Comment on: Zou P, et al. Targeting FoxO1 with AS1842856 suppresses adipogenesis. Cell Cycle 2014; 13(23):3759–67; PMID: 25483084; http://dx.doi.org/10.4161/15384101.2014.965977
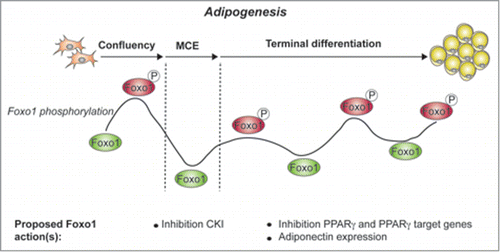
Adipose tissue is increasingly being recognized as a key regulator of whole-body energy homeostasis and consequently as a prime therapeutic target for metabolic syndrome. The differentiation and function of white adipocytes, which can store and release lipids and release hormones (adipokines), and brown adipocytes, which can “burn off” energy, are therefore under intensive study. FOXO1, a member of the Forkhead Box O (FOXO) family of transcription factors, plays an important role in both types of adipocytes. FoxO1+/− mice have smaller, “healthier” white adipocytes when fed a high-fat diet and are protected from developing type 2 diabetes, possibly through de-repression of the adipocyte transcription factor PPARγCitation1 In brown adipose tissue a dominant-negative form of FoxO1 enhances energy expenditure in vivoCitation1 suggesting that inhibition of FoxO1 in white and/or brown adipose tissue may present an attractive therapeutic approach to maintain a healthy energy status. In a recent issue of Cell Cycle, Zou et al.Citation2 now show in a series of in vitro experiments that FoxO1 phosphorylation status – which is inversely associated with its activity – displays a complex, wave-like pattern during adipocyte differentiation, and that inhibition of FoxO1 with the selective inhibitor AS1842856 can suppress differentiation, depending on the timing of addition ()
Figure 1. FoxO1 phoshorylation and activity during 3T3-L1 adipogenesis, as described by Zou et al., indicated are the 3 stages of 3T3-L1 adipogenesis: growing cells to confluency, mitotic clonal expansion (MCE) and terminal differentiation. Active FoxO1 is indicated in green, while inactive, phosphorylated FoxO1 is indicated in red. FoxO1 actions described in previous studies are indicated.Citation1 CKI: cyclin-dependent kinase inhibitor.
Short-term treatment of diabetic db/db mice with AS1842856 ameliorates fasting glycemia, possibly through inhibition of gluconeogenic genes in the liverCitation2 Whether systemic inhibition of FoxO1 in vivo is a realistic therapeutic option warrants further studies, specifically to address the following questions. Firstly, expansion of white adipose tissue may prevent lipid storage in non-adipose organs (liver, muscle, pancreas) and adipose tissue inflammation, processes associated with insulin resistance and type 2 diabetesCitation3 It therefore needs to be established whether AS1842856 suppresses white adipose tissue expansion and harms non-adipose organs. Secondly, FoxO1 inhibition could in principle result in reduced energy expenditure in brown adipose tissue (BAT), and cause weight gain and ultimately type 2 diabetesCitation4,5 Thirdly, FoxO1 inhibition potentially reduces the expression of adiponectin, an adipokine implicated in maintaining a healthy energy balanceCitation6 Fourthly, although it has been suggested that AS1842856 has some specificity for inhibition of FoxO1 and not the other FoxO family members FoxO3 and FoxO4, this has to be further established in vivo. The latter is of high importance, as FOXO transcription factors play crucial roles in controlling fundamental cellular processes (e.g. cell cycle, cell death) and development, and both their negative and positive deregulation has been implicated in several pathologies including cancer, diabetes and stem cell depletionCitation7 Because of these mixed effects of FOXO1 inactivation, both on metabolism and may be on other biological processes, the prime value of selective inhibitors like AS1842856 probably lies in their use as research tools. To dissect the exact role(s) of FoxO1 in whole body energy metabolism, WAT- and BAT-specific FoxO1 knock out mouse models will need to be developed to dissect BAT/WAT phenotypes from systemic metabolic responses evoked by other organs like liver.
References
- Cheng Z, White MF. Antioxid Redox Signal 2011; 14:649–61; PMID:20615072; http://dx.doi.org/10.1089/ars.2010.3370
- Zou P, et al. Cell Cycle 2014; 13:3759–67; PMID:25483084; http://dx.doi.org/10.4161/15384101.2014.965977
- Kim SM, et al. Cell Metab 2014; 20:1049–58; PMID:25456741; http://dx.doi.org/10.1016/j.cmet.2014.10.010
- Nakae J, et al. Diabetes 2008; 57:563–76; PMID:18162510; http://dx.doi.org/10.2337/db07-0698
- Ortega-Molina A, et al. Cell Metab 2012; 15:382–94; PMID:22405073; http://dx.doi.org/10.1016/j.cmet.2012.02.001
- Qiao L, Shao J. J Biol Chem 2006; 281:39915–24; PMID:17090532; http://dx.doi.org/10.1074/jbc.M607215200
- Dansen TB, Burgering BM. Trends Cell Biol 2008; 18:421–9; PMID:18715783; http://dx.doi.org/10.1016/j.tcb.2008.07.004
