Abstract
Stromal fibroblasts are essential for tumor proliferation and invasion. Here we presented a 3-dimensional (3D) microfluidic co-culture device to reconstruct an in vivo-like tumor microenvironment for investigation of the interactions of cancer-associated fibroblasts (CAFs) and bladder cancer cells. With this device, we verified that the cytokines secreted by bladder cancer cells T24 effectively transform the fibroblasts into CAFs. Compared to fibroblasts, the CAFs, which undergo the aerobic glycolysis, showed higher ability to produce lactate and provide energy for bladder cancer cell proliferation and invasion. We also demonstrated that this kind of tumor-promoting effect was associated with the upregulation of monocarboxylate anion transporter 1 (MCT1) and MCT4 expression in CAFs. We concluded that MCT1 and MCT4 are involved in bladder cancer cell proliferation and invasiveness. Moreover, this 3D microfluidic co-culture device allows for the assay to characterize various cellular events in a single device sequentially, facilitating a better understanding of the interactions among heterotypic cells in a sophisticated microenvironment.
Introduction
Bladder cancer is the fifth most commonly diagnosed cancer in USA.Citation1 In China, bladder cancer is one of the most common malignant tumors of urinary malignancies, accounted for 3.2% of the whole body malignant tumors with an increasing trend. In spite of advances in detection and treatment, bladder cancer is still expected to kill nearly 16000 Americans at 2015. Bladder transitional cell carcinoma (BTCC) accounts for 90–95% of bladder cancer. Thus, it is critical to renew information about the cell biology of this cancer, especially the invasion and migration properties, to permit the identification of novel molecular targets for therapy of the disease.
Previous research has shown that a steady source of metabolic energy is required to continue the uncontrolled growth and proliferation in cancer cells.Citation2 Particularly, aerobic glycolysis, which is termed “the Warburg Effect,” has been considered as a hallmark of cancer cell metabolism.Citation3 It means cancer cells obtain sufficient adenosine triphosphate (ATP) even in a hypoxic microenvironment.Citation4 Recently, it has come to the conclusion that fibroblasts, the dominant component of stromal cells, could be activated by cancer cells and transformed into cancer-associated fibroblasts (CAFs).Citation5 Then they undergo the aerobic glycolysis, produce lactate and provide energy for cancer cells.Citation6-9 The tumor-host cell interactions between colon cancer cells and CAFs have been explored,Citation10 however there is a lack of the understanding on the interactions of CAFs and bladder cancer cells.
Monocarboxylate anion transporters (MCTs; also called the solute carrier family 16[SLC16]) are key biosynthetic transporters involved in the lactate transportation and the inhibition of cell apoptosis caused by high concentration lactate.Citation11 It is known that MCT4 (SLC16A3) transports lactate out of fibroblast cellsCitation12,13 and MCT1 (SLC16A1) regulates the entry of lactate into bladder cancer cells.Citation14,15 However, the association between MCTs and bladder cancer cell invasion, proliferation and apoptosis is currently unclear.
For further research in this area, co-culture device becomes measurable and understandable. Although a few methods such as transwell assayCitation16 or bathing cells in conditioned mediumCitation17 were proposed to study the interactions between cancer cells and stromal cells, most of them are still limited because of the poor in vivo-like microenvironment reconstruction.Citation18,19 Recently, microfluidics has attracted intensive attention in molecular and cellular biology research due to its miniaturized size, high throughput detection, low consumption of reagents and especially the accurate reconstruction of 3D in vivo-like microenvironment.Citation1,13 Such devices are particularly suitable for complicated intercellular interaction research with well-controlled spatial parameters.Citation20-22
In this study, we developed a microfluidic device to reconstruct a 3D in vivo-like tumor microenvironment for co-culture of bladder cancer cells and fibroblasts. In this device, bladder cancer cells (T24) and human Foreskin Fibroblast cells (hFF) were co-cultured indirectly in 3D mode, in which cells communicate through diffusible signals. With this novel device, we were able to analyze the expression regulation of MCT1 and MCT4 in 3D co-cultured fibroblasts and the effect of regulated fibroblasts with MCTs-siRNA and MCT1s inhibitors, quercetin (QC)Citation7 and simvastatin (SS),Citation23 on proliferation, apoptosis, migration and invasion of bladder cancer cells in vitro. These data may suggest a possible potential role of MCTs as a novel therapeutic target for bladder cancer.
