How embryonic stem cells (ESCs) retain their pluripotent capacity, commit to specific cell lineages during differentiation and ultimately yield all cell types of a fully formed organism remain major questions in the field. Stem cell differentiation is an essential biological process that influences embryonic development, adult tissue homeostasis, aging and disease. A series of epigenetic mechanisms have been ascribed to the process of ESC differentiation.Citation1 Particularly, epigenetic dynamics involving DNA methylation and demethylation patterns are an integral component of ESC differentiation and remain an active area of research. DNA demethylation occurs passively during progressive cell divisions leading to a successive dilution and consequent disappearance of 5-methylcytosine (5mC).Citation2 Additionally, the loss of 5mC is accomplished through an active DNA demethylation process by the Ten-eleven translocation (TET) enzymes, which are Fe(II)- and 2 oxoglutarate (2OG)-dependent dioxygenases that successively oxidize 5mC into 5-hydroxymethylcytosine (5hmC), 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC), which are then subjected to DNA repair to produce an unmethylated cytosine (5C).Citation3-5 TET enzymes along with its oxidized products are enriched in ESCs; however, their specific functions and mechanisms of action during ESC differentiation remain largely unknown.
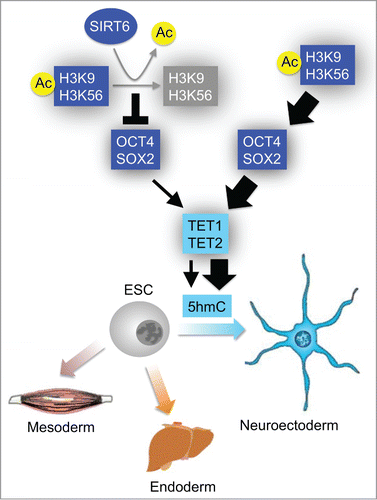
We have recently determined that the histone deacetylase Sirtuin 6 (SIRT6) plays a critical role during ESC differentiation through a mechanism that involves TET-dependent production of 5hmC.Citation6 More specifically, we found SIRT6 to negatively regulate the expression of the core pluripotent genes Oct4, Sox2 and Nanog via deacetylation of histone H3 acetylated at lysine 9 (H3K9ac) and 56 (H3K56ac) (Fig. 1). Concordantly, these core pluripotent genes are upregulated in SIRT6 knockout (S6KO) ESCs and failed to be silenced upon differentiation. OCT4, SOX2 and NANOG are transcription factors essentially required for the maintenance of the pluripotency state.Citation7 Earlier, Koh and coworkers proposed a positive regulatory role for OCT4 and SOX2 affecting the expression of Tet genes.Citation8 We found that S6KO ESCs exhibit an increased recruitment of OCT4 and SOX2 to their targeted sites within Tet genes, thereby supporting a role for these core pluripotent factors as transcriptional activators of Tet gene expression.Citation6 Consistently, TET levels are upregulated in S6KO ESCs along with increased levels of 5hmC, particularly enriched at neural related genes, such as the Hoxa cluster required for development of the neural crest. This underlies a differentiation phenotype in S6KO ESCs that favors the expression of 5hmC-enriched neural genes resulting in an overrepresentation of the neuroectoderm germ layer, thereby supporting a role for 5hmC as an epigenetic mark regulating gene expression rather than a mere DNA demethylation intermediate (Fig. 1). Our studies demonstrated this neuroectoderm developmental bias in vivo through assaying teratoma formation and by generating mouse chimeras, which confirmed the ability of S6KO ESCs to enhance the neural development pathway.Citation6 Notably, we also found SIRT6 to be recruited to Oct4 and Sox2 loci in human ESCs resulting in an upregulation of OCT4 along with TET enzymes and the neuroectoderm specific factor Nestin.Citation6 Thus, the ability of SIRT6 to directly control expression of the core pluripotent genes connected with the regulation of TET enzymes along with neural gene expression is evolutionary conserved from mouse to humans.
