Abstract
Mitosis is orchestrated by several protein kinases including Cdks, Plks and Aurora kinases. Despite considerable progress toward understanding the individual function of these protein kinases, how their activity is coordinated in space and time during mitosis is less well understood. In a recent article published in the Journal of Cell Biology, we show that CDK-1 regulates PLK-1 activity during mitosis in C. elegans embryos through multisite phosphorylation of the PLK-1 activator SPAT-1 (Aurora Borealis, Bora in human). SPAT-1 variants mutated on CDK-1 phosphorylation sites results in severe delays in mitotic entry, mimicking embryos lacking spat-1 or plk-1 function. We further show that SPAT-1 phosphorylation by CDK-1 promotes its binding to PLK-1 and stimulates PLK-1 phosphorylation on its activator T-loop by Aurora A kinase in vitro. Likewise, we find that phosphorylation of Bora by Cdk1 promotes phosphorylation of human Plk1 by Aurora A suggesting that this mechanism is conserved in humans. These results indicate that Cdk1 regulates Plk1 by boosting its kinase activity. Here we discuss these recent findings and open questions regarding the regulation of Plk1/PLK-1 by Cdk1/CDK-1 and Bora/SPAT-1.
Keywords:
Mitotic entry comprises a series of irreversible events such as chromosome condensation and nuclear envelope breakdown and has thus to be tightly regulated. Several protein kinases, including Cyclin-dependent kinase (Cdk1) that makes complexes with CyclinB, Aurora kinases and the Polo-like kinase (Plk1) act in concert to regulate mitotic entry.Citation1 Plk1 is an important regulator of mitotic entry.Citation2,3 In particular, Plk1 promotes mitotic entry by activating Cdc25Citation4 and inactivating Wee1/Myt1,Citation5,6 which contributes to Cdk1-Cyclin B activation.
With its highly stereotypical asynchronous cell division pattern, the early C. elegans embryo represents an ideal system to study the mechanisms regulating mitotic entry in a developmental context and to analyze precisely the effect of small perturbation on this process.Citation7 Furthermore, the regulators of mitotic entry are well conserved in C. elegans. In the early embryo, PLK-1 is required for CDC-25 activation, which in turn leads to CDK-1 activation and mitotic entry. Our recent work indicates that CDK-1 also activates PLK-1 and this is critical for timely mitotic entry in the early embryo.
Overview of Plk1 Structure and Regulation
The Plk1 kinase is composed of an N-terminal kinase domain (KD), a C-terminal Polo box Domain (PBD) containing 2 Polo-Boxes (PB1 and PB2) and an Inter Domain Linker (IDL)Citation8 ().
Figure 1. Cdk1 regulates Plk1 localization and activation. (A) Plk1 primary structure and organization. Plk1 contains a N-terminal serine/threonine kinase domain (blue) and a C-terminal Polo-binding domain (PBD) containing 2 Polo Boxes (PB1 and PB2 in green) separated by a loop (L2, red). The Inter-Domain linker (IDL, orange) links the PBD and the KD. The activating T-loop phosphorylation site (T210) located in the KD is shown with a red line. (B) Mechanisms of Plk1 regulation. In the auto-inhibited Plk1 state (a), the PBD engages in an intramolecular interaction with the KD via the loop region L2, which inhibits the KD, and the IDL prevents access of the activating site in the T-loop to Aurora A kinases. Phospho-peptide binding (b) induces a conformational change of the L2 region that disrupts the KD-PBD interaction and contributes to Plk1 activation. Phosphorylation of the T-loop releases the inhibitory effect of the IDL (c). These two mechanisms contribute to Plk1 activation (d). CDK-1-Cyclin B (yellow) primes several substrates for interaction with the PBD thereby contributing to its basal activation and phosphorylates SPAT-1 to promote its interaction with PLK-1, possibly contributing to displace the IDL, which facilitates T-loop phosphorylation.