Result
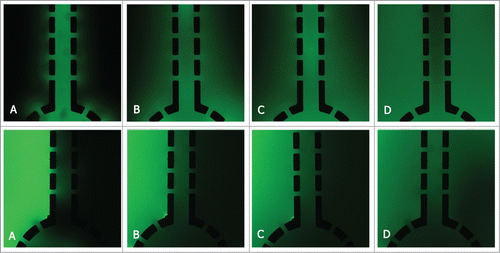
The diffusion of fluorescein isothiocyanate (FITC)-dextran into the metriGel
In this work we aimed to establish a 3D device to keep the co-culture medium flowed through one chamber and diffused into other chambers to exchange nutrients. In order to verify the permeability of metrigel in 3D devices and to see if the soluble factors secreted by cells could diffuse into each chamber, the diffusion of FITC-Dextran was assayed in different ways. depicted the diffusion of FITC-Dextran into or out of the metrigel after being injected for 5 to 120 min, respectively.
Activation of fibroblasts in co-culture with bladder cancer cells
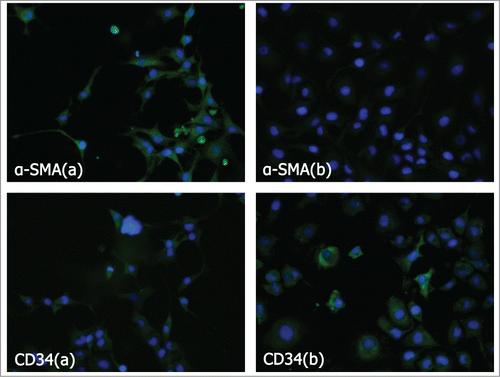
In order to detect whether the bladder cancer cells can activate fibroblasts into CAFs, we carried out an immunofluorescence assay on the hFF cells with or without the induction by T24 cells (experimental and control group cells). The formation of CAFs was detected by differential expression of α-smooth-muscle actin (α-SMA) and CD34.Citation5,Citation24-26 As shown in , the results showed that the α-SMA was expressed abundantly in the experimental group. However, only mild α-SMA expression was detected in the control group cells (P < 0.01). While, the expression of CD34 was just the reverse, it was down-regulated in the experimental group relative to the control group (P < 0.01). This finding suggested that the cytokines secreted by bladder cancer cells were able to activate the fibroblast cells into CAFs.
MCTs was overexpressed in 3D co-culture hFF cells
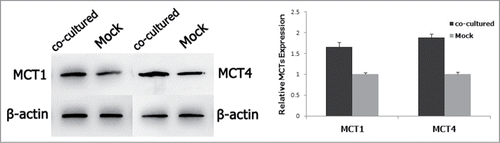
To further determine different expressions of the MCTs in 3D co-culture cells and the control group cells, we measured MCT1 and MCT4 protein level of hFF cells by western blot respectively. As shown in , both MCT1 and MCT4 expression was higher in co-culture hFF cells significantly.
MCTs inhibition decreased the concentration of lactate in the culture medium
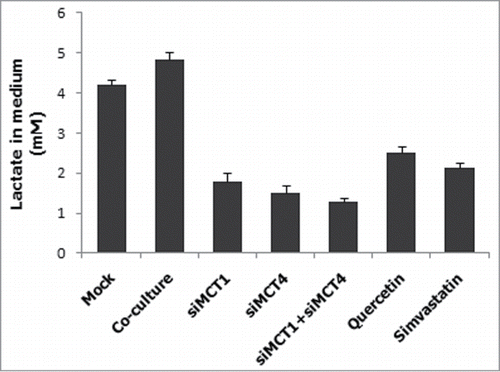
To assess the concentration of lactate in the culture medium, lactate assay was performed. Because MCTs family proteins are associated with the transport of lactate,Citation3 the lactate concentration in each medium was measured. As shown in , lactate concentration correlated with the expression of MCT4 and MCT1. When the MCTs were inhibited by siRNAs, QC or SS, the concentration of lactate would be decreased.