Importantly, by means of genome-wide analyses, we established the hierarchical activity of SIRT6 in having a direct regulatory effect on the core pluripotent genes, which in-turn promote TET expression.Citation6 More specifically, the SIRT6 substrates H3K9ac and H3K56ac are involved in promoting expression of the pluripotent gene network and are not associated with the 5hmC-targeted sites engaged in activating neural gene expression. Therefore, the significance of our work extends the epigenetic field by adding a new layer of complexity involving 5hmC as an epigenetic determinant regulating expression of specific genes whose function is required for neuroectoderm development during differentiation of ESCs. Importantly, we found S6KO neural progenitors (NPCs) to have a higher efficiency of reprogramming toward induced pluripotent stem cells (iPSCs), supporting a role for SIRT6 as a negative regulator controlling expression of the core pluripotent genes.Citation6 The ability of SIRT6 to repress expression of Oct4, Sox2 and Nanog genes becomes more apparent during ESC differentiation, which requires silencing of the pluripotency gene network to allow appropriate cell fate specifications and developmental progression. Since forced expression of these core pluripotent genes is essential for generation of iPSCs,Citation7 our finding impels the idea of SIRT6 to be a roadblock toward reprogramming of somatic cells.Citation6
Collectively, our work supports the concept that TET-dependent oxidized forms, such as 5hmC, may function as new epigenetic determinants needed for regulating gene expression during ESC differentiation, and opened up obvious and intriguing questions for future studies: First, what are the epigenetic readers of these TET-dependent oxidized forms associated with transcriptional regulation of genes required for cell fate specification during ESC differentiation? Second, how other epigenetic marks, such as histone modifications, could influence 5hmC-dependent regulation of gene expression? Third, what is the level of plasticity of 5hmC-dependent regulation of gene expression allowing reversible cell fate transitions, such as somatic cell-induced pluripotency and potentially trans-differentiation from one somatic cell type to another? Finally, as TET enzymes, which are enriched in ESCs, can further oxidize 5hmC into 5fC and 5caC, it remains to be determined whether these oxidized products may also have relevant roles during ESC differentiation.
Figure 1. Schematic representation of the interplay between SIRT6 and TET enzymes during ESC differentiation. The expression of the core pluripotent genes Oct4 and Sox2 is directly repressed by SIRT6-dependent deacetylation of H3K9ac and H3K56ac. This SIRT6-dependent activity controls OCT4:SOX2 heterodimer from over-activating the expression of TET enzymes, which then produce 5hmC at neural-related genes, thereby promoting development of the neuroectoderm germ layer. In the absence of SIRT6, elevated levels of acetylated H3K9 and H3K56 trigger the upregulation of OCT4 and SOX2, which in-turn over-activate expression of TET enzymes resulting in elevated levels of 5hmC at neural-related genes and consequently increasing neuroectoderm development.
References
- Chen T, Dent SY. Nat Rev Genet 2014; 15:93-106; PMID:24366184; http://dx.doi.org/10.1038/nrg3607
- Piccolo FM, Fisher AG. Trens Cell Biol 2014; 24(2):136-143; PMID: 24119665; http://dx.doi.org/10.1016/j.tcb.2013.09.001
- Ito S, et al. Science 2011; 333:1300-1303; PMID:21778364; http://dx.doi.org/10.1126/science.1210597
- He YF, et al. Science 2011; 333:1303-1306; PMID:21817016; http://dx.doi.org/10.1126/science.1210944
- Tahiliani M, et al. Science 2009; 324:930-935; PMID:19372391; http://dx.doi.org/10.1126/science.1170116
- Etchegaray JP, et al. Nature Cell Biol 2015; 17(5):545-557; PMID:25915124; http://dx.doi.org/10.1038/ncb3147
- Takahashi K, Yamanaka, S. Cell 2006; 126: 663-676.
- Koh KP, et al. Cell Stem Cell 2011; 8(2):200-213; PMID:21295276; http://dx.doi.org/10.1016/j.stem.2011.01.008