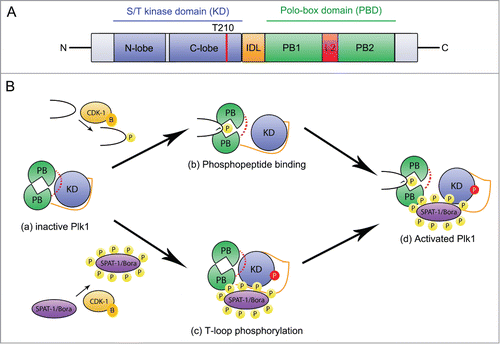
The PBD has a crucial role in the proper subcellular localization of Plk1.Citation9-11 For instance, the PBD fused to the green fluorescent protein (GFP) recapitulates the subcellular localization of endogenous PLK-1 in the early C. elegans embryo.Citation12 The PBD serves as a phospho-peptide binding module that preferentially recognizes a sequence motif of Ser - (pThr/pSer) – Pro/X (X indicates any amino acid), which is phosphorylated by proline-directed kinases such as Cdk1 (Polo-docking site).Citation13-15 Phosphopriming by Cdk1 occurs for several Plk1 targets allowing a coupling between Cdk1 and Plk1 activities. In particular, this mechanism allows Cdk1 to regulate the subcellular localization of Plk1 during mitosis. However, other kinases are also responsible for priming phosphorylation. For instance, in the early C. elegans embryo, the minibrain kinase MBK-2 phosphorylates the cell fate determinant MEX-5 on a polo-docking site to promote PLK-1 binding and its asymmetric localization in the early embryo.Citation12 In other cases, the KD of Plk1 is responsible for the priming phosphorylation and thus creates the phosphorylated site that is bound by the PBD. The PBD can also bind non-phosphorylated targets.Citation11 For instance in Drosophila, the microtubule-associated protein Map205 binds strongly with the PBD in a phospho-independent manner.Citation16 Interestingly in this case, Cdk1-dependent phosphorylation of Map205 is required to disrupt this interaction and to release Map205 from Plk1.Citation16 Interaction of the PBD with its targets can also be regulated by ubiquitination of the PBD.Citation17 The PBD is thus a protein-protein interaction module capable of various types of interactions.Citation11
The kinase activity of Plk1 is regulated by phosphorylation of the KD on the T-loop by Aurora kinases on a conserved threonine residue (T194 in C. e PLK-1 and T210 in H. s Plk).Citation18-22 The KD and the PBD regulate each other's activities allosterically.Citation23,24 Priming phosphorylation of the target allows binding to the PBD, which relieves the auto-inhibition on the KD by the PBD whereas the KD inhibits the binding of the phospho-peptide with the PBD. Autoinhibited Plk1 can thus be activated through phosphorylation of the T-loop by upstream kinases and binding of the PBD to phospho-peptides (). A recent report revealed how phosphorylation of the KD by Aurora B regulates allosterically the binding of the PBD to Map205 in Drosophila, contributing to the release of Map205 from Plk1.Citation25 Plk1 KD is regulated by other phosphorylation events and notably S137 phosphorylation that appears to interfere with the inhibitor interaction of the PBD on the KD.Citation23,24
Plk1 activation is also controlled by its cofactor Bora, which was originally identified in Drosophila in a screen for mutants defective in asymmetric cell division.Citation26 As Bora accumulates in G2 phase of the cell cycle in human cells, it interacts with Plk1 and this interaction likely exposes the activating site located in the Plk1 T loop for Aurora A kinase phosphorylation.Citation19,20 Consistent with this, Bora depletion in human cells interferes with Plk1 activity and affects mitotic entry and progression.Citation19,20,27,28 In C. elegans, the Bora homolog SPAT-1 (Suppressor of PAR-2), which was also originally identified in a polarity screen,Citation29 physically interacts with PLK-1 and its depletion phenocopies plk-1 inactivation.Citation30,31 SPAT-1 and Bora are thus well-established cofactor of PLK-1/Plk1 in C. elegans and humans respectively. However, how their interaction is regulated was unclear.
