Abstract
Positioning the nucleus is critical for many cellular processes including cell division, migration and differentiation. The linker of nucleoskeleton and cytoskeleton (LINC) complex spans the inner and outer nuclear membranes and has emerged as a major factor in connecting the nucleus to the cytoskeleton for movement and positioning. Recently, we discovered that the diaphanous formin family member FHOD1 interacts with the LINC complex component nesprin-2 giant (nesprin-2G) and that this interaction plays essential roles in the formation of transmembrane actin-dependent nuclear (TAN) lines and nuclear movement during cell polarization in fibroblasts. We found that FHOD1 strengthens the connection between nesprin-2G and rearward moving dorsal actin cables by providing a second site of interaction between nesprin-2G and the actin cable. These results indicate that the LINC complex connection to the actin cytoskeleton can be enhanced by cytoplasmic factors and suggest a new model for TAN line formation. We discuss how the nesprin-2G-FHOD1 interaction may be regulated and its possible functional significance for development and disease.
Abbreviations
| LINC | = | linker of nucleoskeleton and cytoskeleton |
| nesprin-2G | = | nesprin-2 giant |
| TAN lines | = | transmembrane actin-dependent nuclear lines |
| SR | = | spectrin repeat |
| KASH | = | Klarsicht |
| ANC-1 | = | Syne homology |
| DRF | = | diaphanous related formin |
| GBD | = | GTPase binding domain |
| DID | = | diaphanous inhibitory domain |
| FH | = | formin homology |
| DAD | = | diaphanous autoregulatory domain |
| ABS | = | actin binding site |
| LPA | = | lysophosphatidic acid |
| GFP-mN2G | = | GFP-mini-nesprin-2G |
| CH | = | calponin homology |
| EDMD | = | Emery-Dreifuss muscular dystrophy |
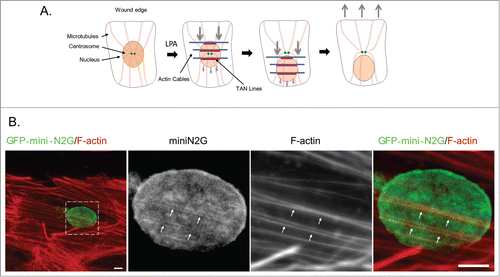
Metazoans actively control the position of the nucleus in the cell to regulate cell division, differentiation, and migration.Citation1 In fibroblasts and myoblasts, actin retrograde flow powers the rearward movement of the nucleus to orient the centrosome and polarize cells for directed migration ().Citation2,3 Many nuclear movements are mediated by connections between the cytoskeleton and the nucleus established by specific LINC complexes.Citation1,4,5 For the actin-dependent nuclear movement in fibroblasts and myoblasts, the specific LINC complex is composed of nesprin-2G, a ˜800 kDa, actin-binding and spectrin repeat (SR)-containing outer nuclear membrane protein and SUN2, an inner nuclear membrane protein that binds the KASH (Klarsicht, ANC-1, Syne homology) domain of nesprin-2G.Citation3,6 The association of these proteins with dorsal actin cables results in their assembly into linear arrays termed TAN lines ().Citation3,6,7 TAN lines are anchored by association of SUN2 with A-type lamins.Citation8 Two inner nuclear membrane proteins, Samp1 and emerin, are also found in TAN lines and may contribute to their anchoring.Citation9,10 Emerin additionally affects actin-dependent nuclear movement by interacting with myosin IIB and organizing the directionality of actin flow.Citation10
Figure 1. TAN line dependent nuclear movement. (A) Model for TAN line dependent nuclear movement in fibroblasts and myoblasts polarizing for migration. In serum-starved wounded monolayers of fibroblasts and myoblasts, LPA stimulation induces the retrograde flow of dorsal actin cables (blue). These actin cables engage nesprin-2G on the nuclear surface to form TAN lines (red) resulting in the rearward movement of the nucleus while the centrosome stays in the center of the cell. This establishes cell polarization by orienting the centrosome toward the wound edge. (B) Immunofluorescence images of TAN lines in NIH3T3 fibroblasts expressing the nesprin-2G chimera, GFP-mini-N2G. Cells were stimulated with LPA for one hr and the formation of TAN lines visualized by staining for GFP-mini-N2G and F-actin. The boxed region in the left panel is shown at higher magnification in the panels to the right. Arrows indicate TAN lines with colocalized GFP-mini-N2G and actin cables. Scale bar, 5 μm.
