Abstract
The application of Next-Generation Sequencing for studying the genetics of papillary thyroid carcinomas (PTC) has recently revealed new somatic mutations and gene fusions as potential new tumor-initiating events in patients without any known driver lesion. Gene and miRNA expression analyses defined clinically relevant subclasses correlated to tumor progression. In addition, it has been shown that tumor driver mutations in BRAF, and RET rearrangements - altogether termed “BRAF-like” carcinomas - have a very similar expression pattern and constitute a distinct category. Conversely, “RAS-like” carcinomas have a different genomic, epigenomic, and proteomic profile. These findings justify the need to reconsider PTC classification schemes.
1
Thyroid carcinomas represent one of the most common malignancies of the endocrine systemCitation1 and the incidence has increased 3-fold over the past 30 years. The vast majority of thyroid cancer (more than 95%) derives from follicular cells.Citation2-4 Well-differentiated tumors of this gland include papillary (PTC) and follicular (FTC) histotypes.Citation2-4 The former accounts for about 90% of all thyroid carcinomas. Conversely, anaplastic carcinomas (ATC) are undifferentiated and very rare (2–5% of cases). The ATC are extremely aggressive and insensitive to conventional radiotherapy and chemotherapy.Citation5,6 The major risk factor for PTC is the exposure to ionizing radiations. Indeed, Hiroshima and Nagasaki survivors, patients treated in the 1950's with head and neck irradiation to cure thymic hyperplasia or mycotic infections, as well as children in Belarus and Ukraine (after the nuclear disaster of Chernobyl in 1986), exhibited an increased PTC incidence.Citation7 Genetic factors, other than the environmental ones, play a key role in thyroid carcinogenesis. Familial forms of thyroid neoplasia associated with tumor syndromes are caused by known germline mutations.Citation8 In addition, common variants in FOXE1 and NKX2 genes have been recently associated to an increased risk (5.7-fold) of both PTC and FTC.Citation9 Non-familial PTC forms are caused by mutations and rearrangements in oncogenes. In particular, mutually exclusive mutations in genes belonging to the mitogen-activated protein kinase (MAPK), such as RET, TRK, RAS and BRAF, have been found in about 70% of PTC cases.Citation10-12 Rearrangements of RET proto-oncogene - with the fusion of its tyrosine kinase (TK) domain with other genes resulting in its constitutive activation - have been found in about 30% of PTCs.Citation13 In the remaining PTC cases, specific point mutations in BRAF (V600E) and RAS genes (K-RAS, H-RAS and N-RAS) have been identified.Citation10,11 Nonetheless, no oncogene mutations have been reported in about 25–30% of PTCs (defined as “dark matter”).
The introduction of Next-Generation Sequencing (NGS) technologies has significantly expanded our view about cancer genetics.Citation14,15 Recent NGS-based studies have explored the mutational landscape and gene expression profiles of PTCs.Citation16-19 Smallridge and colleaguesCitation16 performed RNA-Seq to identify genes differentially expressed between BRAF- and not BRAF-mutated PTCs. They found that about 10% of these genes (i.e., more than 50) were related to immune functions. Through NGS they also identified 4 high-confidence fusion transcripts in PTC samples (i.e., CKLF3-CMTM4, ETV6-NTRK3, MKRN1-BRAF and PPIP5K1-CATSPER2). Notably, CKLF3-CMTM4, ETV6-NTRK3, MKRN1-BRAF gene fusions have been found in 3 different not BRAF-mutated PTC samples, indicating that these may potentially represent new driver events, although with a very rare occurrence.
Similarly, Leeman-Neill and colleaguesCitation18 carried out RNA-Seq to identify new chromosomal rearrangements correlated to ionizing radiations. They found that ETV6-NTRK3, RET/PTC and PAX8-PPARG rearrangements are significantly more common than point mutations in PTCs associated with exposure to 131I.