How is the Interaction between Bora/SPAT-1 and Plk1/PLK-1 Regulated?
Phosphorylated (p)Bora accumulates at the G2-M transition and pBora/pSPAT-1 co-precipitate with Plk1/PLK-1, both in human cellsCitation19,27 and in C. elegans embryos,Citation30 suggesting that the interaction between Bora/SPAT-1 and Plk1/PLK-1 might be regulated by phosphorylation. However, Seki et al. showed that the phospho-Bora/Plk1 complex is resistant to lambda phosphatase treatment indicating that Bora phosphorylation is dispensable for the interaction between Bora and Plk1, or at least, not required to maintain this interaction.Citation19 The finding that, non-modified Bora produced in E. coli, enhanced Aurora A-mediated T-loop phosphorylation of Plk1 is consistent with Bora phosphorylation being dispensable for its interaction with Plk1.Citation19,20 In agreement with these observations, we found that Bora, produced in E. coli, stimulated Aurora A activity toward Plk1.Citation22 However, this effect was greatly enhanced when Bora was pre-phosphorylated by Cdk1-CyclinB, strongly suggesting that Cdk1 facilitates Bora binding to Plk1 and in turn Plk1 phosphorylation by Aurora A.
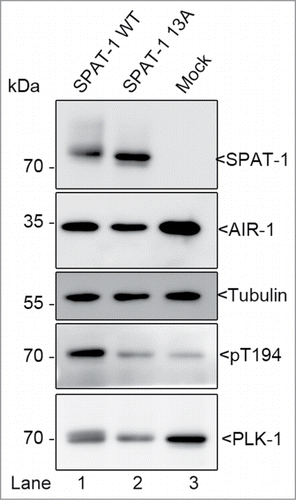
In C. elegans, we found that SPAT-1 phosphorylation by Cdk1-CyclinB is strictly required for PLK-1 phosphorylation of the T-loop by Aurora A. We showed that SPAT-1 phosphorylation by Cdk1-CyclinB is essential to trigger its interaction with PLK-1, and in turn, to promote PLK-1 phosphorylation by the Aurora A kinase in vitro. In contrast to Bora, non-modified SPAT-1 had no effect on PLK-1 phosphorylation by Aurora A, and we recently obtained similar results using C. elegans AIR-1 (the C. elegans homolog of Aurora A) as activating kinase (). It is worth mentioning that it is still unclear which kinase activates PLK-1 in C. elegans. Phenotypic and biochemical analysis revealed that AIR-1 either is not the activating kinase or not the only one.Citation30,31 Therefore, one possibility is that non-modified SPAT-1 might be able to activate PLK-1, similarly to Bora, but AIR-1 is not the physiological kinase and this prevents the detection of this activation in our in vitro assays. Alternatively SPAT-1 might be more dependent than Bora on Cdk1 phosphorylation to interact with and activate PLK-1. Nevertheless, our observations clearly indicate that SPAT-1 and Bora phosphorylation by Cdk1 greatly enhances Plk1/PLK-1 phosphorylation on the activating T-loop by Aurora A/AIR-1 kinase (Citation22 and ).
Figure 2. SPAT-1 wild-type but not the non-phoshorylatable SPAT-1 13A stimulates PLK-1 T-loop phosphorylation by AIR-1. Westernblot analysis of protein extracts obtained from baculovirus-infected Sf9 cells co-expressing AIR-1, PLK-1, and SPAT-1 WT (Lane 1) or SPAT-1 13A (lane 2) or co-expressing only AIR-1 and PLK-1 (Lane 3) revealed with 6xHis (His-AIR-1), SPAT-1, PLK-1, Tubulin and the T-loop phosphospecific antibody (T194 in PLK-1).
What are the SPAT-1 Phospho-sites Required for Plk1 Activation?
