Reproductive risk factors such as nulliparity, early menarchy and late menopause reflect a well-established association between estrogen exposure and breast cancer risk over time. However, not all epidemiological evidence supports this link: In the post-menopausal period for example, estrogen only hormone replacement therapy (HRT) actually appears to reduce breast cancer risk.Citation1 Is it therefore possible that in a proportion of women estrogen is protective, and a more nuanced understanding of breast cancer pathogenesis is now required?
In this regard, examining the role of miRNAs during tumor initiation and progression has provided valuable insight.Citation2 Recently it has emerged that single nucleotide polymorphisms (SNPs) within miRNA binding sites of protein coding genes (miRSNPs) may be important predisposing factors to malignant transformation.Citation3 In breast, a miRSNP which disrupts let-7 binding at the KRAS 3′UTR, is associated with increased overall cancer risk and in particular, Estrogen Receptor negative (ER–ve) disease.Citation4 This KRAS-variant allele is also highly prevalent in women with ovarian cancer diagnosed in the post-menopausal period and appears to mediate alternative and seemingly paradoxical cellular responses to exogenous factors such as chemotherapy drugs.Citation5
This combination of clinical observations led McVeigh and colleagues to investigate how estrogen might impact breast cancer risk and tumor biology for individuals with the KRAS-variant allele.Citation6 In their study, 1712 women with histologically confirmed breast cancer provided saliva samples for KRAS-genotyping and completed questionnaires regarding their reproductive history, use of HRT and personal and family history of cancer. Control samples were provided by women who carried the KRAS-variant allele but were unaffected by cancer.
KRAS-variant patients were no more likely than others to have a family history of breast or ovarian cancer however, they were more likely to have previously undergone oophorectomy or to have relatives diagnosed with multiple primary cancers. Furthermore, it emerged that triple-negative breast cancer (TNBC) was significantly more common in post-menopausal women with KRAS-variant. Notably, the risk of TNBC was particularly elevated in patients who had previously but were no longer using HRT, compared with current users and never users. Past-HRT users with the KRAS-variant were almost 5 times more likely to be diagnosed with TNBC than past-HRT users with non-variant KRAS status; and the tumors were generally of a higher grade. Among past-HRT users, patients with non-variant KRAS were more likely to have ER+ve disease than KRAS-variant patients, and in current and never-user HRT groups, KRAS genotype was not significantly associated with tumor receptor status.
The authors therefore speculated that HRT withdrawal may differentially impact on the subtype and grade of breast cancer depending on KRAS genotype and interrogated their hypothesis by subjecting non-malignant mammary cells to estrogen depletion. Importantly, KRAS-variant isogenic cells underwent transformation on estrogen withdrawal, an effect which was reversed by reintroduction of estrogen to culture media.
When considered together, this evidence raises the possibility that stopping HRT in the post-menopausal period may put women at increased risk of developing breast cancer, particularly if they have KRAS-variant status and/or a previous breast cancer diagnosis. Although this theme was not fully explored, McVeigh and colleagues did establish that KRAS-variant patients exhibit an increased risk of developing multiple primary breast cancers (MPBC) in tandem (synchronous tumors) and over time (metachronous tumors).
The clinical implications of these findings are as follows: Firstly, knowledge of KRAS-variant status may help identify a subset of breast cancer patients at high risk of MPBC who might benefit from more intensive investigation and follow-up. Secondly, as estrogen receptor antagonism is a common component of adjuvant therapy regimes for breast cancer, it is essential to better define the risks associated with KRAS-variant status to ensure the seeds of future malignancies are not sewn during treatment for the index cancer.
On a broader point, more detailed understanding of the molecular underpinnings of cancer may enhance drug development programs and further encourage a shift toward personalized treatment choices. In this respect the current study resonates with previous research which established the link between cetuximab resistance and KRAS mutation status, and changed clinical practice in colorectal cancer.Citation7 With this precedent in mind and with further research, miRNA binding site polymorphisms, which appear to be promising markers of cancer risk and treatment efficacy, may prove to have considerable utility in the clinical setting.Citation5
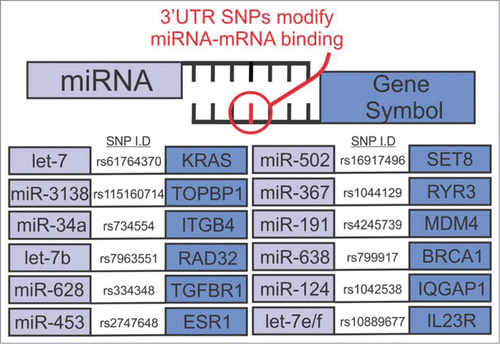
Figure 1. Summary of breast cancer associated SNPs at putative miRNA binding sites; adapted from Cipollini et al.Citation5
References
- Anderson GL, et al. Jama 2004; 291:1701–12; PMID:15082697; http://dx.doi.org/10.1001/jama.291.14.1701
- Eastlack SC, et al. Non-Coding RNA 2015; 1:17–43; http://dx.doi.org/10.3390/ncrna1010017
- Nicoloso MS, et al. Cancer Res 2010; 70:2789–98; PMID:20332227; http://dx.doi.org/10.1158/0008-5472.CAN-09-3541
- Paranjape T, et al. Lancet Oncol 2011; 12:377–86; PMID:21435948; http://dx.doi.org/10.1016/S1470-2045(11)70044-4
- Cipollini M, et al. Pharmacogenomics Pers Med 2014; 7:173–91; PMID:25114582
- McVeigh T, et al. Cell Cycle 2015; PMID:25961464
- Lievre A, et al. Cancer Res 2006; 66:3992–5; PMID:16618717; http://dx.doi.org/10.1158/0008-5472.CAN-06-0191