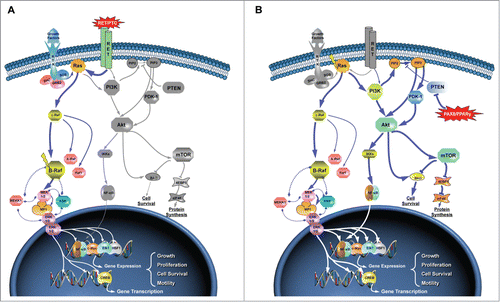
Recently, the seminal work of The Cancer Genome Atlas (TCGA) Research Network has explored more than 400 PTCs.Citation17 In this study, the authors describe a comprehensive multiplatform analysis of the genetic landscape of PTC, performed by SNP arrays, exomes, RNA-Seq, miRNA-Seq, DNA methylation and targeted sequencing. One of the most significant advances was the identification of somatic alterations (single nucleotide variants, INDELSs and gene fusions) as potential new tumor-initiating events - i.e. the “dark matter” - in patients without any known driver lesion. In particular, the authors identified EIF1AX, PPM1D and CHEK2 as new driver PTC genes, and also discovered TERT promoter mutations in a subset of aggressive and less-differentiated PTCs, strongly correlated to a high risk of recurrence. Gene and miRNA expression analysis also allowed defining clinically relevant subclasses potentially correlated to loss of differentiation and tumor progression (e.g. over-expressed miR-21 in association with aggressive tall cell variant of PTC). In addition, handling information about the mutation status of these samples, they could define their gene expression signature, determining the differential signaling consequences of BRAF and RAS driver mutations on the activation of MAPK signaling (schematized in ). Through the combination of multi-level molecular data, this study has demonstrated that BRAF-like and RAS-like PTCs differ in their genomic, epigenomic, and proteomic profiles, and that RAS-like papillary carcinomas are more similar to the follicular ones. These findings justify the need to reconsider PTC classification schemes.
Figure 1. Driver genetic lesions in papillary thyroid carcinoma. In both panels, colored and gray proteins indicate activated or non-activated proteins, respectively. Line thickness is proportional to the extent of activation. (A) As depicted, BRAF exerts its activity via MEK1 and MEK2 proteins, which in turn activate ERK1 and ERK2. Activated proteins translocate into the nucleus where activate distinct nuclear transcription factors, enhancing gene transcription. BRAFV600E mutation, common in PTCs as well as other epithelial-derived tumors, leads to the constitutive activation of BRAF protein, which leads to the hyper-activation of downstream effectors of the MAPK pathway, as well as to non-responsiveness to ERK inhibitory feedback on BRAF itself. PTC patients with RET gene fusions (namely RET/PTC) show a phenotype - and a gene expression profile - that is similar to BRAFV600E PTCs. (B) RAS protein(s) operates upstream the MAPK and phosphoinositide-3 kinase (PI3K) pathways. Specific somatic mutations in RAS genes (HRAS, KRAS and NRAS) maintain RAS protein in a constitutive active state. Such hyper-activation mediates its cellular effects either by the activation of the MAPK cascade and the PI3K pathway, while PAX8-PPARg gene fusion leads to the inhibition of the PTEN inhibitory effect and to the activation of PI3K signaling. Modified from Pathway Central (Qiagen).