Phosphorylated forms of SPAT-1, modified at multiple sites, co-immunoprecipitate with PLK-1 suggesting that the interaction between SPAT-1 and PLK-1 may require multisite phosphorylation of SPAT-1 by CDK-1. Consistently, we found that SPAT-1 is phosphorylated at 13 Sp/Tp sites by Cdk1 in vitro, among which at least 7 are also phosphorylated in vivo. By expressing GFP-SPAT-1 transgenes resistant to RNA interference, we could test the contribution of these sites to SPAT-1 function in vivo upon depletion of endogenous SPAT-1 by RNAi. Inactivation of SPAT-1 in WT animals delays mitotic entry in the early embryo, which can be easily monitored by time-lapse Nomarski microscopy. This delay in mitotic entry was rescued by expressing a GFP::SPAT-1 wild-type transgene but not a non-phosphorylatable GFP::SPAT-1 13A mutant indicating that SPAT-1 phosphorylation is required for timely mitotic entry. We then mapped the critical phospho-sites and found that phosphorylation sites located in the most conserved N-terminal part of the protein are critical for SPAT-1 function. Embryos expressing a GFP::SPAT-1 7AN mutant transgene, containing 7 Sp/Tp residues in the N-terminal part of the protein mutated into Alanine, phenocopy embryos expressing a non-phosphorylatable GFP::SPAT-1 13A mutant, mutated on the 13 phosphorylation sites.Citation22 In contrast, embryos expressing a GFP::SPAT-1 6AC transgene containing the 6 remaining Sp/Tp residues in the C-terminal part of the protein mutated to alanine failed to show any significant difference from GFP::SPAT-1 wild-type, suggesting that phospho-sites in the C-terminal part are largely dispensable for SPAT-1 function in vivo. However, these might still play an important role in fine-tuning the activation status of SPAT-1 and therefore PLK-1, for instance by influencing the phosphorylation status of the N-terminal part of the protein.
The N-terminal region of SPAT-1 contains a polo-docking site (S251) and phosphorylation of this site by Cdk1 regulates the interaction of SPAT-1 with the PLK-1 PBD.Citation22,30 However, mutation of this site did not prevent binding of SPAT-1, phosphorylated by Cdk1, to PLK-1 in vitro and a GFP::SPAT-1 S251A transgenes rescued endogenous SPAT-1 depletion.Citation22 Consistent with the polo-docking site of SPAT-1 being dispensable for PLK-1 activation in worms, Seki et al showed that the phospho-peptide-binding site in the Plk1 PBD is not required for the activation of Plk1 by Bora and Aurora A Citation19. These observations suggest that the interaction of Bora/SPAT-1 with the Plk1/PLK-1 PBD is dispensable for the ability of these proteins to activate Plk1/PLK-1 phosphorylation by upstream kinases.
Among the remaining N-terminally located residues, we found that at least 2 residues, S119 and S190, contribute to SPAT-1 function. Unfortunately, we could not test the contribution of T229 because as we were not able to obtain transgenic lines expressing this mutated version. However, work is ongoing to test the role of this phosphorylation site and to test whether the combined S119A, S190A and eventually T229A mutations recapitulates the phenotype observed upon expression of GFP::SPAT-1 7AN or 13A mutant transgenes.