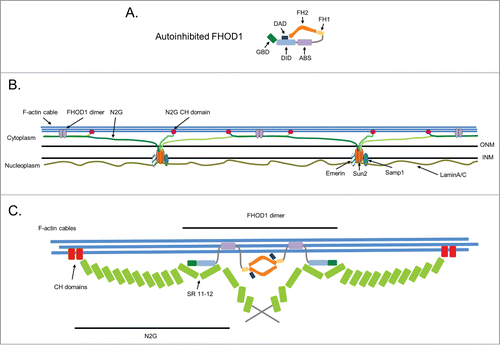
TAN lines resemble other membrane adhesions, such as focal adhesions and cadherin cell-cell adhesions, in that they form by actin-dependent clustering of membrane proteins and transmit force. The large number of proteins associated with focal and cell-cell adhesions suggests that additional proteins are likely to be associated with TAN lines. In a recent study,Citation11 we identified the diaphanous related formin (DRF) FHOD1 as interaction partner of nesprin-2G and a new component of TAN lines. Like other DRFs, FHOD1 is composed of an N-terminal GTPase binding domain (GBD), followed by the diaphanous inhibitory domain (DID), the central formin homology (FH) domains FH1 and the actin binding FH2, and the C-terminal diaphanous autoregulatory domain (DAD) (). Despite a similar domain organization, FHOD1 exhibits features that distinguish it from other DRFs. Importantly, It does not stimulate actin polymerization in vitro, but instead appears to cap actin filaments.Citation12 Also unlike other DRFs, FHOD1 bundles actin filaments both in vivo and in vitro.Citation12-15 This bundling activity requires dimerization provided by the FH2 domain, but also a second F-actin binding domain in the N-terminus between the DID and the FH1 domain that is unique to FHOD1 among the 15 mammalian formins (). This second actin binding site (ABS) also endows FHOD1 with the ability to bind along the length of actin filaments, unlike other DRFs, which interact transiently with actin filament barbed ends. FHOD1 is regulated by autoinhibition which is accomplished by binding of the DAD to the DID. Although for most DRFs release of autoinhibition is mediated by binding of specific Rho GTPase(s) to the GBD, for FHOD1 release of autoinhibition occurs by phosphorylation of the DAD (see below).
Figure 2. Multi-site attachment model for TAN lines. (A) Schematic of the autoinhibited form of FHOD1 (shown as a monomer to emphasize domains). Individual domains are described in the text. (B) Model for the multi-site attachment of nesprin-2G (N2G) and FHOD1 to actin cables in TAN lines. The interaction of nesprin-2G with FHOD1 forms a branched connection between nesprin-2G and the actin cable with one connection provided by nesprin-2G's CH domains and the other by FHOD1s unique ABS. This branched connection is proposed to strengthen the association between the nesprin and the actin cable. In the perinuclear space between the inner (INM) and outer nuclear membrane (ONM), KASH domains of nesprin-2G interact with the SUN2 trimer. In the nucleoplasm, SUN2 is anchored by interaction with lamin A/C of the lamina and with the INM proteins Samp1 and emerin. (C) A detailed view showing FHOD1 interacting with SRs 11–12 of nesprin-2G through its N-terminal GBD and DID and to the actin cable through its ABS. The dimeric nature of FHOD1 may bring multiple nesprin-2Gs together.
The FHOD1 N-terminus (residues 1–339) is structurally different from other DRFsCitation13 and we used it as a bait in a yeast 2-hybrid screen for FHOD1 interacting proteins. We found that a fragment of human nesprin-2G (residues 1340–1669) interacted with the N-terminal fragment of FHOD1.Citation11 By GST-pulldown and co-immunoprecipitation assays we confirmed the interaction of the proteins in mammalian cells. The interaction of the full length proteins was shown by co-immunoprecipitation of endogenous nesprin-2G with over-expressed FHOD1. A directed yeast 2-hybrid interaction screen with nesprin-2G fragments covering the length of nesprin-2G and additional GST-pulldown assays revealed that the only site of FHOD1 interaction on nesprin-2G was with SRs 11 and 12.