A more recently published paper has confirmed, by RNA-Seq, that gene expression profiles of BRAF-mutated and RET/PTC samples are very similar among them, and that they significantly differ from RAS-mutated PTCs.Citation19 Based on RNA-Seq data they could assign, by gene expression profiles' similarity, PTC samples without known genetic lesions to RAS- and BRAF-like categories. Despite the relatively small number of analyzed PTC samples (n = 18), integrating RNA-Seq expression data to known driver events data (both mutations and gene rearrangements), they found that BRAF-like gene expression levels correlate more with the mutation pattern than with tumor staging. Combining RNA-Seq and targeted Sanger sequencing they also identified in PTC samples new missense mutations in well-established cancer driver genes as CBL, SMARCA4 and NOTCH1, and new mutations in driver genes, already described in other cancer type (DICER1, MET and VHL). Notably, the DICER1E1813G mutation affects the metal binding site of RNase IIIb domain, which is involved in miRNA strand cutting. Even though somatic mutations in DICER1 are rare in cancer patients, hot-spot mutations in DICER1 have been recently identified in a range of nonepithelial ovarian cancers.Citation20 Such missense variations, although do not abrogate DICER1 function, alter its functionality in specific cell types, making this protein oncogenic. In addition, the same authors describe the identification of a new chimeric transcript generated by the fusion between the exon 1 of WNK1 gene, that encodes a lysine deficient protein kinase 1, and the exon 2 of B4GALNT3, encoding the β1,4-N-acetylgalactosaminyltransferase III, an enzyme that promotes the formation of GalNAcβ1,4GlcNAc (LacdiNAc).Citation19 Such genetic alteration appears to be a rare event. Indeed, it was identified in one out 18 analyzed PTCs. Interestingly, this gene fusion was not associated to any other known genetic alteration typical of PTC and its presence correlated with a significant over-expression of B4GALNT3 gene. Notably, B4GALNT3 overexpression has been reported in more than 70% of colon carcinomas compared with their normal counterparts.Citation21 Its over-expression resulted in enhanced cell-extracellular matrix adhesion, migration, anchorage-independent cell growth, and invasion of colon cancer cells. It also increased tumor growth and metastasis and decreased survival of tumor-bearing nude mice, suggesting that its up-regulation may promote tumor malignancy through integrin and MAPK signaling pathways. Moreover, a recent study has confirmed the oncogenic role of B4GALNT3 acting through an altered EGFR glycosylation and signaling.Citation22
Therefore, it would be interesting to assess whether this new rearrangement is also present in other cancer types, such as colon carcinoma. Of note, our preliminary results indicate that such a gene fusion is present quite frequently in colon carcinomas. Then, determining how such a genetic alteration is early in this process would be of crucial relevance. However, we should also mention that, at odds with the oncogenic activity of B4GALNT3 in colon cancer, Hsu and colleagues proposed this gene as tumor suppressor in human neuroblastoma. Its overexpression was correlated with favorable histologic profile and early clinical staging, and predicted a more favorable prognosis.Citation23 In addition, the rescue of B4GALNT3 in neuroblastoma cell lines suppressed cell proliferation and migration by decreasing b1 integrin signaling and leading to decreased phosphorylation of focal adhesion kinase, Src, paxillin, Akt and ERK1/2. The concomitant expression of constitutively active Akt or MEK reversed the B4GALNT3-mediated suppression of cell migration and invasion. Such apparent discrepancy in the oncogenic or tumor suppressor ability B4GALNT3 is likely to be associated to the distinct origin of the affected tissues and/or to the presence of other tissue-specific predisposing factors.
In conclusion, the above-mentioned papers have provided increased knowledge of the molecular pathogenesis of PTCs, and propose a re-classification scheme that more accurately reflects the genotypic and phenotypic differences between (and within) RAS-like and BRAF-like papillary carcinomas. They also have highlighted the presence of new mutations in PTC samples, also indicating that some of them may accumulate in patients with already known driver genetic lesions. The proteins encoded by these genes may potentially represent good candidates for adjuvant therapies in PTC treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin 2008; 58:71-96; PMID:18287387
