Abstract
We have previously demonstrated that the neural stem-cell marker nestin is expressed in hair follicle stem cells located in the bulge area which are termed hair-follicle-associated pluripotent (HAP) stem cells. HAP stem cells from mouse and human could form spheres in culture, termed hair spheres, which are keratin 15-negative and CD34-positive and could differentiate to neurons, glia, keratinocytes, smooth muscle cells, and melanocytes in vitro. Subsequently, we demonstrated that nestin-expressing stem cells could effect nerve and spinal cord regeneration in mouse models. In the present study, we demonstrated that HAP stem cells differentiated to beating cardiac muscle cells. We separated the mouse vibrissa hair follicle into 3 parts (upper, middle, and lower), and suspended each part separately in DMEM containing 10% FBS. All three parts of hair follicle differentiated to beating cardiac muscle cells as well as neurons, glial cells, keratinocytes and smooth muscle cells. The differentiation potential to cardiac muscle is greatest in the upper part of the follicle. The beat rate of the cardiac muscle cells was stimulated by isoproterenol and inhibited by propanolol. HAP stem cells have potential for regenerative medicine for heart disease as well as nerve and spinal cord repair.
Introduction
The stem cell marker nestin is expressed in hair follicles in cells located above the bulge area, below the sebaceous gland. The nestin-expressing hair follicle cells were discovered in transgenic mice with nestin-driven green fluorescent protein (ND-GFP).Citation1-4 The nestin-expressing cells can differentiate into neurons, glial cells, smooth muscle cells, keratinocytes and other cell types.Citation3 We have termed these cells hair follicle-associated pluripotent (HAP) stem cells.Citation5
HAP stem cells originate in the bulge area (BA) and migrate to the DP. HAP stem cells from the DP and BA cells differentiated into neuronal and glial cells after transplantation to the injured spinal cord and enhanced injury repair and locomotor recovery within 4 weeks.Citation6-8
In Gelfoam® histoculture, HAP stem cells from mouse whisker follicles formed nerve-like structures contained β-III tubulin-positive fibers.9 The growing fibers had growth cones on their tips expressing F-actin, indicating they were growing axons. These results suggest a major function of HAP stem cells is for growth of the follicle sensory nerve.Citation9 HAP stem cells can be cryopreserved with full function.Citation10
In the present study, we demonstrate that HAP stem cells can differentiate to beating cardiac muscle cells.
Results
The differentiation potential to cardiac muscle cells is greatest in the upper part of the hair follicle
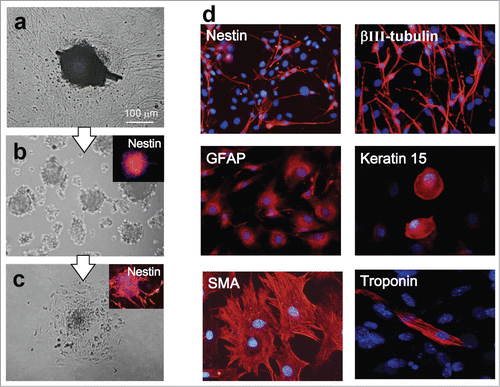
We divided the mouse vibrissa hair follicle into 3 parts (upper, middle, and lower part), and suspended each part separately in DMEM containing 10% FBS (). Two weeks after culture all 3 parts (upper, middle, and lower part) of the hair follicle cells that differentiated to troponin- and desmin-positive cardiac muscle cells appeared. The number of cardiac muscle cells differentiating from the upper part of the hair follicle was significantly higher compared to the middle, and lower parts of the hair follicle (). Fluorescence-activated cell sorting (FACS) analysis showed that all 3 parts of hair follicle could produce troponin-positive cardiac muscle cells as well as βIII-tubulin-positive neurons, K15-positive keratinocytes, smooth muscle actin-positive cells, and GFAP-positive glial cells. The differentiation potential to form all these cell types was greatest in the upper part of the hair follicle compared to the middle, and lower parts (, ).
Figure 1. (A) Schematic of the separated mouse vibrissa hair follicle. The upper part of the vibrissa hair follicle contains nestin-expressing, CD34-positive, K15-negative hair-follicle-associated-pluripotent (HAP) stem cells. (B) The mouse vibrissa hair follicle was separated into 3 parts (upper, middle, and lower part). Each hair follicle part was separately suspended in DMEM containing 10% FBS. (C) Two weeks after culture all 3 parts (upper, middle, and lower) of the hair follicle, incubated in DMEM with 10% FBS cells differentiated to troponin- and desmin-positive cardiac muscle cells. The number of cardiac muscle cells was significantly higher in the upper part compared to the middle, and lower part of the hair follicle. Bars 100 μm.