MCTs inhibition by QC, SS or siMCTs of CAFs in co-culture devices suppressed T24 cells proliferation
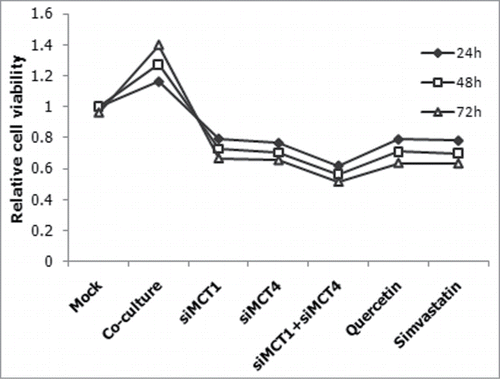
To explore the effect of MCT1 and MCT4 on human bladder cancer, we treated the co-cultured CAFs with QC, a known MCT1 inhibitor, and SS, a known MCT4 inhibitor, and measured the proliferation of T24 cells by CCK8 assay. Incubating CAFs with QC or SS both led to dose-dependent (data not shown) and time-dependent decreases of the cells' viability of co-cultured T24 cells. The similar experiments were done with siMCT1 and/or siMCT4 simultaneously. As predicted, it showed that QC, SS or siMCTs transfection resulted in a significant reduction of proliferation. Compared to mono-transfection, the co- transfection targeting MCT1 and MCT4 reduced cells' viability more significantly (P < 0.05)().
siRNA or inhibitors mediated down-regulation of MCTs in CAFs induces apoptosis of T24 cells in 3D co-cultue devices
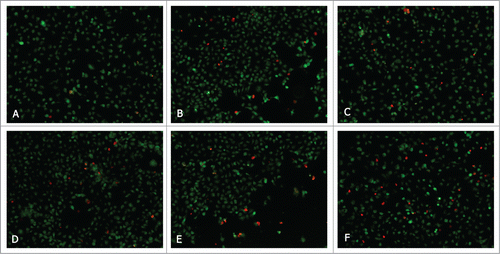
To explore the functional effect of MCTs on cell survival, we measured apoptosis of T24 cells from 3D co-culture devices in which the CAFs were transfected with siMCT1 and/or siMCT4, or inhibited by QC or SS. Downregulation of MCT1 and MCT4 increased apoptosis in T24 cells in the 3D co-culture device, as measured by fluorescence microscopy of cells for acridine orange (AO) and ethidium bromide (EB) staining (). QC and SS were used to induce apoptosis 24 hours after co-culture. Apoptosis was much higher in co-transfection group than mono-transfection groups (P < 0.05). The effect of QC and SS is similar to the mono-transfection of siMCTs. These data suggest that MCTs down-regulation in CAFs can induce T24 cells apoptosis.