Because of the importance of nesprin-2G for centrosome orientation and nuclear movement in fibroblasts, we tested if FHOD1 was required for these processes by knocking it down with siRNA oligonucleotides in NIH3T3 fibroblasts. Similar to the phenotype of nesprin-2G knockdown cells, FHOD1-depleted cells did not orient their centrosomes due to a failure to move their nuclei rearward after stimulation with lysophosphatidic acid (LPA), a serum factor that stimulates these processes in starved NIH3T3 fibroblasts.Citation16 Defects in centrosome orientation and nuclear movement in FHOD1-depleted cells were rescued by re-expression of wild-type FHOD1 but not by re-expression of FHOD1 lacking residues 1–339 that interact with nesprin-2G. Consistent with the importance of the interaction between FHOD1 and nesprin-2G, overexpression of the interacting fragments of nesprin-2G and FHOD1 inhibited centrosome orientation and nuclear movement.
Rearward nuclear movement requires the formation and retrograde flow of dorsal actin cables that engage the nucleus through TAN lines.Citation6,10 Despite its proposed role in regulating the actin cytoskeleton, FHOD1 knockdown cells did not have significant differences in total levels of F-actin, the number of dorsal actin cables above the nucleus, or the velocity of actin retrograde flow after LPA stimulation compared to control cells. However, TAN line formation was severely impaired in FHOD1 knockdown cells. Importantly, expressed RFP-FHOD1 localized to TAN lines, which were visualized by staining of either expressed GFP-mini-nesprin-2G (GFP-mN2G, a minimal functional nesprin-2G containing the N-terminal calponin homology (CH) domains and the C-terminal KASH domainCitation6) or endogenous nesprin-2G.
We further dissected the role of FHOD1 in TAN line formation by expressing various forms of FHOD1 in FHOD1 depleted cells. We found that a FHOD1 construct comprising the entire N-terminus (residues 1–569) and lacking the canonical formin FH1 and FH2 domains localized in TAN lines, rescued centrosome orientation and partially rescued nuclear movement in FHOD1 deficient cells. In addition to a nesprin-2G binding site, FHOD1 1–569 contains the unique ABS of FHOD1. This consideration raised the possibility that FHOD1 bound to nesprin-2G contributes to TAN line formation by providing a second F-actin binding site in addition to the actin binding CH domains of nesprin-2G. To test this possibility, we fused the nesprin-2G interacting site of FHOD1 (residues 1–339) with the F-actin binding CH domains of α-actinin. Expression of this chimera rescued the centrosome orientation and nuclear movement defects in FHOD1 knockdown cells as well as FHOD1 1–569, implying that the FHOD1-nesprin-2G interaction provides a second linkage to actin cables in addition to that provided by the CH domains of nesprin-2G.
These findings support a new model for TAN line engagement of actin cables (). In this model, nesprin-2G attaches to actin cables by 2 sites of interaction: its own CH domains and the unique ABS in the N-terminus of FHOD1. We hypothesize that this branched connection reinforces the nesprin-actin interaction and allows it to resist the high forces necessary to move such a large organelle as the nucleus. The model also implies that the orientation of nesprin-2G relative to the actin cable is likely to be nearly parallel (at least for the region between the CH domains and the SR11–12) rather than the end-on orientation that was originally envisioned.Citation6,7 This conclusion is based on the extended structure of SR proteins and average 6 nm length of individual SRs, yielding a distance of at least 60 nm between the CH domains and SRs11–12 of nesprin-2G.Citation17,18 Coupled with the predicted length of the FHOD1 N-terminal fragment that interacts with both nesprin-2G and the actin cable (<10 nm),Citation13 the only way that both proteins can interact with the actin cable is if nesprin-2G lies along the axis of the actin cable. Lastly, FHOD1 is hypothesized to form a dimer through its FH2 domains.Citation14 Thus, FHOD1 has the potential to bring two nesprin-2Gs together. In this way, FHOD1 may additionally contribute to the assembly of TAN lines by enhancing the avidity of nesprin-2G for the actin cables. Perhaps, this explains why monomeric constructs based on the N-terminus of FHOD1 did not fully rescue nuclear movement.