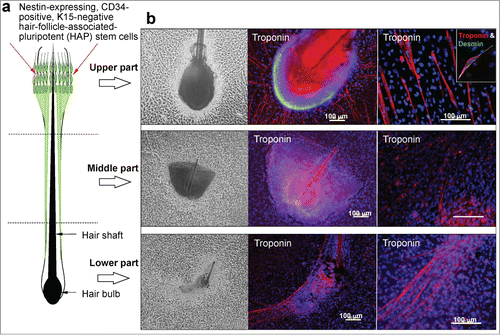
Figure 2. (A) Total cell number differentiated from the upper, middle and lower part of the hair follicle. (B) The number of cardiac muscle cells differentiated from the upper, middle and lower parts of the hair follicle. *P < 0.01 vs upper part.
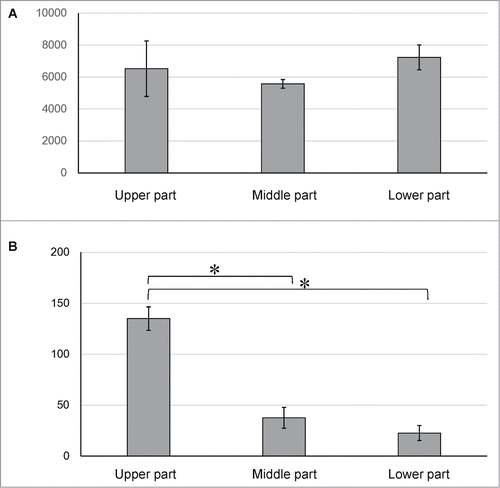
Figure 3. Fluorescence-activated cell sorting (FACS) analysis showed that all 3 parts of the hair follicle differentiated to troponin-positive cardiac muscle cells, βIII-tubulin-positive neurons, K15-positive keratinocytes, smooth muscle actin-positive cells, and GFAP-positive glial cells.
Table 1. Percentage of cardiac muscle cells and other cell types differentiate from the separated upper, middle and lower parts of the mouse whisker follicle
Isoproterenol increases the spontaneous beating rate in cardiac muscle cells differentiated from the hair follicle
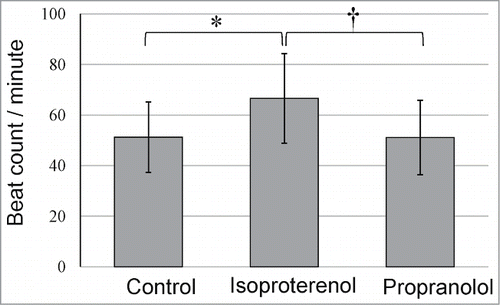
The spontaneous, unstimulated beating rate of cardiac muscle cells differentiated from the whisker hair follicle ranged from 51.3 to 66.6 (n = 10; average 51.3 ± 14) beats/minute (Supplemental video). The spontaneous beating rate increased significantly by 130.3% with isoproterenol treatment. Propranolol reduced the isoproterenol-induced increase in the beating rate by 99.2% ().
Cardiac muscle cells differentiated from hair-spheres
Four weeks after culture of the upper part of the hair follicle in DMEM with 10% FBS, out-growing cells were transferred to DMEM/F12 without fetal bovine serum (FBS). One week after culture in DMEM/F12 without FBS, the growing cells formed many hair spheres containing nestin-expressing HAP stem cells (). Two days after transfer to DMEM with FBS, the hair spheres began to differentiate (). One week after transfer to DMEM/F12 without FBS, the hair spheres differentiated to troponin- and desmin-positive cardiac muscle cells as well as nestin- and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes and actin-positive smooth muscle cells ().
Figure 5. (A) The upper part of hair follicle was cultured for 4 weeks in DMEM with 10% FBS. (B) Cells growing out from the upper part of the hair follicle were transferred to DMEM/F12 without FBS. Two weeks later, the growing cells formed many nestin-expressing hair spheres. (C) Two days after transfer to DMEM with 10% FBS, the hair spheres started to differentiate. (D) One week after switching to DMEM containing 10% FBS, the hair spheres differentiated to desmin-positive cardiac muscle cells, nestin- and βIII-tubulin-positive neurons, GFAP-positive glial cells, K15-positive keratinocytes, and actin-positive cells.