Expression of MCTs in CAFs correlates with the invasion function of T24 cells
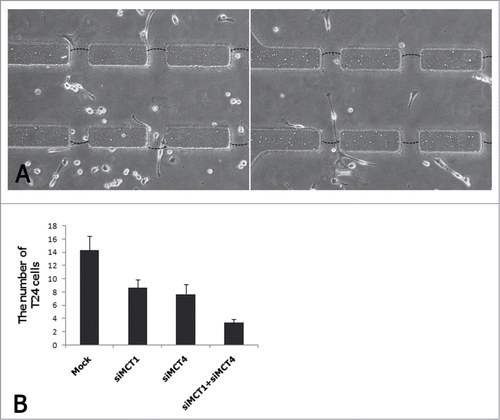
Inhibition of basigin (BSG) expression reduces tumor cell invasion and BSG is tightly associated with MCT1 and MCT4.Citation27 Therefore, we speculated that the invasion activity was positively correlated with MCTs expression. To assess the effect of MCTs in the invasion activity of T24 cells, we used specific siRNA targeting MCT1 and MCT4 in CAFs to inhibit MCT1 and MCT4 in CAFs. We assessed invasion activities by comparing the numbers of T24 cells invading through the matrigel channels (). We found that the invasion activity of T24 cells was strongly and positively correlated with MCTs expression in 3D co-culture environment ().
Discussion
Recently, the role of ‘Warburg effect’ has been one of the renewed interests in human cancer. A growing number of evidence suggests that energy metabolism plays the key role in malignant progression of cancer and represents an important target for cancer therapy. Although some targeted drugs have been commercialized, the outcome of application could not match the expectations. The heterogeneity of tumor cells could be one of the reasons. It is different subunits even in one tumor that lead to the decline of sensibility of targeted drugs. In our opinion, the overlook of tumor microenvironment has inescapable relationship with tough problem. In vivo, cancer cells were cultured in dishes in conventional methods such as transwell assayCitation16 or bathing cells in conditioned medium,Citation17 but with poor in vivo-like microenvironment reconstruction.Citation18,19 In the present study, a 3D microfluidic device was developed to reconstruct an in vivo-like tumor microenvironment for bladder cancer cells.
In the 3D microfluidic device, bladder cancer cells and fibroblasts were co-cultured with indirect contact in 3D mode. Compared to the conventional methods, our device has the following advantages. First, to mimic the co-microenvironment in vivo, we embedded hFF cells and T24 cells in a physically relevant 3D microenvironment. It has been confirmed that cells in 3D culture environment represent more real morphogenesis and gene expression profiles that closely resemble the in vivo biological activities than 2D culture environment.Citation28,29 So we think that embedding fibroblasts and bladder cancer cells in matrix, receiving the soluble factors secreted by each other continuously, is more faithful and visual to investigate the function of CAFs. Second, medium inlet was used to supply and transfer medium, buffers, and even waste products from cellular metabolism, thus resembling the function of human circulatory system. Third, the microfluidic device allows for real-time imaging of the interactions between multiple cell types exposed to both autocrine and paracrine signaling molecules, all within a 3D ECM environment.
We come to realize that CAFs, the major component of the extracellular matrix, was involved in tumor occurrence, proliferation, evolution and metastasis in various types of cancers.Citation30,31 Cancer cells induce fibroblasts into CAFs with a transformation of dysfunctional mitochondria,Citation7 therefore, CAFs rely on enhanced glycolysis, leading to increased lactate to produce energy for the cancer cells.Citation8,32 In the present study, fibroblasts was induced into CAFs by T24 cells, which was characterized by the upregulated α-SMA and downregulated CD34.
Lactate, identified as a major energy fuel in tumors, is abundantly synthesized by aerobic glycolysis to offer sufficient ATP for tumor microenvironment.Citation15 To avoid acidosis and to keep metabolic steady, a series of transporters must be employed in the transmembrane transportation of metabolite. MCT1 and MCT4 are regards as the prominent path for lactate metabolism by bidirectional bladder cancer cells and CAFs.Citation33 As representative rate-limiting enzyme, MCT4 transports lactate out of the cell and MCT1 regulates the entry of lactate into tumor cells. It has been reported that accumulation of lactate within tumors is associated with a poor clinical outcome.Citation33,34 So, we conjectured that MCT1 and MCT4 were involved in the proliferation and metastasis of bladder cancer. It was verified by the evidence that MCT1 and MCT4 were overexpressed in CAFs and inhibition of them resulted in the suppression of T24 cell proliferation and invasion and the decrease of lactate production.