In muscle cells there are 2 splice variants of FHOD1 in addition to the widely expressed version in fibroblasts: one variant contains a 28 aa insert in the ABS, the other variant is a short N-terminal form that includes a unique 7 aa insert and terminates within the ABS.Citation19 Neither of these splice variants have been functionally characterized, but it is interesting to note that the short variant is similar to the construct that we used to rescue the nuclear movement defect in FHOD1 depleted cells. Additional studies to refine the location of the actin binding site may reveal whether this short variant retains the capacity to bind actin filaments. If so, it has the potential to reinforce the nesprin-2G-actin cable interaction; if not, it may act as an endogenous inhibitor of the interaction.
The N-terminal site on FHOD1 that interacts with nesprin-2G is in the same region that is involved regulating its activity. This raises interesting questions concerning how FHOD1 interaction with nesprin-2G may be regulated and whether nesprin-2G interaction with FHOD1 may maintain it in an active state. Like other DRFs, FHOD1 is regulated by auto-inhibition through an intra-molecular interaction between its N-terminal DID and C-terminal DAD.Citation20 By co-immunoprecipitation of FHOD1 transfected 293T cells, in preliminary experiments we have observed that a nesprin-2G fragment containing SR11–12 co-immunoprecipitated the constitutively active FHOD1 ΔDAD more efficiently than it did wild-type FHOD1. This implies that the intra-molecular interaction between FHOD1's DID and DAD reduces the interaction between FHOD1 and nesprin-2G and that the release of FHOD1's autoinhibition may be a pre-requisite for efficient FHOD1-nesprin-2G interaction.
There have been several mechanistic models proposed for how the autoinhibition of FHOD1 is released. The GBD of FHOD1 interacts with Rac1-GTP, but this does not seem to activate FHOD1.Citation13,20,21 Phosphorylation of FHOD1 at three serine/threonine residues by ROCK1 partially releases autoinhibtion.Citation14,22 Additionally, phosphorylation of FHOD1 at Y99 by Src was shown to be a prerequisite for phosphorylation of the DAD by ROCK1.Citation23 Phosphorylation of FHOD1 by Src and ROCK1 to release autoinhibition might be relevant to activate FHOD1 for TAN line formation. However, expression of dominant negative RhoA does not inhibit centrosome orientation, suggesting that activation of FHOD1 by ROCK is not required for rearward nuclear movement.Citation16 Perhaps for FHOD1's function in linking nesprin-2G to actin cables in TAN lines, only the phosphorylation of the N-terminus by Src is required. It will be interesting to test whether Src is required for TAN line formation and nuclear movement.
SRs11–12 are in one of 2 clusters of SRs in nesprin-2G (SRs11–13 and SRs 49–53) that are phylogenetically conserved and are predicted to interact with proteins based on their probability of surface exposure.Citation24,25 Interestingly, an additional protein meckelin, which is localized in plasma membrane and ER, was found to bind to the SRs 11–13.Citation26-28 Taking this into account, FHOD1 and meckelin binding to the SRs could work independently, cooperatively, or competitively to regulate the formation of TAN lines. While SRs 11–13 are close to the CH domains at the N-terminus of nesprin-2G, SRs 49–53 are at the other end of the molecule near the transmembrane domain.Citation24 A number of proteins bind to this regionCitation29 raising the possibility that additional bridging proteins will be important for TAN line formation and nuclear movement.