Wada et al.Citation11 reported that induced cardiomyocyte-like cells (iCMs) can be directly generated from mouse cardiac fibroblasts in vitro and vivo by transduction of 3 transcription factors: Gata4, Mef2c, and Tbx5. Berry et al.Citation12 reported that new cardiomyocytes formed in the dystrophic heart and that nestin-expressing interstitial cells could generate them in addition to other cells of the cardiac lineage. Wang et al.Citation13 showed that transplantation of mesenchymal stem cells facilitated cardiac muscle repair.
In the present study, the hair follicle differentiated to multiple cell types, including beating cardiac muscle cells expressing troponin. The differentiation potential to form beating cardiac muscle cells is greatest in the upper part of the hair follicle which is enriched in HAP stem cells above the bulge. Hair spheres, consisting of nestin-expressing HAP stem cells formed from the upper part of hair follicle also differentiated to cardiac muscle cells as well as neurons, glial cells, keratinocytes, smooth and muscle cells.
HAP stem cells are autologous, easily accessible and can be cryopreserved for banking,Citation10 making them highly attractive for regenerative medicine for heart disease as well as nerve and spinal cord repair.
Materials and Methods
C57BL/6-mice
C57BL/6 mice (CLEA Japan, Tokyo Japan) were used to isolate the vibrissa hair follicles. All animal experiments were conducted according to the Guidelines for Animal Experimentation at Kitasato University.
Isolation and division of vibrissa hair follicles
To isolate vibrissa hair follicles from C57BL/6 mice, the upper lip containing the vibrissa pad was cut under anesthesia, and the inner surface was exposed. Whole vibrissae hair follicles were dissected under a binocular microscope and plucked from the pad by pulling them gently by the neck with fine forceps. The isolated vibrissae were washed in DMEM/F12 (GIBCO, Grand Island, NY) with 50 μg/ml gentamicin (GIBCO). All surgical procedures were done under a sterile environment. The vibrissa hair follicles were surgically separated into 3 parts, upper, middle, and lower under a binocular microscope.
The divided vibrissa hair follicle part were resuspended into fresh DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS), 50 μg/ml gentamicine, 2 mM L-glutamin (Gibco), 10 mM HEPES (MP Biomedicals, Santa Ana, CA).
Differentiation of hair-follicle-associated-pluripotent (HAP) stem cells from different parts of the whisker hair follicle
Four weeks after culture of the separated parts of the whisker with in DMEM with FBS as described above, they were transferred to DMEM/F12 without FBS. One week after culture in DMEM/F12 without FBS, the cells growing out from the follicle formed hair spheresCitation14 containing HAP stem cells. Two days after transfer to DMEM with 10% FBS, the hair spheres began to differentiate and one week later, the HAP stem cells differentiated to multiple types of cells.
Immunofluorescence staining and FACS of differentiated cells
The primary antibodies used were: anti-βIII-tubulin monoclonal (1:500, Tuj1 clone; Covance, San Diego, CA); anti-glial fibrillary acidic protein (GFAP) chicken polyclonal (1:300; Abcam, Kinxton, Cambridge, UK); anti-keratin 15 (K15) monoclonal (1:100; Lab Vision, Fremont, CA); anti-smooth muscle actin (SMA) monoclonal (1:200; Lab Vision); anti-cardiac toroponin T monoclonal (1:500; GeneTex, Hsinchu City, Taiwan); anti-desmin rabbit polyclonal (1:25; Cell Signaling, Tokyo, Japan); and anti-nestin monoclonal (1:200, rat401 clone; Millipore, Tokyo, Japan).
Secondary antibodies for immunofluorescence were Alexa Fluor® 568-conjugated goat anti-mouse (1:400; Molecular Probes, Eugene, Oregon); Alexa Fluor® 488-conjugated goat anti-rabbit (1:400; Molecular Probes) and Alexa Fluor® 568-conjugated goat anti-chicken (1:1000; Molecular Probes). For immunofluorescence staining quantification of the percentage of cells producing a given marker protein, in any given experiment at least 4 fields were photographed, and the number of positive cells determined relative to the total number of cells.