The results of this study demonstrate several interesting issues. First, the cytokines from bladder cancer cells effectively transformed the co-cultured fibroblasts into CAFs by indirect contact. Second, the expression of MCT1 and MCT4 in CAFs could be elevated by the co-culture with bladder cancer cells, increasing concentration of lactate and then supply energy for bladder cancer cells by aerobic glycolysis. Third, the overexpression of MCT1 and MCT4 in CAFs is tightly associated with the proliferation and metastasis of bladder cancer cells. Fourth, inhibition of MCTs by siRNA and inhibitors, quercetin or simvastatin suppressed the proliferation, promoted apoptosis and reduced the invasion ability of T24 cells, and blocked lactate transportation. All these results suggested that over-expression of MCT1 and MCT4 in CAFs were involved in the proliferation and metastasis activity of bladder cancer cells in the 3D microenvironment.
In summary, we described a simple microfluidic device for the functional investigation of the response of CAFs induced by bladder cancer cells. We observed that elevated MCT1 and MCT4 expression in CAFs is associated with the proliferation and metastasis of bladder cancer cells. These findings suggest that MCT1 and MCT4 may be potentially used as a novel biomarker and even a bladder cancer therapeutic target. The methodology reported is straightforward and easy to operate. It can be used not only to investigate the cellular events between fibroblasts and cancer cells, but can also provide an effective method for interaction between multiple heterotypic cells and mimic the sophisticated microenvironment in vivo.
Materials and Methods
Design and fabrication of the microfluidic co-culture device
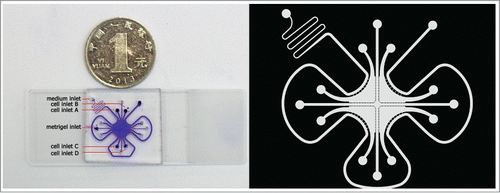
The microfluidic device was fabricated in PDMS (Sylgard 184, Dow Corning, USA) by standard lithography methodsCitation35 and was irreversibly bonded to a glass slide assisted by oxygen plasma surface treatment. This device consisted of 4 separated culture chambers for the culture of 2 groups of cells simultaneously. As shown in , 6 inlets (200 μm) were drilled on this device, with one inlet for medium loading, one inlet for metrigel loading and the other 4 inlets for cell loading. In order to balance the pressure, each inlet may correspond to an outlet. Microchannel was employed as a microbridge so each chamber could be linked together. A series of micro-pillars (100 μm × 50 μm) with the micro-gaps, the main component of microchannel, were placed to block the cells cross from the adjacent chambers. As a result, only the soluble secreted from the cells could move through to each other chamber of 3D cell culture device. In a word, the device was mainly used to supply the cells mimicking the 3D co-culture microenvironment in vivo.
3D co-culture of T24 cells and hFF cells on the microfluidic device to induce the fibroblasts to be CAFs
In our study, the human bladder cancer cell line T24 and the human fibroblast cell line hFF were used to verify the hypothesis we presented. The two types of cells were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium (Hyclone, USA), supplemented with 10% fetal bovine serum (Gibco, USA) and 1% antibiotic (Hyclone, USA) at 37°C with 5% CO2 and 95% relative humidity.
In order to test whether the bladder cancer cells can induce the fibroblasts to be CAFs, the bladder cancer cells were divided into experimental group and control group. In the experimental group, the suspension of T24 cells and hFF cells in medium which was premixed with metrigel (356234, Corning, USA) (3:1) were infused into different 3D culture chambers through matched inlets. In the control group, only hFF cells were infused into cell culture chamber while T24 cells were substituted by medium-metrigel mixture. The metrigel channel was infused by medium-metrigel mixture (1:1) to mimic the extracellular matrix.