Despite the importance of the FHOD1-nesprin-2G interaction for nuclear movement and polarity regulation of cells in culture, it remains to be tested whether the FHOD1-nesprin-2G interaction is involved in animal development, differentiation or pathogenesis. Nesprin-2 has numerous splice isoforms from the giant nesprin-2G to shorter isoforms that lack actin interacting CH domains or even the transmembrane KASH domain.Citation30 When the exon of Syne2 (the gene encoding nesprin-2) specifying the KASH domain is deleted, mice exhibit defects in laminar formation in the cerebral cortex and hippocampus during brain development resulting in poor learning skills.Citation31 Nesprin-1G is structurally similar to nesprin-2G, but lacks the set of nesprin-2G conserved SRs that interact with FHOD1.Citation24,25 Unlike the KASH null nesprin-2 mouse, the KASH null nesprin-1 mouse does not have brain developmental defects.Citation30 This difference in phenotype may reflect FHOD1's specific interaction with nesprin-2G and raises the possibility that the nesprin-2G-FHOD1 interaction is important for brain development.
There is an intriguing correlation between proteins contributing to TAN line- dependent nuclear movement in fibroblasts and myoblasts and variants in their genes causing Emery-Dreifuss muscular dystrophy (EDMD). Mutations in genes encoding emerinCitation32-34 and A-type laminsCitation35 were the first to be associated with EDMD, but more recent studies have identified variants in nesprin-2Citation36 and SUN2.Citation37 In addition to skeletal dystrophy, EDMD frequently leads to dilated cardiomyopathy. Furthermore, mice with KASH null nesprin-1 and 2 genes in cardiomyocytes develop cardiomyopathy, and various mutations of nesprin-2 have been found in cardiomyopathy patients.Citation38 Expression of EDMD variants of lamin A and SUN2 or depletion of emerin, as occurs with many EDMD emerin variants, leads to defective nuclear movement and centrosome orientation in fibroblasts.Citation8,10,37 Expression of FHOD1 is found in many tissues and like nesprin-2G it is particularly high in heart, skeletal muscle, and spleen.Citation19,30 The coincidence of expression patterns of FHOD1 and nesprin-2G and their interaction suggest that FHOD1 like nesprin-2 plays a pivotal role in the maintenance of striated muscle, and the disruption of the interaction between nesprin-2G and FHOD1 may lead to muscular dystrophies. Although variants of FHOD1 have not been associated with disease, FHOD1's closet relative, FHOD3 is expressed in the heart and a missense variant of FHOD3 has been found in a patient case with adult-onset familial dilated cardiomyopathy.Citation39 Interestingly, FHOD1 localizes in intercalated discs and costameres in cardiomyocytes, unlike FHOD3's cross-striated pattern,Citation40,41 suggesting a distinct role for FHOD1 in cardiomyocyte function. It will be interesting to see if FHOD1 mutations are associated with skeletal or cardiac muscle diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Our work on nuclear movement is supported by NIH (grant GM 099481 and NS059352 to GGG) and the Deutsche Forschungsgemeinschaft (grant FA 378/ 15–1 to OTF).
References
- Gundersen GG, Worman HJ. Nuclear positioning. Cell 2013; 152:1376-89; PMID:23498944; http://dx.doi.org/10.1016/j.cell.2013.02.031
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 2005; 121:451-63; PMID:15882626; http://dx.doi.org/10.1016/j.cell.2005.02.022
- Chang W, Antoku S, Ostlund C, Worman HJ, Gundersen GG. Linker of nucleoskeleton and cytoskeleton (LINC) complex-mediated actin-dependent nuclear positioning orients centrosomes in migrating myoblasts. Nucleus 2015; 6:77-88; PMID:25587885
- Razafsky D, Zang S, Hodzic D. UnLINCing the nuclear envelope: towards an understanding of the physiological significance of nuclear positioning. Biochem Soc Trans 2011; 39:1790-4; PMID:22103527; http://dx.doi.org/10.1042/BST20110660
