Thanks to the development of antiretroviral therapy (ART) in the past 30 years, HIV has become a controllable disease with significantly reduced mortality rate, although it is still considered as an incurable disease. This is mainly because of the lack of effective method to completely remove HIV from its host reservoir cells. The fundamental reason mainly ascribes to the nature of the life cycle of HIV, which integrates into the host genome and acts as a latent provirus to evade host immunity. This persistence of HIV burden necessitates costly lifelong ART-based treatment in order to suppress the HIV viral titer in the patient blood stream. In addition, daily medication can elicit several issues, such as toxicity to long-term drug use or development of HIV strains resistant to ART due to the high mutation rate of the virus. These lingering issues keep challenging scientists to discover alternative methodologies to cure the HIV disease.Citation1
Fundamentally, there are 2 types of cures defined in the HIV therapies (). First, a sterilizing (or so called eradicating) cure completely disrupts all viruses from the patient body. Second, a functional cure, in contrast, can eliminate the existence of actively replicating virus from patient blood stream, but without eradication of all latent viruses from the entire body.Citation1 Although current ART treatments belong to the latter, an ideal functional cure should help patients get rid of the viral symptoms without daily medication for the rest of their lives. Here, we summarize the prospects of achieving these 2 types of cures beyond ART in light of the recent development of stem cell technology and an emerging gene-editing technique based on the use of the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein-9 nuclease (Cas9).
Figure 1. Potential functional and sterilizing cures of HIV by CRISPR/Cas9-mediated gene editing in human stem cells. CIRSPR/Cas9 can be used to functionally cure the HIV disease by disrupting the CCR5 gene. It can also be used as a sterilizing cure by excising the HIV-1 genome from host cells. When applying this CRISPR/Cas9 anti-HIV technology into stem cells, the therapy can be long lasting and has the potential to help patients avoid lifelong ART treatments.
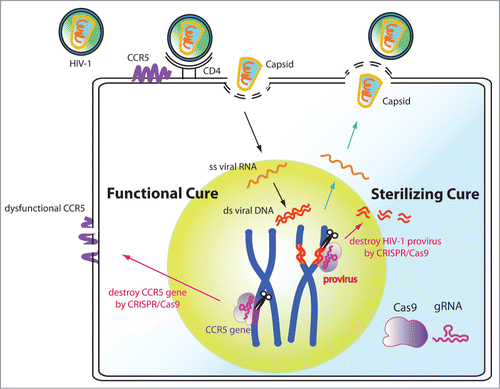
Stem cells have the abilities to self-renew and also to differentiate into a diversity of specialized cells. Autologous transplantation of haematopoietic stem or progenitor cells (HSPCs) that have undergone genetic modifications offers a potential functional cure for HIV. The C-C motif chemokine receptor 5 (CCR5) found on the surface of white blood cells acts as a co-receptor for HIV entry to the host cells. Genetic disruption of the CCR5 gene in either primary CD4+ T cells or HSPCs by zinc-finger nuclease (ZFNs) mediated gene editing has been shown to render the cells resistant to CCR5-tropic HIV strains.Citation2 These pioneering proof-of-concept studies prove that precision genome editing in stem cells offers an exciting new avenue of anti-viral therapy.
Originally derived from a bacterial adaptive immune system against foreign DNA, CRISPR/Cas9 has been successfully adapted as a new gene editing technology in mammalian cells and in several model organisms. Due to the RNA-directed nature of the CRISPR/Cas9 system, its advantages include the relatively easy assembly and the possibility to target multiple loci at the same time. Several groups have applied the CRISPR/Cas9 system to genetically modify the CCR5 co-receptor of HIV. The CCR5-disrupted immune cells showed a significant reduction of HIV infection rate. This shows that as in the case of ZFNs, CRISPR/Cas9 genome editing technology also has a great potential as a simply “functional cure” to control the HIV infection.Citation3,4
However, the ZFN studies shown that although dysfunction of CCR5 in either primary CD4+ T cells or HSPCs confers resistance to multiple CCR5-tropic HIV strains, it does not control the viral level of CXCR4-tropic HIV-1 strain.Citation2 More importantly, the CCR5 disruption does not directly remove latent HIV viruses from the genome of infected cells. These caveats beckon new methodology for “sterilizing cure” that can remove integrated HIV genome from patients.
We and other groups have been working on using the CRISPR-based strategies to eradicate the latent HIV genome from its reservoirs.Citation5,6 As a proof of concept, the CRISPR/Cas9 system was successfully applied for the excision of the HIV provirus by targeting in the viral LTR region.Citation7 Multiplex targeting/disruption of the HIV genome enhances the reduction of HIV expression in physiological-relavent cell types.Citation5,6 Moreover, human stem cells with an engineered anti-HIV CRISPR/Cas9 system can be differentiated into monocyte and macrophage lineages, and remain protected against HIV in these viral reservoirs.Citation6 These studies pave a new avenue for novel research and clinical strategies that combine CRISPR/Cas9 with stem cell technologies to treat a wide range of viral infectious diseases.
With the recent advance in gene therapy, HIV has moved closer to a potential cure. Whether the CRISPR based stem cell therapy can further advance the HIV medicine from a functional cure to a sterilizing cure, which completely eradicates HIV from the patient, is the next exciting question.
References
- Abou-El-Enein M, et al. Trends Mol Med 2014; 20:632-42; PMID:25262540; http://dx.doi.org/10.1016/j.molmed.2014.08.004
- Holt N, et al. Nat Biotechnol 2010; 28:839-47; PMID:20601939; http://dx.doi.org/10.1038/nbt.1663
- Wang W, et al. PloS One 2014; 9:e115987; PMID:25541967; http://dx.doi.org/10.1371/journal.pone.0115987
- Li C, et al. J Gen Virol 2015.
- Hu W, et al. Proc Natl Acad Sci U S A 2014; 111:11461-6; PMID:25049410; http://dx.doi.org/10.1073/pnas.1405186111
- Liao HK, et al. Nat Commun 2015; 6:6413; PMID:25752527; http://dx.doi.org/10.1038/ncomms7413
- Ebina H, et al. Sci Rep 2013; 3:2510; PMID:23974631; http://dx.doi.org/10.1038/srep02510
