressing breast cancers: potential clinical implications. Am J Pathol 2010; 176:2139-49; PMID:20228224; http://dx.doi.org/10.2353/ajpath.2010.090477
- Wiechec E. Implications of genomic instability in the diagnosis and treatment of breast cancer. Expert Rev Mol Diagn 2011; 11:445-53; PMID:21545260; http://dx.doi.org/10.1586/erm.11.21
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001; 414:105-11; PMID:11689955; http://dx.doi.org/10.1038/35102167
- Zhang M, Rosen JM. Stem cells in the etiology and treatment of cancer. Curr Opin Genet Dev 2006; 16:60-4; PMID:16377171; http://dx.doi.org/10.1016/j.gde.2005.12.008
- Tan S, Ng Y, James DE. Next-generation Akt inhibitors provide greater specificity: effects on glucose metabolism in adipocytes. Biochem J 2011; 435:539-44; PMID:21348862; http://dx.doi.org/10.1042/BJ20110040
- Chan CK, Jans DA. Using nuclear targeting signals to enhance non-viral gene transfer. Immunol Cell Biol 2002; 80:119-30; PMID:11940112; http://dx.doi.org/10.1046/j.1440-1711.2002.01061.x