- Carcangiu ML, Zampi G, Rosai J. Poorly differentiated (“insular”) thyroid carcinoma. A reinterpretation of Langhans' “wuchernde Struma”. Am J Surg Pathol 1984; 8:655-68; PMID:6476195
- LiVolsi VA, Asa SL. The demise of follicular carcinoma of the thyroid gland. Thyroid 1994; 4:233-6; PMID:7920009; http://dx.doi.org/10.1089/thy.1994.4.233
- Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 2006; 6:292-306; PMID:16557281
- Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol 1999; 16:64-9; PMID:9890741
- Yau T, Lo CY, Epstein RJ, Lam AK, Wan KY, Lang BH. Treatment outcomes in anaplastic thyroid carcinoma: survival improvement in young patients with localized disease treated by combination of surgery and radiotherapy. Ann Surg Oncol 2008; 15:2500-5; PMID:18581185
- Williams D. Cancer after nuclear fallout: lessons from the Chernobyl accident. Nat Rev Cancer 2002; 2:543-9; PMID:12094241
- Lindor NM, Greene MH. The concise handbook of family cancer syndromes. Mayo. Familial Cancer Program. J Natl Cancer Inst 1998; 15;90:1039-71. Update in: J Natl Cancer Inst Monogr 2008; 38: 1–93
- Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, He H, Blondal T, Geller F, Jakobsdottir M, Magnusdottir DN, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nat Genet 2009; 41:460-4; PMID:19198613
- Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003; 63:1454-7; PMID:12670889
- Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Máximo V, Botelho T, Seruca R, Sobrinho-Simões M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene 2003; 22:4578-80; PMID:12881714; http://dx.doi.org/10.1038/sj.onc.1206706
- Frattini M, Ferrario C, Bressan P, Balestra D, De Cecco L, Mondellini P, Bongarzone I, Collini P, Gariboldi M, Pilotti S, et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene 2004; 23:7436-40; PMID:15273715; http://dx.doi.org/10.1038/sj.onc.1207980
- Santoro M, Melillo RM, Carlomagno F, Vecchio G, Fusco A. Minireview: RET: normal and abnormal functions. Endocrinology 2004; 145:5448-51; PMID:15331579; http://dx.doi.org/10.1210/en.2004-0922
- Costa V, Aprile M, Esposito R, Ciccodicola A. RNA-Seq and human complex diseases: recent accomplishments and future perspectives. Eur J Hum Genet 2013; 21:134-42; PMID:22739340
- Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, Leiserson MDM, Niu B, McLellan MD, Uzunangelov V, et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014 158:929-44; PMID:25109877; http://dx.doi.org/10.1016/j.cell.2014.06.049
- Smallridge RC, Chindris AM, Asmann YW, Casler JD, Serie DJ, Reddi HV, Cradic KW, Rivera M, Grebe SK, Necela BM, et al. RNA sequencing identifies multiple fusion transcripts, differentially expressed genes, and reduced expression of immune function genes in BRAF (V600E) mutant vs BRAF wild-type papillary thyroid carcinoma. J Clin Endocrinol Metab 2014; 99:E338-47; PMID:24297791
- Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014; 159:676-90; PMID:25417114
- Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, Evdokimova VN, Hatch M, Zurnadzy LY, Nikiforova MN, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014; 120:799-807; PMID:24327398; http://dx.doi.org/10.1002/cncr.28484
- Costa V, Esposito R, Ziviello C, Sepe R, Bim LV, Cacciola NA, Decaussin-Petrucci M, Pallante P, Fusco A, Ciccodicola A. New somatic mutations and WNK1-B4GALNT3 gene fusion in papillary thyroid carcinoma. Oncotarget 2015; 6:11242-51.
- Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L, Fejes AP, Chow C, Tone A, Kalloger SE, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med 2012; 366:234-42; PMID:22187960
- Huang J, Liang JT, Huang HC, Shen TL, Chen HY, Lin NY, Che MI, Lin WC, Huang MC. Beta1,4-N-acetylgalactosaminyltransferase III enhances malignant phenotypes of colon cancer cells. Mol Cancer Res 2007; 5:543-52; PMID:17579116
- Che MI, Huang J, Hung JS, Lin YC, Huang MJ, Lai HS, Hsu WM, Liang JT, Huang MC. β1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget 2014; 5:3673-84; PMID:25003232
- Hsu WM, Che MI, Liao YF, Chang HH, Chen CH, Huang YM, Jeng YM, Huang J, Quon MJ, Lee H, et al. B4GALNT3 expression predicts a favorable prognosis and suppresses cell migration and invasion via β1 integrin signaling in neuroblastoma. Am J Pathol 2011; 179:1394-404; PMID:21741930