Overall, these results indicate that phosphorylation of SPAT-1 by CDK-1 on at least 2 sites located in the most conserved N-terminal part of the protein is critical for PLK-1 activation. Multisite phosphorylation of proteins is a recurrent theme in numerous signaling systems including the cell cycle. Multisite phosphorylation has been proposed to transform a graded protein kinase signal into an ultrasensitive switch-like response.Citation32,33 For instance, Cdc25 phosphatase and the Wee1 kinase are both regulated by multisite phosphorylation by Cdk1.Citation34-36 Wee1 and Cdk1 inactivate each other through inhibitory phosphorylation. Cdk1 phosphorylates the N-terminal part of Wee1 on 5 sites among which 2 inactivate Wee1.Citation34 Therefore, among the 32 possible phosphorylation states, only 8 inactivate Wee1. Furthermore, the sites that are not inactivating Wee1 tend to be phosphorylated first. In such a scenario, Cdk activity has to reach a certain threshold to inactivate Wee1, contributing to render the system bistable. It is tempting to propose that a similar type of regulation might take place in the case of the interaction between SPAT-1 and PLK-1. We speculate that CDK-1-dependent multisite phosphorylation of SPAT-1 might contribute to render PLK-1 and CDK-1 activation ultrasensitive. In such a model, the feedback loop between CDK-1 and PLK-1 would be activated exclusively when CDK-1 activity reaches a certain threshold contributing to make the timing of mitotic entry highly reproducible.
SPAT-1 and Bora Regulation by Phosphorylation and Ubiquitin-Mediated Degradation
Although the role of Plk1 in activating Cdk1-CyclinB via Cdc25Citation4 and Wee1/Myt1 regulationCitation5,6 was well established, the role of Cdk1 in Plk1 activation was less well understood. In Xenopus egg extract, phosphorylation of Bora on a Cdk consensus site T52 blocks Bora degradation by the SCFΒTrCP E3-ligase.Citation37 Cdk1 may thus activate Plk1 not only by promoting the interaction between Bora and Plk1, but also indirectly, by stabilizing Bora. Such a regulation might be required to maintain sufficient Bora levels to sustain Plk1 activity during mitosis.Citation28
Whether CDK-1 similarly controls SPAT-1 stability in nematodes awaits further investigation. SPAT-1 is degraded by the proteasome upon PLK-1 activation (ref.Citation30 and our unpublished data). However, the E3-ligase targeting SPAT-1 for degradation remains to be found. In contrast to Bora, SPAT-1 does not contain a DSGxxS phosphodegron, which is typically recognized by the SCFβTrCP E3-ligaseCitation27,38 and LIN-23 (βTrCP in worm) inactivation does not affect mitotic entry.Citation39 SPAT-1 stability is thus likely controlled by another E3-ligase in worms.
Our results suggest that CDK-1 is the major Cdk phosphorylating SPAT-1 but we cannot exclude that other Cdk, and possibly other kinases may also play a role. Whereas C. elegans SPAT-1 contains 13 Sp/Tp sites, the human Bora homolog contains 26 sites, strongly suggesting that Bora might integrate several signaling pathways to regulate mitotic entry. Interestingly, in human cells Bora is phosphorylated by the GSK3 kinase at the G2-M transition and these phosphorylations contribute to Plk1 activation and mitotic entry but the underlying mechanism remains to be found.Citation40
Finally, although we provided strong evidences that CDK-1 controls SPAT-1 phosphorylation to regulate PLK-1 activation, we presently do not know the identity of the cyclin subunits that associate with CDK-1 to phosphorylate SPAT-1. Noteworthy, CyclinB2 overexpression in mouse causes Aurora A-mediated Plk1 hyperactivation, resulting in accelerated centrosome separation and lagging chromosomes leading ultimately to cancer formation.Citation41 Based on our results, it is tempting to speculate that Bora phosphorylation by CyclinB2/Cdk1 might contribute to Plk1 and Aurora A hyperactivation and tumorogenesis.