a-SMA and CD34 assay on hFF cells by immunofluorescence imaging
Expression of α-smooth muscle actin (α-SMA) and CD34, well accepted markers for CAFs,Citation25,26 was detected and used to examine the effect of induction by cancer cells. Briefly, after 3 d of co-culture, hFF cells were fixed in 2.5% glutaraldehyde for 20 min, permeabilized in phosphate buffer saline (PBS) containing 0.1% Triton X-100 for 15 min, and blocked with 1% bovine serum albumin for 30 min. Then, the cells were incubated with primary antibody (Boster, China, 1:400) over night at 4°C. The next day, they were rinsed with PBS twice, incubated with FITC green-conjugated secondary antibody (Boster, China, 1:100) for 1 h. The nuclei were labeled with 4,6-diamidino-2-phenylindole (Solarbio, China), and the cells were visualized using fluorescence microscopy (Nikon, Japan).
Lactate measurement
To measure the concentration of lactate in the medium and cell lysates of different chambers in 3D co-culture devices, EnzyChromTM Lactate Assay kit (ECLC-100) (BioAssay Systems, Hayward, USA) was used according to the manufacturer's instructions. Briefly, the standard curve was created with a series of known concentration of lactate solution. Then, the concentration of lactate in 20 uL culture medium or cell lysates was detected by spectrophotometry at 565 nm. For the scattergram, the concentration of lactate in the medium without cells was subtracted.
Knockdown MCT1 and MCT4 with siRNA
The sequence of the MCT1-targeted siRNA (5′- GGAAGAGACCAGUAUAGAU dTdT -3′) and MCT4-targeted siRNA (5′- CGGCAUCACUAACCUGCUU dTdT -3′) were chosen out of 3 individual siRNAs respectively because of the high efficiency in reducing MCT1, MCT4 at mRNA and protein level. We used a scrambled-sequence siRNA duplex as a negative siRNA control. All siRNAs were designed and synthesized by Guangzhou Ribobio Company (China). hFF cells were transiently transfected with 50 nM siMCT1 and/or siMCT4 in 3D co-culture devices using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, USA) according to the manufacturer's instructions. Transfected cells were used for quantitative real-time reverse transcriptase PCR, protein gel blotting, cell cycle analysis, proliferation assay, apoptosis assay and invasion assay.
Western blot
MCT1 and MCT4 proteins were detected in the membrane fraction of the cells. For this purpose, cells were washed with PBS and then ice-cold lysis buffer was immediately transferred into co-culture chambers for 30 min. Then the protein samples were purified by centrifugation at 15000 g for 5 min at 4°C. Protein samples (10 ul) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8% gel. Samples were blotted onto PVDF membrance (Bio-Rad, USA). The membrane was blocked with 5% nonfat dry milk in tris buffered saline with 0.1% Tween 20 (TBST) for 2 h at room temperature. The blots were incubated with the specific antibody at 4°C overnight. The following antibodies were used: antibodies against human MCT1, MCT4 and β-actin at 1:1000 dilution (Proteintech Group, USA). After washing in TBST for 3 × 10 minutes, the blots were incubated with anti-rabbit IgG horseradish peroxidase conjugate at 1:5000 dilution (Abgent, San Diego, USA) for 2 hours at room temperature. Antigen was detected using standard chemical luminescence methodology (UVP, USA).
Cell proliferation assay
Cell proliferation was determined with the Cell Counting Kit-8 (CCK-8) assay (Yiyuanbiotech, China). Twenty four hour after transfected with siRNA or inhibited by QC and SS as described above, the T24 cells from co-culture devices were suspended at a final concentration of 3 × 10Citation3 cells/well and cultured in 96-well plates. After overnight culture, the CCK-8 reagent (10 uL) was added to each well of a 96-well plate containing 100 uL culture medium and the plate was incubated for 2 hours at 37°C. Viable cells were evaluated by absorbance measurements at 470 nm. After 48 h and 72 h of dispose, the cells were stained in the same way.
Cell apoptosis assay
T24 cells and hFF cells were co-cultured at the 3D co-culture devices as previously mentioned. The experimental group's hFF cells were transfected with siRNA or inhibited by MCT inhibitors respectively. After an additional incubation of 48 hours, the co-cultured T24 cells were harvested, stained with acridine orange (AO) and ethidium bromide (EB), and visualized using fluorescence microscopy (Nikon, Japan).