Secondary antibodies for fluorescence-activated cell sorting (FACS) were goat anti-mouse IgG H&L phycoerythrin (1:500; Abcam), and goat anti-chicken IgY biotinylated (1:1000; R&D Systems, Minneapolis, MN), Brilliant Violet 421TM streptavidin (1:1000; BioLegend, San Diego, CA) was also used. Immunofluorescence staining and FACS were repeated in triplicate.
Effect of isoproterenol and proterenol on cardiac muscle cells differentiated from HAP stem cells
Cardiac muscle cells differentiated from HAP stem cells were treated with isoproterenol (Sigma-Aldrich) (200 nm) and propranolol (Sigma-Aldrich) (200 nM). The beat rate per minute of the cardiac muscle cells was recorded by video microscopy (ScopPad-500, GelleX, Tokyo, Japan).
Statistical analysis
The experimental data are expressed as the mean ± SD. Statistical analyses were performed with the paired Student's t test with a p-value of ≤ 0.05 considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1042633_supplemental_movie.wmv
Download (3.3 MB)Funding
This work was partially supported by Grant-in-Aid for Scientific Research (C) 23591653 from the Ministry of Education, Science, Sports, and Culture of Japan, a grant from the Ministry of Education, Culture, Sports, Science, and Technology of the Japan (Assistance for Strategic Creation of Research Basis, 2014–2016), and the Terumo Life Science Foundation (to Y. Amoh) and by National Institute of Neurological Disorders and Stroke grant NS086217.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G, Hoffman RM. Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 2003; 100:9958-61; PMID:12904579; http://dx.doi.org/10.1073/pnas.1733025100
- Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA 2004; 101:13291-95; PMID:15331785; http://dx.doi.org/10.1073/pnas.0405250101
- Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA 2005; 102:5530-34; PMID:15802470; http://dx.doi.org/10.1073/pnas.0501263102
- Amoh Y, Li L, Katsuoka K, Hoffman RM. Chemotherapy targets the hair-follicle vascular network but not the stem cells. J Invest Dermatol 2007; 127:11-15; PMID:16841031; http://dx.doi.org/10.1038/sj.jid.5700486
- Hoffman RM. Nestin-expressing hair follicle-accessible pluripotent (HAP) stem cells for nerve and spinal cord repair. Cells Tissues Organs; 2015; 200:42-47; PMID:25766743
- Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L, Koenig K, Duong J, Aki R, Saito N, et al. The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 2011; 10:830-39; PMID:21330787; http://dx.doi.org/10.4161/cc.10.5.14969
- Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells which support repair of severed peripheral nerves. Proc Natl Acad Sci USA 2005; 102:17734-38; PMID:16314569; http://dx.doi.org/10.1073/pnas.0508440102
- Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 2008; 7:1865-69; PMID:18583926; http://dx.doi.org/10.4161/cc.7.12.6056
- Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y, Saito N, Katsuoka K, Hoffman RM. The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 2013; 114:1674-84; PMID:23444061; http://dx.doi.org/10.1002/jcb.24509
- Kajiura S, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K, Li L, Katsuoka K, Hoffman RM, Amoh Y. Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair follicle-associated pluripotent (HAP) stem cells. Tissue Engineering, Part C; in press
- Wada R, Muraoka N, Inagawa K, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Kaneda R, Suzuki T, Kamiya K, et al. Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc Natl Acad Sci USA 2013; 110:12667-72; PMID:23861494; http://dx.doi.org/10.1073/pnas.1304053110
- Berry SE, Andruszkiewicz P, Chun JL, Hong J. Nestin expression in end-stage disease in dystrophin-deficient heart: implications for regeneration from endogenous cardiac stem cells. Stem Cells Transl Med 2013; 2:848-61; PMID:24068741; http://dx.doi.org/10.5966/sctm.2012-0174
- Wang M, Zhang G, Wang Y, Liu T, Zhang Y, An Y, Li Y. Crosstalk of mesenchymal stem cells and macrophages promotes cardiac muscle repair. Int J Biochem Cell Biol 2015; 58:53-61; PMID:25462160; http://dx.doi.org/10.1016/j.biocel.2014.11.003
- Yu H, Fang D, Kumar SM, Li L, Nguyen TK, Acs G, Herlyn M, Xu X. Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol 2006; 168:1879-88; PMID:16723703; http://dx.doi.org/10.2353/ajpath.2006.051170