Conclusion and Future Directions
In conclusion, we have shown that phosphorylation of SPAT-1 regulates its binding to PLK-1, which promotes PLK-1 phosphorylation on its activating T-loop by Aurora A. Likewise, Bora phosphorylation by Cdk1 greatly enhances Plk1 phosphorylation by Aurora A, suggesting that the mechanism we have uncovered in C. elegans is evolutionarily conserved between worms and humans. It will be critical to identify the phospho-sites in human Bora that contribute to Plk1 activation and to determine their precise contribution in vitro and in vivo. Based on the recent structural analysis of Plk1, it is plausible that Bora phosphorylation facilitates the accessibility of the activation loop of Plk1 to upstream kinases. Clearly, structural analysis of Plk1 in complex with Bora is needed to resolve this issue. Overexpression of Plk1 has been observed in many human cancers,Citation42 therefore, understanding the precise mechanism by which Bora phosphorylation changes Plk1 accessibility to upstream kinases should facilitate the design of novel drugs for cancer therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
NT was supported by a PhD fellowship from the Fondation ARC pour la Recherche sur le Cancer, the City of Paris and from the Who am I? Laboratory of Excellence no. ANR-11-LABX-0071, supported by the French Government through its Investments for the Future program operated by the French National Research Agency (ANR) under grant no. ANR-11-IDEX-0005–01. Work in the laboratory of MG is supported by the Swiss National Science Foundation (Grant number 31003A_156013) and the University of Geneva. Work in the laboratory of LP is supported by the French National Research Agency under grant no. ANR-2012-BSV2–0001–01 and by the Foundation for Medical Research “Equipe FRM DEQ20140329538.”
References
- Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol 2009; 185:193-202; PMID:19364923; http://dx.doi.org/10.1083/jcb.200812045
- Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 2009; 10:265-75; PMID:19305416; http://dx.doi.org/10.1038/nrm2653
- Zitouni S, Nabais C, Jana SC, Guerrero A, Bettencourt-Dias, M. Polo-like kinases: structural variations lead to multiple functions. Nat Rev Mol Cell Biol 2014; 15:433-52; PMID:24954208; http://dx.doi.org/10.1038/nrm3819
- Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep 2002; 3:341-8; PMID:11897663; http://dx.doi.org/10.1093/embo-reports/kvf069
- Watanabe N, Arai H, Nishihara Y, Taniguchi M, Watanabe N, Hunter T, Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFbeta-TrCP. Proc Natl Acad Sci U S A 2004; 101:4419-24; PMID:15070733; http://dx.doi.org/10.1073/pnas.0307700101
- Inoue D, Sagata N. The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J 2005; 24:1057-67; PMID:15692562; http://dx.doi.org/10.1038/sj.emboj.7600567
- Noatynska A, Tavernier N, Gotta M, Pintard L. Coordinating cell polarity and cell cycle progression: what can we learn from flies and worms? Open Biol 2013; 3:130083; PMID:23926048; http://dx.doi.org/10.1098/rsob.130083
- Kothe M, Kohls D, Low S, Coli R, Cheng AC, Jacques SL, Johnson TL, Lewis C, Loh C, Nonomiya J, Sheils AL, Verdries KA, Wynn TA, Kuhn C, Ding YH. Structure of the catalytic domain of human polo-like kinase 1. Biochemistry 2007; 46:5960-71; PMID:17461553; http://dx.doi.org/10.1021/bi602474j
- Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Mutation of the polo-box disrupts localization and mitotic functions of the mammalian polo kinase Plk. Proc Natl Acad Sci U S A 1998; 95:9301-6; PMID:9689075; http://dx.doi.org/10.1073/pnas.95.16.9301