- Starr DA. A nuclear-envelope bridge positions nuclei and moves chromosomes. J Cell Sci 2009; 122:577-86; PMID:19225124; http://dx.doi.org/10.1242/jcs.037622
- Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 2010; 329:956-9; PMID:20724637; http://dx.doi.org/10.1126/science.1189072
- Luxton GW, Gomes ER, Folker ES, Worman HJ, Gundersen GG. TAN lines: a novel nuclear envelope structure involved in nuclear positioning. Nucleus 2011; 2:173-81; PMID:21818410; http://dx.doi.org/10.4161/nucl.2.3.16243
- Folker ES, Ostlund C, Luxton GW, Worman HJ, Gundersen GG. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A 2011; 108:131-6; PMID:21173262; http://dx.doi.org/10.1073/pnas.1000824108
- Borrego-Pinto J, Jegou T, Osorio DS, Aurade F, Gorjanacz M, Koch B, Mattaj IW, Gomes ER. Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci 2012; 125:1099-105; PMID:22349700; http://dx.doi.org/10.1242/jcs.087049
- Chang W, Folker ES, Worman HJ, Gundersen GG. Emerin organizes actin flow for nuclear movement and centrosome orientation in migrating fibroblasts. Mol Biol Cell 2013; 24:3869-80; PMID:24152738; http://dx.doi.org/10.1091/mbc.E13-06-0307
- Kutscheidt S, Zhu R, Antoku S, Luxton GW, Stagljar I, Fackler OT, Gundersen GG. FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat Cell Biol 2014; 16:708-15; PMID:24880667; http://dx.doi.org/10.1038/ncb2981
- Schonichen A, Mannherz HG, Behrmann E, Mazur AJ, Kuhn S, Silvan U, Schoenenberger CA, Fackler OT, Raunser S, Dehmelt L, et al. FHOD1 is a combined actin filament capping and bundling factor that selectively associates with actin arcs and stress fibers. J Cell Sci 2013; 126:1891-901; PMID:23444374; http://dx.doi.org/10.1242/jcs.126706
- Schulte A, Stolp B, Schonichen A, Pylypenko O, Rak A, Fackler OT, Geyer M. The human formin FHOD1 contains a bipartite structure of FH3 and GTPase-binding domains required for activation. Structure 2008; 16:1313-23; PMID:18786395; http://dx.doi.org/10.1016/j.str.2008.06.008
- Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J 2008; 27:618-28; PMID:18239683; http://dx.doi.org/10.1038/emboj.2008.7
- Koka S, Neudauer CL, Li X, Lewis RE, McCarthy JB, Westendorf JJ. The formin-homology-domain-containing protein FHOD1 enhances cell migration. J Cell Sci 2003; 116:1745-55; PMID:12665555; http://dx.doi.org/10.1242/jcs.00386
- Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol 2001; 11:1536-41; PMID:11591323; http://dx.doi.org/10.1016/S0960-9822(01)00475-4
- Muthu M, Richardson KA, Sutherland-Smith AJ. The crystal structures of dystrophin and utrophin spectrin repeats: implications for domain boundaries. PloS One 2012; 7:e40066; PMID:22911693; http://dx.doi.org/10.1371/journal.pone.0040066
- Ylanne J, Scheffzek K, Young P, Saraste M. Crystal structure of the alpha-actinin rod reveals an extensive torsional twist. Structure 2001; 9:597-604; PMID:11470434; http://dx.doi.org/10.1016/S0969-2126(01)00619-0
- Tojo H, Kaieda I, Hattori H, Katayama N, Yoshimura K, Kakimoto S, Fujisawa Y, Presman E, Brooks CC, Pilch PF. The Formin family protein, formin homolog overexpressed in spleen, interacts with the insulin-responsive aminopeptidase and profilin IIa. Mol Endocrinol 2003; 17:1216-29; PMID:12677009; http://dx.doi.org/10.1210/me.2003-0056
- Westendorf JJ. The formin/diaphanous-related protein, FHOS, interacts with Rac1 and activates transcription from the serum response element. J Biol Chem 2001; 276:46453-9; PMID:11590143
- Gasteier JE, Madrid R, Krautkramer E, Schroder S, Muranyi W, Benichou S, Fackler OT. Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J Biol Chem 2003; 278:38902-12; PMID:12857739
- Hannemann S, Madrid R, Stastna J, Kitzing T, Gasteier J, Schonichen A, Bouchet J, Jimenez A, Geyer M, Grosse R, et al. The Diaphanous-related Formin FHOD1 associates with ROCK1 and promotes Src-dependent plasma membrane blebbing. J Biol Chem 2008; 283:27891-903; PMID:18694941
