During B-cell ontogeny and maturation, Ig loci undergo successive transcription coupled remodeling events: V(D)J recombination and class switch recombination (CSR) for the IgH locus, and VJ recombination for IgL loci. Both loci also undergo somatic hypermutation (SHM) to improve antibody affinity. RAG-mediated V(D)J recombination occurs during the early antigen-independent step of B-cell development. AID-mediated CSR and SHM occur later, and require interaction with cognate antigen. These transcription-induced events involve a succession of DNA breaks and repairs, and thus represent potentially oncogenic events, hence implying a strict regulation. Ig cis-regulatory elements and especially transcriptional enhancers are major locus regulators. While 5′ cis-regulatory elements (Eμ and Eκi for IgH and Igκ locus, respectively) control V(D)J recombination, SHM is controlled by the IgH 3′ regulatory region (3′RR)Citation1 (for IgH locus) and the 3′Eκ (for Igκ locus). The 3′RR is also the master regulator of CSR.Citation2 Although less documented, transcriptional enhancers are present in the Igλ locus.Citation3 Chromosomal translocations linking oncogenes to these elements are implicated in various B-cell malignancies.Citation3-5
If the deregulation of the translocated oncogene by enhancer elements located in cis was extensively studied,Citation4,5 little is known about a potential trans effect of the Ig enhancers, for example the role of the IgH 3′RR on lymphomagenesis caused by an oncogene translocation to the IgL locus. Indeed, studies have shown that deletion of the 3′RR modulates the B-cell fate by lowering the BCR signaling,Citation6 which is of key importance in B-cell malignancies. We recently studied the impact of the absence of the 3′RR in mice carrying a transgene mimicking the translocation of c-myc under the transcriptional control of the Igλ enhancers.Citation7 Our results indicate a wide alteration of B-cell lymphoma phenotypes. While wt mice indifferently develop mature or immature lymphomas, 3′RR-deficient mice exhibit a strong preference for immature lymphomas. Furthermore, 3′RR-deficiency leads to a lowered frequency of CD43+ activated mature B-cell lymphomas and to an increased frequency of CD5+ B-cell lymphomas. Transcription level of the c-myc transgene is identical in B-cell lymphomas from wt and 3′RR-deficient mice, suggesting that the 3′RR does not directly regulate the expression of the oncogene bring under the control of the Igλ enhancers.Citation7
Two main models are suggested to explain the heterogeneity of B-cell malignancies: the various tumor subtypes may arise from different mutations within the same target B-cell or from the same mutation occurring during different stages of B-cell differentiation. Although these models are not mutually exclusive, our results reinforce the second hypothesis that the same initial oncogenic event occurring in B-cells with different maturity degree can lead to different B-lymphoma subtypes. Indeed, while wt and 3′RR deficient mice bear the same “mini-locus translocation” (i.e. c-myc under the transcriptional control of Igλ enhancers), they develop different tumor subtypes. The only difference that could explain this variation is the slightly altered B-cell maturation and the abolition of CSR and SHM in 3′RR-deficient mice. B-cells from both genetic backgrounds over express the myc oncogene, but need to accumulate secondary mutations to develop B-cell lymphomas (). Secondary mutations principally occur during the different genetic events that punctuate B-cell ontology. In wt B-cells, these oncogenic events can occur either during early step of maturation (i.e., during V(D)J recombination) or during the later stages (i.e. during CSR or SHM). CSR and SHM are abrogated in 3′RR-deficient mice,Citation1,2 lowering the probability of oncogenic mutations during these later stages of B-cell maturation, hence their increased frequency of immature B-cell lymphomas as compared with wt mice.
Figure 1. Schematic hypothesis on the implication of the 3′RR in the development of B-cell lymphomas induced by oncogene translocation in IgL locus. While not directly impacting the expression of the translocated oncogene, the absence of the 3′RR modifies B-cell lymphoma phenotypes by impairing the later steps of B-cell ontology.
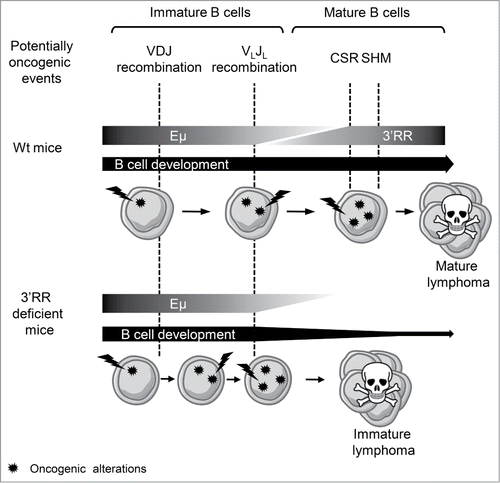
Taken together (), our results confirm the importance of considering both the cell of origin and the genetic alterations to determine the B-cell lymphoma phenotype and its behavior. Indeed, B-cells bearing the same initial oncogenic events can lead to different B-cell lymphoma subtypes if their development is impaired, while different mutations occurring in the same cell of origin can induce similar tumors.Citation4 In this context, we also show that the 3′RR can affect the phenotype of B-cell lymphomas due to oncogene translocation in other loci than the IgH one, by acting on B-cell maturation and regulating potentially oncogenic recombination and mutations during late B-cell differentiation.
References
- Rouaud P, et al. J Exp Med 2013; 21:1501-7; http://dx.doi.org/10.1084/jem.20130072
- Vincent-Fabert C, et al. Blood 2010; 116:1895-8; PMID:20538806; http://dx.doi.org/10.1182/blood-2010-01-264689
- Gerbitz A, et al. Oncogene 1999; 4:1745-53; http://dx.doi.org/10.1038/sj.onc.1202468
- Rouaud P, et al. Oncotarget 2012; 3:586-93; PMID:22592113
- Amin R, et al. Oncotarget 2014; 5:8995-9006; PMID:25229630
- Saintamand A, et al. Oncotarget 2015; 6:4845-52; PMID:25742787
- Saad F, et al. Oncotarget 2015; PMID:25742787