- Jang YJ, Lin CY, Ma S, Erikson RL. Functional studies on the role of the C-terminal domain of mammalian polo-like kinase. Proc Natl Acad Sci U S A 2002; 99:1984-9; PMID:11854496; http://dx.doi.org/10.1073/pnas.042689299
- Park JE, Soung NK, Johmura Y, Kang YH, Liao C, Lee KH, Park CH, Nicklaus MC, Lee KS. Polo-box domain: a versatile mediator of polo-like kinase function. Cell Mol Life Sci 2010; 67:1957-70; PMID:20148280; http://dx.doi.org/10.1007/s00018-010-0279-9
- Nishi Y, Rogers E, Robertson SM, Lin R. Polo kinases regulate C. elegans embryonic polarity via binding to DYRK2-primed MEX-5 and MEX-6. Development 2008; 135:687-97; PMID:18199581; http://dx.doi.org/10.1242/dev.013425
- Elia AE, Cantley LC, Yaffe MB. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 2003; 299:1228-31; PMID:12595692; http://dx.doi.org/10.1126/science.1079079
- Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. The molecular basis for phosphodependent substrate targeting and regulation of Plks by the Polo-box domain. Cell 2003; 115:83-95; PMID:14532005; http://dx.doi.org/10.1016/S0092-8674(03)00725-6
- Cheng KY, Lowe ED, Sinclair J, Nigg EA, Johnson LN. The crystal structure of the human polo-like kinase-1 polo box domain and its phospho-peptide complex. EMBO J 2003; 22:5757-68; PMID:14592974; http://dx.doi.org/10.1093/emboj/cdg558
- Archambault V, D'Avino PP, Deery MJ, Lilley KS, Glover DM. Sequestration of Polo kinase to microtubules by phosphopriming-independent binding to Map205 is relieved by phosphorylation at a CDK site in mitosis. Genes Dev 2008; 22:2707-20; PMID:18832073; http://dx.doi.org/10.1101/gad.486808
- Beck J, Maerki S, Posch M, Metzger T, Persaud A, Scheel H, Hofmann K, Rotin D, Pedrioli P, Swedlow JR, Peter M, Sumara I. Ubiquitylation-dependent localization of PLK1 in mitosis. Nat Cell Biol 2013; 15:430-9; PMID:23455478; http://dx.doi.org/10.1038/ncb2695
- Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol 1999; 19:8625-32; PMID:10567586
- Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 2008; 320:1655-8; PMID:18566290; http://dx.doi.org/10.1126/science.1157425
- Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 2008; 455:119-23; PMID:18615013; http://dx.doi.org/10.1038/nature07185
- Archambault V, Carmena M. Polo-like kinase-activating kinases: Aurora A, Aurora B and what else? Cell Cycle 2012; 11:1490-5; PMID:22433949; http://dx.doi.org/10.4161/cc.19724
- Tavernier N, Noatynska A, Panbianco C, Martino L, Van Hove L, Schwager F, Leger T, Gotta M, Pintard L. Cdk1 phosphorylates SPAT-1/Bora to trigger PLK-1 activation and drive mitotic entry in C. elegans embryos. J Cell Biol 2015; PMID:25753036
- Xu J, Shen C, Wang T, Quan J. Structural basis for the inhibition of Polo-like kinase 1. Nat Struct Mol Biol 2013; 20:1047-53; PMID:23893132; http://dx.doi.org/10.1038/nsmb.2623
- Archambault V, Lepine G, Kachaner D. Understanding the Polo Kinase machine. Oncogene 2015; PMID:25619835; http://dx.doi.org/10.1038/onc.2015.451
- Kachaner D, Pinson X, El Kadhi KB, Normandin K, Talje L, Lavoie H, Lepine G, Carreno S, Kwok BH, Hickson GR, Archambault V. Interdomain allosteric regulation of Polo kinase by Aurora B and Map205 is required for cytokinesis. J Cell Biol 2014; 207:201-11; PMID:25332165; http://dx.doi.org/10.1083/jcb.201408081
- Hutterer A, Berdnik D, Wirtz-Peitz F, Zigman M, Schleiffer A, Knoblich JA. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev Cell 2006; 11:147-57; PMID:16890155; http://dx.doi.org/10.1016/j.devcel.2006.06.002