Cell invasion assay
As previously mentioned, T24 cells and hFF cells were seeded into each staggered chamber. Metrigel was full filled into the cross microchannel in order to block the cells from crossing the adjacent chambers. But as is well known, tumor cells, secreting MMP (Matrix Metallo Preteinases), could invade through the metrigel. So in our study, we transfected the CAFs, co-cultured with T24 cells, with siM CT1 and/or siMCT4. Then we compared the numbers of T24 cells invading through the matrigel channels with control group after 24 h.
Statistical analyses
Student's t-test and one-way ANOVA were used for statistical significance, respectively, with values of P < 0.005 considered statistically significant. Two-sided tests were used throughout the analyses. All statistical evaluations were performed with SPSS, ver. 19.0 (SPSS. Inc., Chicago, USA).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a grant from National Natural Science Foundation of China (Nos. 30901481, 81372752, 81472411); Wu JiePing Medical Foundation (320.6750.13261); Natural Science Foundation of Shandong Province, China (ZR2014HM088).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5-29; PMID:25559415
- Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy 2006; 53:233-56; http://dx.doi.org/10.1159/000104457
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009; 324:1029-33; PMID:19460998; http://dx.doi.org/10.1126/science.1160809
- Rivera LB, Bergers G. Targeting vascular sprouts. Science 2014; 344:1449-50; PMID:24970064; http://dx.doi.org/10.1126/science.1257071
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6:392-401; PMID:16572188; http://dx.doi.org/10.1038/nrc1877
- Ghesquière B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature 2014; 511:167-76; http://dx.doi.org/10.1038/nature13312
- Martinez-Outschoorn UE, Balliet RM, Rivadeneira D, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer K, Lin Z, Witkiewicz A. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 2010; 9:3276-96; http://dx.doi.org/10.4161/cc.9.16.12553
- Guido C, Whitaker-Menezes D, Lin Z, Pestell RG, Howell A, Zimmers TA, Casimiro MC, Aquila S, Ando S, Martinez-Outschoorn UE. Mitochondrial fission induces glycolytic reprogramming in cancer-associated myofibroblasts, driving stromal lactate production, and early tumor growth. Oncotarget 2012; 3:798; PMID:22878233
- Rattigan YI, Patel BB, Ackerstaff E, Sukenick G, Koutcher JA, Glod JW, Banerjee D. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp Cell Res 2012; 318:326-35; PMID:22178238; http://dx.doi.org/10.1016/j.yexcr.2011.11.014
- Karagiannis GS, Petraki C, Prassas I, Saraon P, Musrap N, Dimitromanolakis A, Diamandis EP. Proteomic signatures of the desmoplastic invasion front reveal collagen type XII as a marker of myofibroblastic differentiation during colorectal cancer metastasis. Oncotarget 2012; 3:267; PMID:22408128
- Halestrap AP. The monocarboxylate transporter family—structure and functional characterization. IUBMB Life 2012; 64:1-9; PMID:22131303; http://dx.doi.org/10.1002/iub.573
- Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P, Chiarugi P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res 2012; 72:5130-40; PMID:22850421; http://dx.doi.org/10.1158/0008-5472.CAN-12-1949
- Végran F, Boidot R, Michiels C, Sonveaux P, Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res 2011; 71:2550-60; http://dx.doi.org/10.1158/0008-5472.CAN-10-2828
- Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol 2009; 92:329-33; PMID:19604589; http://dx.doi.org/10.1016/j.radonc.2009.06.025
- Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 2008; 118:3930; PMID:19033663