- Iskratsch T, Yu CH, Mathur A, Liu S, Stevenin V, Dwyer J, Hone J, Ehler E, Sheetz M. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev Cell 2013; 27:545-59; PMID:24331927; http://dx.doi.org/10.1016/j.devcel.2013.11.003
- Autore F, Pfuhl M, Quan X, Williams A, Roberts RG, Shanahan CM, Fraternali F. Large-scale modelling of the divergent spectrin repeats in nesprins: giant modular proteins. PloS One 2013; 8:e63633; PMID:23671687; http://dx.doi.org/10.1371/journal.pone.0063633
- Simpson JG, Roberts RG. Patterns of evolutionary conservation in the nesprin genes highlight probable functionally important protein domains and isoforms. Biochem Soc Transact 2008; 36:1359-67; PMID:19021556; http://dx.doi.org/10.1042/BST0361359
- Dawe HR, Adams M, Wheway G, Szymanska K, Logan CV, Noegel AA, Gull K, Johnson CA. Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J Cell Sci 2009; 122:2716-26; PMID:19596800; http://dx.doi.org/10.1242/jcs.043794
- Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T, et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet 2007; 16:173-86; PMID:17185389; http://dx.doi.org/10.1093/hmg/ddl459
- Wang M, Bridges JP, Na CL, Xu Y, Weaver TE. Meckel-Gruber syndrome protein MKS3 is required for endoplasmic reticulum-associated degradation of surfactant protein C. J Biol Chem 2009; 284:33377-83; PMID:19815549
- Chang W, Worman HJ, Gundersen GG. Accessorizing and anchoring the LINC complex for multifunctionality. J Cell Biol 2015; 208:11-22; PMID:25559183; http://dx.doi.org/10.1083/jcb.201409047
- Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci 2005; 118:673-87; PMID:15671068; http://dx.doi.org/10.1242/jcs.01642
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 2009; 64:173-87; http://dx.doi.org/10.1016/j.neuron.2009.08.018
- Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet 1994; 8:323-7; PMID:7894480
- Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum Mol Genet 1996; 5:801-8; PMID:8776595; http://dx.doi.org/10.1093/hmg/5.6.801
- Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, Hayashi YK, Tsukahara T, Arahata K. Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat Genet 1996; 12:254-9; PMID:8589715
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 1999; 21:285-8; PMID:10080180
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, et al. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 2007; 16:2816-33; PMID:17761684; http://dx.doi.org/10.1093/hmg/ddm238
- Meinke P, Mattioli E, Haque F, Antoku S, Columbaro M, Straatman KR, Worman HJ, Gundersen GG, Lattanzi G, Wehnert M, et al. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet 2014; 10:e1004605; PMID:25210889; http://dx.doi.org/10.1371/journal.pgen.1004605
- Banerjee I, Zhang J, Moore-Morris T, Pfeiffer E, Buchholz KS, Liu A, Ouyang K, Stroud MJ, Gerace L, Evans SM, et al. Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet 2014; 10:e1004114; PMID:24586179; http://dx.doi.org/10.1371/journal.pgen.1004114
- Arimura T, Takeya R, Ishikawa T, Yamano T, Matsuo A, Tatsumi T, Nomura T, Sumimoto H, Kimura A. Dilated cardiomyopathy-associated FHOD3 variant impairs the ability to induce activation of transcription factor serum response factor. Circul J 2013; 77:2990-6; PMID:24088304; http://dx.doi.org/10.1253/circj.CJ-13-0255
- Al Haj A, Mazur AJ, Radaszkiewicz K, Radaszkiewicz T, Makowiecka A, Stopschinski BE, Schonichen A, Geyer M, Mannherz HG. Distribution of formins in cardiac muscle: FHOD1 is a component of intercalated discs and costameres. Eur J Cell Biol 2015; 94:101-13; PMID:25555464; http://dx.doi.org/10.1016/j.ejcb.2014.11.003
- Dwyer J, Pluess M, Iskratsch T, Dos Remedios CG, Ehler E. The formin FHOD1 in cardiomyocytes. Anat Record 2014; 297:1560-70