- Chan EH, Santamaria A, Sillje HH, Nigg EA. Plk1 regulates mitotic Aurora A function through betaTrCP-dependent degradation of hBora. Chromosoma 2008; 117:457-69; PMID:18521620; http://dx.doi.org/10.1007/s00412-008-0165-5
- Bruinsma W, Macurek L, Freire R, Lindqvist A, Medema RH. Bora and Aurora-A continue to activate Plk1 in mitosis. J Cell Sci 2014; 127:801-11; PMID:24338364; http://dx.doi.org/10.1242/jcs.137216
- Labbe JC, Pacquelet A, Marty T, Gotta M. A genomewide screen for suppressors of par-2 uncovers potential regulators of PAR protein-dependent cell polarity in Caenorhabditis elegans. Genetics 2006; 174:285-95; PMID:16816419; http://dx.doi.org/10.1534/genetics.106.060517
- Noatynska, A, Panbianco, C, Gotta, M. SPAT-1/Bora acts with Polo-like kinase 1 to regulate PAR polarity and cell cycle progression. Development 2010; 137:3315-25; PMID:20823068; http://dx.doi.org/10.1242/dev.055293
- Hachet V, Busso C, Toya M, Sugimoto A, Askjaer P, Gonczy P. The nucleoporin Nup205/NPP-3 is lost near centrosomes at mitotic onset and can modulate the timing of this process in Caenorhabditis elegans embryos. Mol Biol Cell 2012; 23:3111-21; PMID:22740626; http://dx.doi.org/10.1091/mbc.E12-03-0204
- Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 2001; 414:514-21; PMID:11734846; http://dx.doi.org/10.1038/35107009
- Ferrell JEJ, Ha SH. Ultrasensitivity part II: multisite phosphorylation, stoichiometric inhibitors, and positive feedback. Trends Biochem Sci 2014; 39:556-69; PMID:25440716; http://dx.doi.org/10.1016/j.tibs.2014.09.003
- Kim SY, Song EJ, Lee KJ, Ferrell JEJ. Multisite M-phase phosphorylation of Xenopus Wee1A. Mol Cell Biol 2005; 25:10580-90; PMID:16287869; http://dx.doi.org/10.1128/MCB.25.23.10580-10590.2005
- Kim SY, Ferrell JEJ. Substrate competition as a source of ultrasensitivity in the inactivation of Wee1. Cell 2007; 128:1133-45; PMID:17382882; http://dx.doi.org/10.1016/j.cell.2007.01.039
- Trunnell NB, Poon AC, Kim SY, Ferrell JEJ. Ultrasensitivity in the Regulation of Cdc25C by Cdk1. Mol Cell 2011; 41:263-74; PMID:21292159; http://dx.doi.org/10.1016/j.molcel.2011.01.012
- Feine O, Hukasova E, Bruinsma W, Freire R, Fainsod A, Gannon J, Mahbubani HM, Lindqvist A, Brandeis M. Phosphorylation-mediated stabilization of Bora in mitosis coordinates Plx1/Plk1 and Cdk1 oscillations. Cell Cycle 2014; 13:1727-36; PMID:24675888; http://dx.doi.org/10.4161/cc.28630
- Seki A, Coppinger JA, Du H, Jang CY, Yates JR, Fang G. Plk1- and β-TrCP-dependent degradation of Bora controls mitotic progression. J Cell Biol 2008; 181:65-78; PMID:18378770; http://dx.doi.org/10.1083/jcb.200712027
- Peel N, Dougherty M, Goeres J, Liu Y, O'Connell KF. The C. elegans F-box proteins LIN-23 and SEL-10 antagonize centrosome duplication by regulating ZYG-1 levels. J Cell Sci 2012; 125:3535-44; PMID:22623721; http://dx.doi.org/10.1242/jcs.097105
- Lee YC, Liao PC, Liou YC, Hsiao M, Huang CY, Lu PJ. Glycogen synthase kinase 3 β activity is required for hBora/Aurora A-mediated mitotic entry. Cell Cycle 2013; 12:953-60; PMID:23442801; http://dx.doi.org/10.4161/cc.23945
- Nam HJ, van Deursen JM. Cyclin B2 and p53 control proper timing of centrosome separation. Nat Cell Biol 2014; 16:538-49; PMID:24776885; http://dx.doi.org/10.1038/ncb2952
- Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene 2005; 24:267-76; PMID:15640842; http://dx.doi.org/10.1038/sj.onc.1208273