- Buess M, Nuyten D, Hastie T, Nielsen T, Pesich R, Brown PO. Characterization of heterotypic interaction effects in vitro to deconvolute global gene expression profiles in cancer. Genome Biol 2007; 8:R191; PMID:17868458
- Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res 2010; 70:6945-56; PMID:20699369; http://dx.doi.org/10.1158/0008-5472.CAN-10-0785
- Hsu T-H, Kao Y-L, Lin W-L, Xiao J-L, Kuo P-L, Wu C-W, Liao W-Y, Lee C-H. The migration speed of cancer cells influenced by macrophages and myofibroblasts co-cultured in a microfluidic chip. Integr Biol 2012; 4:177-82; PMID:22179425; http://dx.doi.org/10.1039/C2IB00112H
- Wong AP, Perez-Castillejos R, Love JC, Whitesides GM. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials 2008; 29:1853-61; PMID:18243301; http://dx.doi.org/10.1016/j.biomaterials.2007.12.044
- Kaji H, Yokoi T, Kawashima T, Nishizawa M. Controlled cocultures of HeLa cells and human umbilical vein endothelial cells on detachable substrates. Lab Chip 2009; 9:427-32; PMID:19156292; http://dx.doi.org/10.1039/B812510D
- Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim H-Y, Putnam AJ, Jeon NL. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip 2009; 9:1740-8; PMID:19495458; http://dx.doi.org/10.1039/b818401a
- Hsu TH, Xiao JL, Tsao YW, Kao YL, Huang SH, Liao WY, Lee CH. Analysis of the paracrine loop between cancer cells and fibroblasts using a microfluidic chip. Lab Chip 2011; 11:1808-14; PMID:21491053; http://dx.doi.org/10.1039/c1lc20090a
- Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse γ-hydroxybutyric acid. AAPS J 2008; 10:311-21; PMID:18523892; http://dx.doi.org/10.1208/s12248-008-9035-6
- Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle 2009; 8:1461-5; PMID:19395855; http://dx.doi.org/10.4161/cc.8.10.8557
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol 2003; 200:429-47; PMID:12845611; http://dx.doi.org/10.1002/path.1398
- Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res 2009; 69:369-78; PMID:19118022
- Izumi H, Takahashi M, Uramoto H, Nakayama Y, Oyama T, Wang KY, Sasaguri Y, Nishizawa S, Kohno K. Monocarboxylate transporters 1 and 4 are involved in the invasion activity of human lung cancer cells. Cancer Sci 2011; 102:1007-13; PMID:21306479; http://dx.doi.org/10.1111/j.1349-7006.2011.01908.x
- Håkanson M, Textor M, Charnley M. Engineered 3D environments to elucidate the effect of environmental parameters on drug response in cancer. Integr Biol 2011; 3:31-8; http://dx.doi.org/10.1039/C0IB00074D
- Abbott A. Cell culture: biology's new dimension. Nature 2003; 424:870-2; PMID:12931155; http://dx.doi.org/10.1038/424870a
- Chaudhri VK, Salzler GG, Dick SA, Buckman MS, Sordella R, Karoly ED, Mohney R, Stiles BM, Elemento O, Altorki NK. Metabolic Alterations in lung cancer–associated fibroblasts correlated with increased glycolytic metabolism of the tumor. Mol Cancer Res 2013; 11:579-92; PMID:23475953; http://dx.doi.org/10.1158/1541-7786.MCR-12-0437-T
- Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. The J Clin Invest 2009; 119:3613; PMID:19920354; http://dx.doi.org/10.1172/JCI38988
- Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal 2012; 16:1264-84; PMID:21883043; http://dx.doi.org/10.1089/ars.2011.4243
- Zhao Z, Wu MS, Zou C, Tang Q, Lu J, Liu D, Wu Y, Yin J, Xie X, Shen J. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-κB pathway. Cancer Lett 2014; 342:150-8; PMID:24012639; http://dx.doi.org/10.1016/j.canlet.2013.08.042
- Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000; 60:916-21; PMID:10706105
- Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat‐plate bioreactor. Biotechnol Bioeng 2001; 73:379-89; PMID:11320508; http://dx.doi.org/10.1002/bit.1071